Published online Nov 27, 2023. doi: 10.4254/wjh.v15.i11.1237
Peer-review started: August 22, 2023
First decision: September 6, 2023
Revised: September 22, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 27, 2023
Processing time: 94 Days and 5.8 Hours
Chronic Hepatitis C (CHC) affects 71 million people globally and leads to liver issues such as fibrosis, cirrhosis, cancer, and death. A better understanding and prognosis of liver involvement are vital to reduce morbidity and mortality. The accurate identification of the fibrosis stage is crucial for making treatment decisions and predicting outcomes. Tests used to grade fibrosis include histolo
To identify potential biomarkers that might stratify these lesions and add information about the molecular mechanisms involved in the disease.
Plasma samples were collected from 46 patients with hepatitis C and classified into fibrosis grades F1 (n = 13), F2 (n = 12), F3 (n = 6), and F4 (n = 15). To ensure that the identified biomarkers were exclusive to liver lesions (CHC fibrosis), healthy volunteer participants (n = 50) were also included. An untargeted metabolomic technique was used to analyze the plasma metabolites using mass spectrometry and database verification. Statistical analyses were performed to identify differential biomarkers among groups.
Six differential metabolites were identified in each grade of fibrosis. This six-metabolite profile was able to establish a clustering tendency in patients with the same grade of fibrosis; thus, they showed greater efficiency in discriminating grades.
This study suggests that some of the observed biomarkers, once validated, have the potential to be applied as prognostic biomarkers. Furthermore, it suggests that liquid biopsy analyses of plasma metabolites are a good source of molecular biomarkers capable of stratifying patients with CHC according to fibrosis grade.
Core Tip: Chronic Hepatitis C affects 71 million people globally and leads to liver fibrosis, cirrhosis, cancer, and death. The accurate staging of fibrosis is crucial for treatment decisions and outcome prediction. Blood markers are a relevant source of information, and various molecular biomarkers have been investigated to characterize liver fibrosis. We analyzed plasma metabolites by mass spectrometry in 50 healthy participants, and in 46 patients with hepatitis C and classified them into fibrosis grades F1-F4. Six differential metabolites were identified in each grade of fibrosis; their biochemical pathways were analyzed and suggests molecular mechanisms involved in the disease.
- Citation: Ferrasi AC, Lima SVG, Galvani AF, Delafiori J, Dias-Audibert FL, Catharino RR, Silva GF, Praxedes RR, Santos DB, Almeida DTM, Lima EO. Metabolomics in chronic hepatitis C: Decoding fibrosis grading and underlying pathways. World J Hepatol 2023; 15(11): 1237-1249
- URL: https://www.wjgnet.com/1948-5182/full/v15/i11/1237.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i11.1237
Chronic hepatitis C (CHC) is an infectious disease caused by the hepatitis C virus (HCV) and is a serious public health problem, affecting an estimated 71 million people worldwide[1-3].
Approximately 50%-80% of HCV-infected individuals develop CHC, which can trigger a chronic inflammatory disease process leading to liver fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and death[4].
Natural progression of CHC occurs with sustained inflammation due to repetitive liver injury, followed by the activation of hepatic stellate cells, deposition of fibrillar collagen in the extracellular matrix (ECM), and progressive fibrosis[5,6]. These progressive processes may result in ECM degradation and, consequently, vascular and architectural alterations, leading to cirrhosis (10%-20% of patients)[7] or HCC (1%-5%)[8].
Early diagnosis and treatment can prevent liver cirrhosis and HCC, especially with screening and recent advances in CHC treatment based on direct-acting antiviral therapy. However, effective reduction of disease morbidity and mortality requires better characterization of liver involvement, more accurate prognosis, and follow-up[9]. Under this scenario, accurate identification of the liver fibrosis stage is critical for the clinical management of HCC, guiding therapeutic options and helping to predict prognosis[10]. However, this approach is challenging. Tests used to stage fibrosis include histological analysis of liver biopsies and imaging tests. Liver biopsy is considered the “gold standard” for the diagnosis and staging of liver fibrosis. However, it is an invasive and uncomfortable procedure with a risk of minor (10%-20%) or serious (0.5%-1%) complications[7,11]. In addition, the interpretation of histological results is subject to sampling errors and inter-observer subjectivity in the interpretation of histological results[7,12,13]. For staging the grades of fibrosis in biopsied liver tissue, the 0-4 scale of the Metavir classification system[14] is commonly used; however, the main limitations are related to the representativeness of liver samples and histopathological interpretation. Conventional imaging tests include ultrasonography, computed tomography, and magnetic resonance imaging. Although they represent important tools for detecting cirrhosis, nodules on the liver surface, and splenomegaly, they present low sensitivity for moderate or even advanced fibrosis. Newer acoustic technologies, such as hepatic elastography, can increase the accuracy of imaging techniques. For these tests, acoustic vibrations are applied to the abdomen and, according to how quickly these vibrations are transmitted along the liver tissue, the stiffness (fibrosis) of the liver is indicated. However, conditions other than fibrosis also increase liver stiffness[7], which requires further study and standardization. Another important limitation is the cost of the equipment[15], which is unaffordable in places with limited financial resources. In clinical practice, blood markers should be considered a relevant source of information. Current approaches are limited to combining commonly available tests (e.g., aspartate transaminase, alanine aminotransferase, albumin, serum bilirubin, and international normalized ratio) with clinical information (e.g., age, body mass index, and diabetes) and, in some cases, direct markers of liver function. However, this approach is most useful in distinguishing between two levels of fibrosis: Absent to minimal vs moderate to severe and fails to stratify the grades.
Undeniably, the search for blood biomarkers is a less invasive method for diagnosis and prognosis, and as blood circulates through most tissues, it can be a relevant source of information about diseases. Therefore, different molecular biomarkers, particularly those with easier and more accessible analytical methodologies, have been investigated for the characterization of liver fibrosis[16-20].
The present study focused on analyzing the plasma metabolome of patients with CHC with different grades of fibrosis aiming to identify potential biomarkers for stratifying these lesions. The metabolome is the set of endogenously synthesized metabolites in a specific physiological condition and may represent the final product of gene expression. Thus, as a secondary aim of this study, we analyzed the pathways linked to the main metabolites detected, contributing information about the molecular mechanisms involved in the disease.
This study was approved by the Ethics Committee on Research of São Paulo State University in accordance with the provisions of the Declaration of Helsinki. Plasma samples from 46 volunteer participants diagnosed with hepatitis C were obtained from peripheral blood. The inclusion criteria were as follows: Patients > 18 years, unrelated, diagnosed by detection of HCV RNA, with identification of HCV genotype, naïve patients (with no previous hepatitis C treatment), and patients with a known fibrosis stage or clinical diagnosis of cirrhosis by imaging. The exclusion criteria were as follows: Volunteers with a history of liver transplantation, hepatic steatosis unrelated to chronic hepatitis C and other liver diseases. To ensure that the biomarkers identified were exclusive to liver lesions (hepatitis C fibrosis), 50 healthy volunteer blood bank donors [healthy control group (CG)] were included in this study. Participants were recruited from the Viral Hepatitis Outpatient Clinic of Botucatu Medical School, UNESP, Brazil. The demographic and clinical characteristics of the study participants are summarized in Table 1.
| Variables | Healthy control | Fibrosis grade (Metavir)1 | |||
| F1 | F2 | F3 | F4 | ||
| Age (yr) | 44 ± 12.2 | 50 ± 10.78 | 49 ± 8.6 | 55 ± 8.8 | 54 ± 11.22 |
| Sex | |||||
| Male | 24 (65.0) | 8 (61.5) | 7 (58.3) | 5 (100) | 9 (60.0) |
| Female | 26 (52.0) | 5 (38.5) | 5 (41.7) | 0 | 6 (40.0) |
| BMI (kg/m2) | 22.9 ± 2.98 | 28.3 ± 8.89 | 26.5 ± 6.26 | 26.9 ± 3.60 | 27.4 ± 7.14 |
| HCV genotype | |||||
| 12 | - | 10 (77.0) | 8 (66.7) | 3 (50.0) | 12 (80.0) |
| Not 13 | - | 3 (23.0) | 4 (33.3) | 3 (50.0) | 3 (20.0) |
Fibrosis was classified based on the Metavir score[14]. Liver samples were collected by percutaneous biopsy before treatment and analyzed histologically. Peripheral blood was collected at the same time as the liver biopsy.
Samples were collected in tubes with ethylenediamine tetraacetic acid anticoagulant, followed by centrifugation to separate the plasma, which was stored at -80 ℃ until metabolite extraction. At the time of extraction, 20 μL of blood plasma was solubilized in 200 μL of tetrahydrofuran, vortexed, and centrifuged at 3200 rpm for 5 min. Then, the collected supernatant was solubilized in 780 μL of methanol and again centrifuged as above. Afterward, 50 μL of this supernatant was solubilized in 500 μL methanol q.s., homogenized, and subjected to chemical ionization with 0.1% formic acid.
For mass spectrometry analysis, the ionized solution was directly injected into an LTQ Mass Spectrometer (ESI-LTQ-XL Discovery, Thermo Fisher Scientific, Waltham, MA, United States) using electrospray ionization. Ten replicates were used for each biological replicate. The parameters for analysis were set as the following configuration: Sample flow rate of 10 μL/min, capillary temperature of 180 ℃, 7 kV spray voltage, and carrier gas of 2 arbitrary units. After direct injection, the samples were analyzed in the positive ion mode in the mass range of 100-1400 (mass-to-charge ratio), and the signal intensity was detected, which resulted in a set of ions m/z for each sample. XCalibur software (v. 2.4, Thermo Scientific) was used to acquire and process the spectrometer data, which were submitted for statistical analysis.
Statistical analysis was performed using MetaboAnalyst 4.0 platform[21], in which raw data were evaluated using partial least squares discriminant analysis (PLS-DA). As a result, a list of markers was generated according to the intensity of the most differential and important markers for each group evaluated; that is, the variable importance score (VIP score) was obtained. From this, six ions with the highest VIP score for each grade of fibrosis, with scores > 2.0, were selected. The accuracy of the identified biomarkers was assessed by receiver operating characteristic (ROC) curve analysis.
From the selected biomarkers, a search was performed using the METLIN online metabolomics database (http://metlin.scripps.edu) to identify molecules compatible with the mass/charge values selected for each grade of fibrosis. The molecules of interest were added to the candidate list and fragmented in silico using the MassFrontier tool (v. 6.0, Thermo Fisher Scientific). After the fragmentation in silico, the molecules whose fragments were compatible with those generated experimentally were selected.
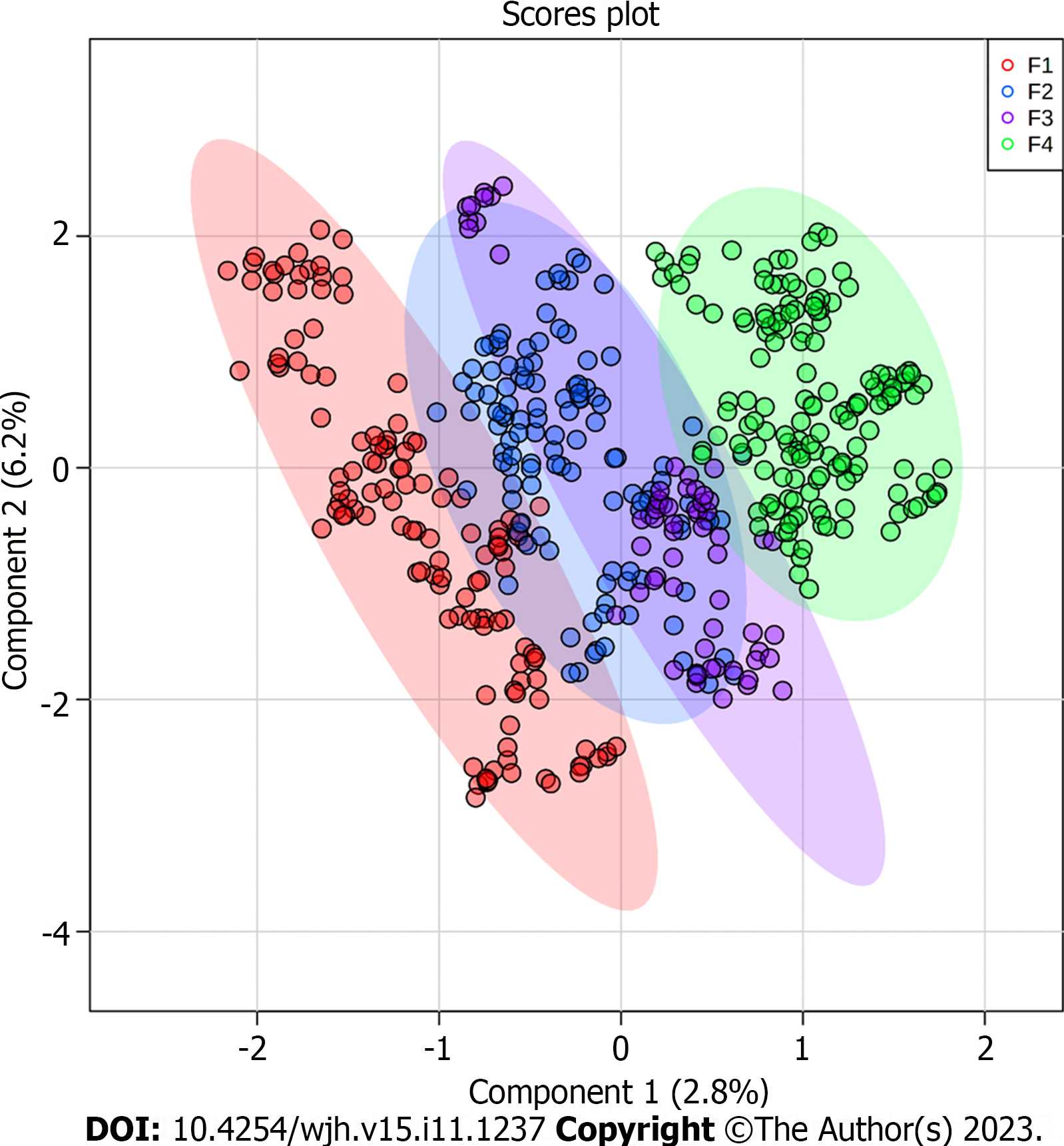
Based on the PLS-DA, the ions were grouped according to the signal intensity profile within each staging grade, making it possible to analyze the separation between fibrosis grades, as represented in the PLS-DA score plot (Figure 1).
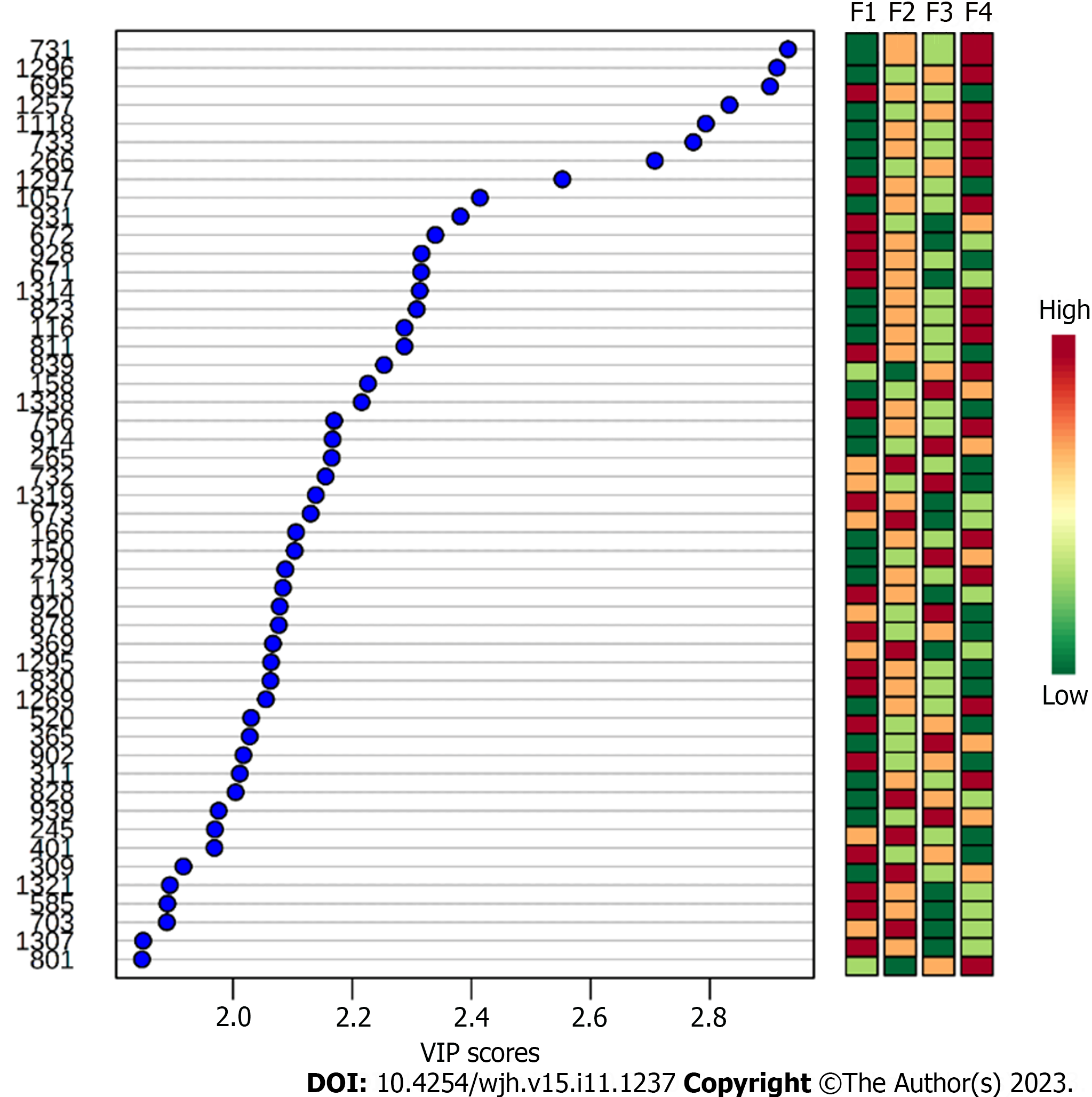
To identify the biomarkers responsible for the separation between the groups (Figure 1), a VIP score was used in the projection. This score allows visualization of the relevance of each marker within each grade analyzed according to the mass/charge ratios of the metabolites[22]. Considering a VIP score of > 2.0 (Figure 2), the six most important ions were selected for each group (Table 2).
| Class | Molecule | Ion | VIP score | Formula | Adducts | MSMS | Metlin ID |
| F1 | |||||||
| Sterols | 18:0 Cholesteryl ester | 671 | 2.3 | C45H80O2 | [M+NH4]+ | 303-369-583-437-147-161-135-109-95 | 83955 |
| 20:5 Cholesteryl ester | 672 | 2.35 | C47H74O2 | [M+H]+ | 41710 | ||
| Glycerolipids | DG(44:12)1 | 695 | 2.9 | C47H68O5 | [M+H-H2O]+ | 311-119-95-81-69-57-97-113-339 | 4681 |
| DG(42:7)1 | C45H74O5 | [M+H]+ | 311-119-95-81-69-57-97-113-339-437 | 4605/59181 | |||
| Coenzyme A | cis,cis-3,6-Dodecadienoyl-CoA | 928 | 2.35 | C33H54N7O17P3S | [M+H-H2O]+ | 95-112-119-720-184 | 58193 |
| Polypeptide | Angiotensin III | 931 | 2.4 | C46H66N12O9 | [M+H]+ | 400-311-112-113-96-97-437-659-720-146-147 | 58017 |
| Unknown | - | 1297 | 2.55 | - | - | - | - |
| F2 | |||||||
| Methyladenosine | N6-Methyladenosine | 265 | 2.18 | C11H15N5O4 | [M+H-H2O]+ | 81-85-63-99-117-135-149-163-177 | 58196 |
| 3'-O-Methyladenosine | 81-85-63-117-135-163 | 58340 | |||||
| 1-Methyladenosine | 81-84-63-99-117-135-163 | 6888 | |||||
| 2'-O-Methyladenosine | 81-85-63-99-117 | 58235 | |||||
| O6-Methyl-2'-deoxyguanosine | 81-85-63-99-149-163-177 | 66286 | |||||
| Eicosanoids | 8,15-diHPETE | 369 | 2.08 | C20H32O6 | [M+H]+ | 95-81-109-147-161-135-69-93-107 | 75001 |
| 5S,15S-diHPETE | 75023 | ||||||
| 5,15-diHPETE | 75000 | ||||||
| 15S-hydroperoxy-PGD2 | 74985 | ||||||
| 20-hydroxy-PGD2 | 74981 | ||||||
| Sphingolipids | Cer (42:1)1 | 673 | 2.15 | C42H83NO3 | [M+Na]+ | 303-370-95-81-109-60-69-93-107 | 41569 |
| Unknown | - | 828 | 2.05 | - | - | - | 0 |
| F3 | |||||||
| Aminoacid derivative | (S)-2,3,4,5-Tetrahydropiperidine-2-carboxylate | 150 | 2.1 | C6H9NO2 | [M+Na]+ | 106-134-84-61-56-52-105-120 | 62803 |
| Prenol lipid | Farnesylcysteine | 365 | 2.05 | C18H31NO2S | [M+K]+ | 203-185-112-81-71-307 | 62388 |
| Glycerophospholipid | PE(34:5)1 | 732 | 2.17 | C39H68NO8P | [M+Na]+ | 184-437-438-660-83-113-133-97 | 60844 |
| Coenzyme A | S-2-Octenoyl CoA | 914 | 2.18 | C29H48N7O17P3S | [M+Na]+ | 86-80-119-112-95-67-104-184-720 | 58140 |
| Unknown | - | 158 | 2.2 | - | - | - | - |
| 920 | 2.1 | ||||||
| F4 | |||||||
| Glycerophospholipids | PE(36:1)1 | 731 | 3 | C41H80NO7P | [M+H]+ | 184-659-437-393-113 | 62180 |
| PE(34:6)1 | C39H66NO8P | [M+Na]+ | 184-659-437-393-316-113 | 77367 | |||
| PE(O-36:1)1 | 733 | 2.6 | C41H82NO7P | [M+H]+ | 184-97-304-437-369 | 77526 | |
| PE(P-36:0)1 | 77623 | ||||||
| PE(34:5)1 | C39H68NO8P | [M+Na]+ | 184-97-304-437-675-369 | 60812 | |||
| Coenzyme A | CoA(22:2)1 | 1118 | 2.8 | C43H74N7O17P3S | [M+CH3OH+H]+ | 453-703-437-338-113-780-675-799 | 75415 |
| Acyl-carnitines | Malonylcarnitine | 266 | 2.7 | C10H17NO6 | [M+NH4]+ | 172-94-95-116-57-90-204 | 6484 |
| Unknown | - | 1257 | 2.85 | - | - | - | - |
| 1296 | 2.95 | ||||||
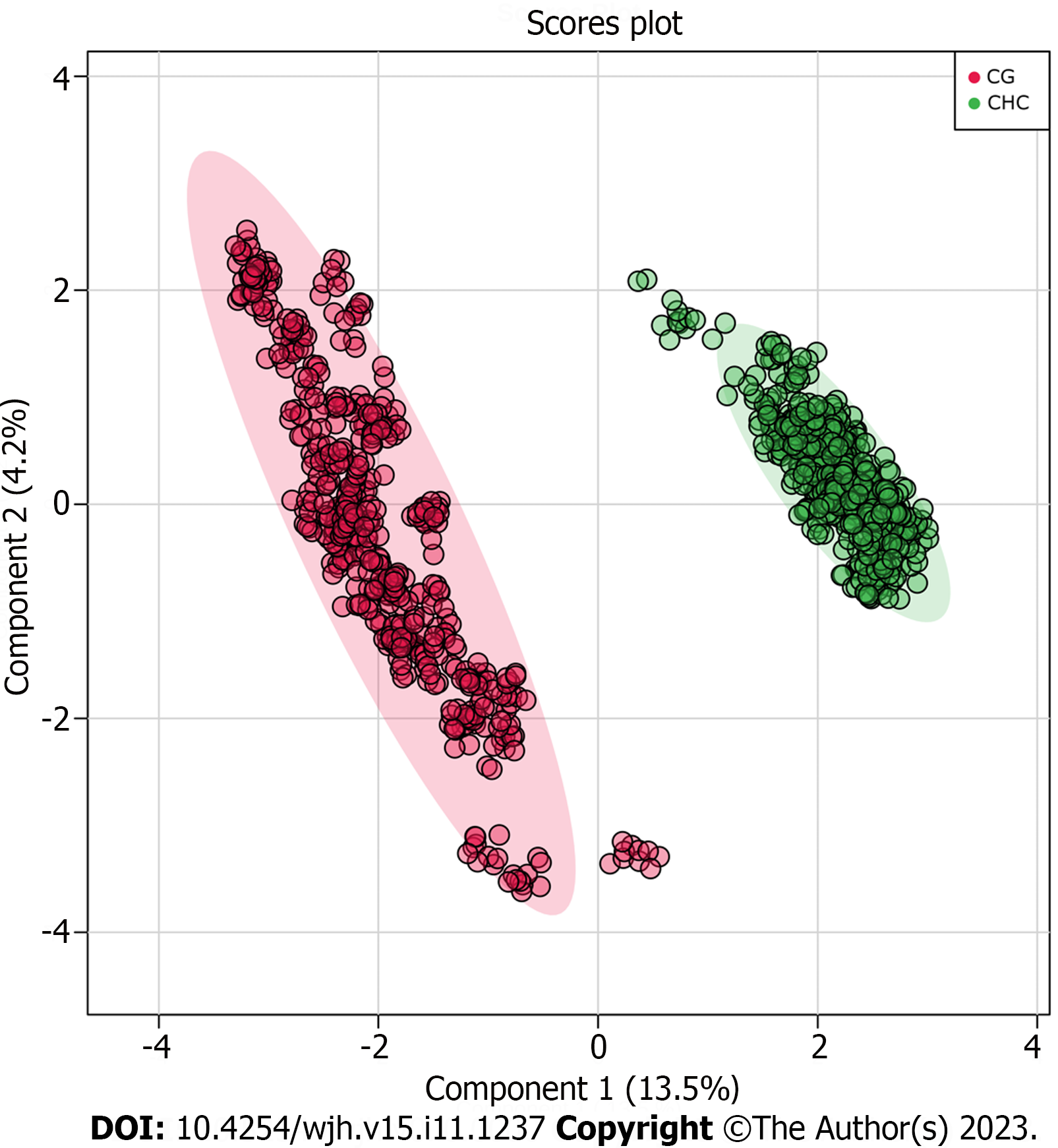
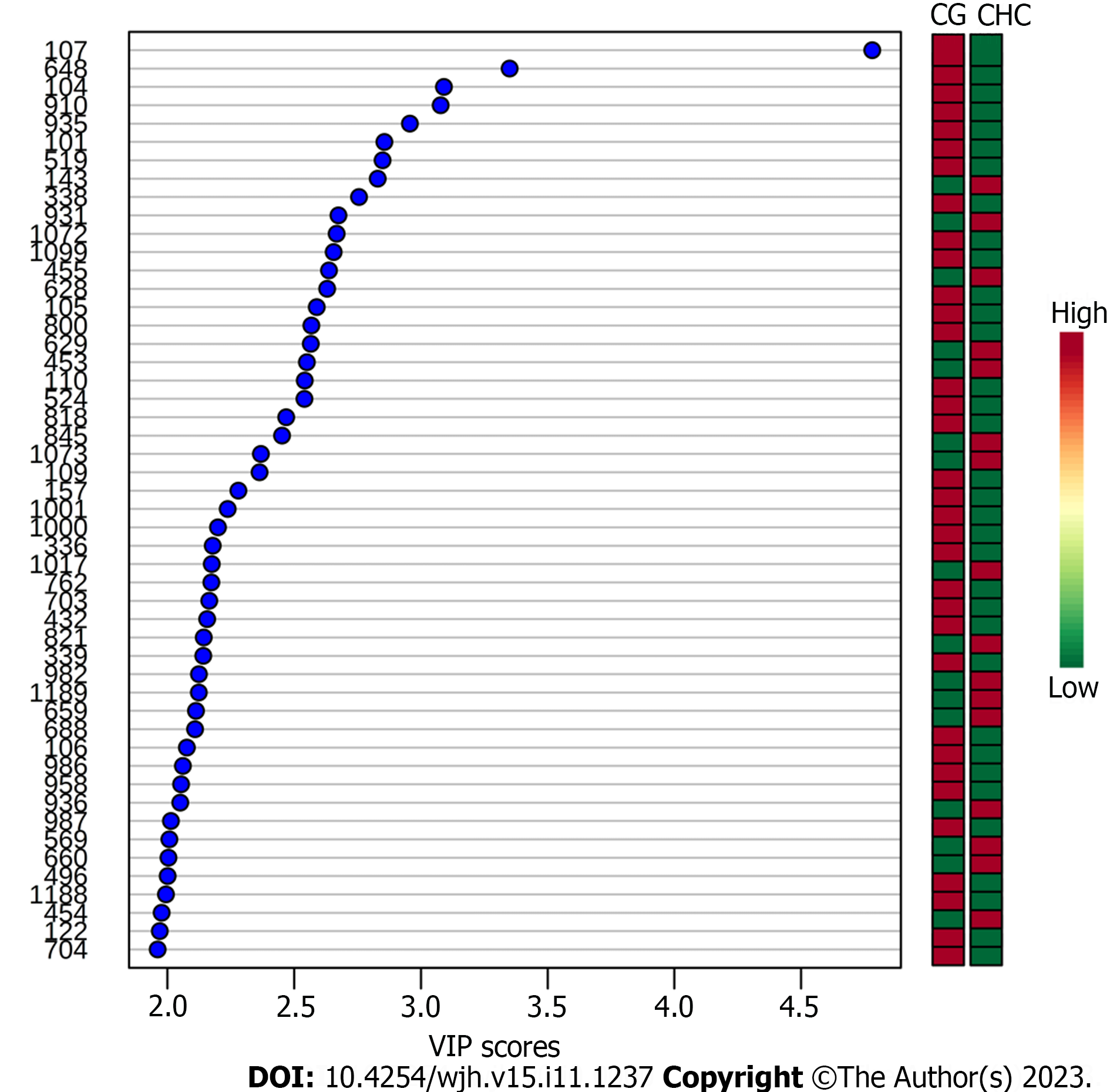
To ensure that the identified biomarkers were exclusive to liver lesions (fibrosis, CHC), CG were included. The plasma samples from the two groups (CHC vs CG) were compared and this analysis showed that the fibrosis biomarkers (Table 2) were not detected in CG. The PLS-DA and VIP score graphs comparing the two groups are shown in Figures 3 and 4, respectively.
The most relevant biomarkers, represented by m/z values, were identified according to fibrosis grade, as shown in Table 2.
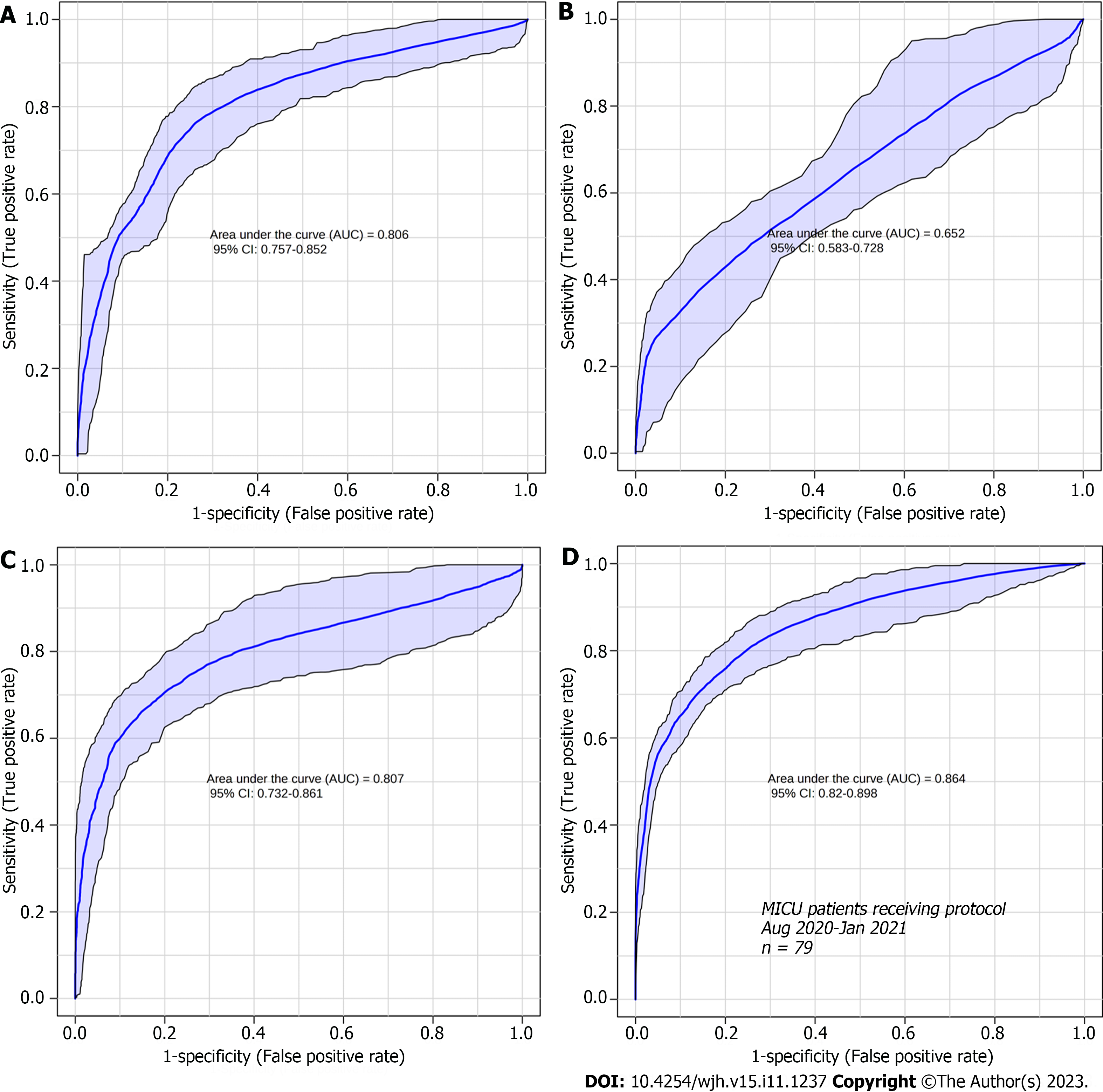
The accuracy of the biomarkers was assessed using the ROC curve analysis of the sets of metabolites identified for each fibrosis grade (Figure 5). ROC curves were used to analyze the sensitivity, specificity, and area under the curve (AUC) of each group of metabolites identified at each grade of fibrosis. The ROC curve of the selected metabolites for F1 (AUC = 0.806) was plotted with a sensitivity of 82% and a specificity of 68%, and the other selected metabolite groups for F2 (AUC = 0. 652), F3 (AUC = 0.807), and F4 (AUC = 0.864) showed sensitivities of 62%, 82%, and 83% and specificities of 57%, 74%, and 76%, respectively.
The metabolome was analyzed to identify new prognostic and diagnostic biomarkers. Thus, the present study investigated the differential metabolites in blood plasma as potential biomarkers of fibrosis stages. Our analysis identified potential biomarkers for each grade of liver fibrosis, which will increase our knowledge about the progression of CHC and highlight targets for further investigation. The identified biomarkers were able to establish a clustering tendency in patients with the same grade of fibrosis despite some overlap. The score plot analysis showed greater efficiency in discriminating between the extreme grades (F1 and F4), with an overlap in grades F2 and F3. This result may be related to the analytical bias of histological classification, as the formation of groups was based on this criterion-Metavir[12-14], which is subject to some bias related to inadequate sample acquisition, incorrect sample representation or inter-observer variability[7,12,13]. To ensure that the identified biomarkers were exclusive to liver lesions caused by CHC, we compared them with the plasma samples of healthy donors (Figures 3 and 4). None of the biomarkers found in the patient within the CHC group were detected in the plasma of healthy controls, which reinforces their potential as biomarkers exclusive to the disease.
Analysis of the accuracy of the most relevant metabolites in each grade showed that the sets associated with grades F1, F3, and F4 were good biomarkers (AUC 0.806, 0.807, and 0.864, respectively; Figure 5) and had good sensitivity and specificity scores. However, the metabolites identified as grade F2 were less specific and showed poor sensitivity. Such findings could be useful for distinguishing grades F1, F3, and F4, where uncertainty exists when the analyses are based solely on histology. Some serum markers of fibrosis validated in patients with hepatitis C and correlated with liver biopsy as a reference standard showed a mean AUC suitable for clinical practice (> 0.80)[23]; however, an overlap was also observed between adjacent grades of liver fibrosis, particularly the lower grade[24].
Despite the histological bias, our analysis identified different metabolites from diverse chemical classes, including sterols, fatty acids, lipids, and coenzymes. However, for each grade of fibrosis, a metabolite profile has been identified, and as observed in Figure 2, the relevance of each molecule changes according to the fibrosis grade and may intensify or decrease during the disease.
Some studies have demonstrated the potential of metabolomics analyses for different scenarios in diverse diseases, particularly in cancer management[25]. One of the great achievements of metabolomics is the assessment of therapeutic responses and tumor progression, as shown by Rattner et al[26], in which serum blood metabolites indicated positive or negative responses to chemotherapy using gas chromatography-mass spectrometry. In addition, some methods for metabolomics analysis, such as nuclear magnetic resonance and multisegment injection-capillary electrophoresis-mass spectrometry, have also shown impressive results, and have also been used to evaluate the metabolome of serum samples from patients with CHC with fibrosis of different grades[27]. This study identified markers for the highest grades of fibrosis, which are compatible with our results, such as glycerophospholipid and acyl-carnitine markers. Therefore, the use of metabolomics approaches for liquid biopsies show promise as diagnostic, prognostic, and therapeutic monitoring tools.
In the context of viral infection, viruses are known to synthesize fatty acids by benefitting from their intermediate products. HCV alters the expression of lipid-related genes associated with cholesterol biosynthesis[28,29]. Interestingly, some metabolites found in different grades of fibrosis are associated with lipid alterations[30-32].
For grade F1, biomarkers that may be more related to HCV infection than to the development of fibrosis were observed when compared to patients with more advanced fibrosis. Thus, the first molecule identified in F1 belonged to the sterol class, with specific signatures for cholesterol ester (CE) (m/z = 671 and m/z = 672). Previous studies have pointed out that CE is a critical component of lipoviral particles whose synthesis has been linked to HCV infection in vitro when cholesterol and triglyceride accumulation is observed[29]. In agreement with our results, we suggest that HCV may modulate the environment, promoting a higher density and infectivity of viral particles and viral spread in the hepatic tissue, which intensifies infection[28,33,34].
Considering lipid metabolism and accumulation, it was possible to identify the sphingolipid class in intermediate-grade F2, represented by ceramide (m/z = 673). It is a central molecule in sphingolipid metabolism with anti-proliferative and pro-apoptotic effects[30]. In the context of HCV infection, lipid accumulation and, consequently, ceramide accumulation occur and may lead to steatosis[35], which may contribute to the development of liver fibrosis[5,35-37].
In addition, a glycerolipid was also identified in F1, specified as diacylglycerol (DG) (m/z = 695). Recent studies have shown that the conversion of DG to phosphatidic acid (mediated by diacylglycerokinases) results in lysophosphatidic acid production, which is involved in many chronic inflammatory diseases, including fibrosis and cancer[38,39]. Therefore, the present study highlights a potential relationship between high levels of DG and a less fibrotic state (low-grade fibrosis) compared to F4, where fibrosis is accentuated.
Another lipid class, glycerophospholipids, was identified in intermediate-grade F3 and advanced-grade F4, in which the biomarkers were identified as phosphoethanolamines (PE) (m/z = 731, m/z = 732, and m/z = 733). Some studies have suggested that PE gradually increases according to the grade of liver fibrosis and acts as a potential marker of carcinogenesis[40,41]. This finding suggests that patients diagnosed with F3 could be at the beginning of the carcinogenesis process; however, this hypothesis needs to be further investigated.
Other biomarkers related to changes in lipid signaling pathways have also been identified. One of these belongs to the eicosanoid class (m/z = 369) identified in F2. This molecule is a biologically active lipid that has several implications in biological processes and is a potent mediator of inflammation in infectious diseases and HCC[42,43]. In addition, it is associated with liver fibrosis staging and is a potential biomarker[44-46]. Another class of lipids, prenol lipids, was identified as F3, represented by farnesylcysteine (m/z = 365). This marker participates in the process of liver carcinogenesis by directly acting on the activity of oncogenic rat sarcoma virus protein[47,48]. Thus, these results encourage investigations into the use of this metabolite as a potential biomarker of the risk of tumor development.
Different intermediate metabolites of the coenzyme A (CoA) class have also been identified, and they are typically involved in the β-oxidation of medium- and long-chain fatty acids to acyl-CoA, a key intermediate in lipid metabolism. Some studies suggest the existence of a disruption in fatty acid lipid metabolic pathways during HCV infection[49,50]. This process results in the accumulation of acyl-CoA and fatty acid metabolic intermediates, such as the three molecules identified in the present study, described as follows. The cis,cis-3,6-dodecadienoyl-CoA (m/z = 928) was identified in the F1 cases in our study. For F3, the marker S-2-octenoyl CoA (m/z = 914) was found[51,52], and in advanced grade (F4), a CoA metabolite (m/z = 1118) was identified. Because different acyl-CoAs isoenzymes are expressed in the liver, some of which are overexpressed in activated hepatic stellate cells[51,53], the results of the present study indicate that there is a disruption in lipid metabolism throughout the infection; however, this is unclear and requires further investigation. Considering the presence of acyl-CoAs in three different fibrosis grades, these molecules are not good candidates for the classification of fibrosis stages but highlight their importance in CHC.
Another marker involved in β-oxidation was found in patients with F4, represented by malonyl carnitine (m/z = 266). Tumors require more energy for cell proliferation, which may lead to dysregulation of energy-supplying metabolic pathways, such as β-oxidation of fatty acids[54,55]. In the context of HCC, alterations in the metabolism of acylcarnitine are directly related to the worsening of the disease and to alterations of β-oxidation[56], which results in the accumulation of Acyl-CoA[57], as discussed previously. Thus, malonylcarnitine can be considered a potential HCC biomarker; however, further studies are needed to validate this hypothesis.
In addition to the lipid biomarkers, the polypeptide angiotensin III (Ang III) (m/z = 931) was identified in F1, which, according to some studies, exhibits physiologically relevant effects similar to those of angiotensin II. In the context of CHC and liver fibrosis, Ang III participates in the increase in collagen production through its interaction with the angiotensin type 2 receptor[58,59] Therefore, this pathway may be involved at the beginning of the fibrotic process once Ang III is identified in F1.
The last two metabolites were identified as intermediate grades: methyladenosine (m/z = 265) in F2 and (S)-2,3,4,5-tetrahydropiperidine-2-carboxylate (m/z = 150) in F3. Adenosine methylation is a post-transcriptional modification of mRNAs that affects various biological functions[60-62]. In HCV infection, methyladenosine may represent an RNA modification that enhances the production of infectious particles by interacting with viral proteins[62-64]. These findings suggest that these modifications are involved in the progression of infections and liver fibrosis. Finally, (S)-2,3,4,5-tetrahydropiperidine-2-carboxylate identified in F3 may be related to the degradation of enzymatically inactive proteins and viral assembly[65]. Although this study related amino acid residues to the progression of infection and consequent worsening of fibrosis staging, further studies are necessary to clarify the actions of these protein residues in the viral cycle.
The main limitation of this research was the sample size. This study covered a regional sample and were limited to a single center, which may limit external generalization. However, the results encourage further research with a larger casuistry and the application of this methodology to other liver diseases.
The current study has innovative potential for the detection of markers in plasma, an easily accessible biological fluid. Besides, liquid biopsy could be used side by side with the other noninvasive tests (like elastography) for achieving more accuracy in predicting prognosis.
In conclusion, the results from this study suggest that some of the observed biomarkers, once validated, have the potential to be applied as prognostic biomarkers. In addition, they suggest that liquid biopsy analyses of plasma metabolites are a good source of molecular biomarkers capable of stratifying patients with CHC according to fibrosis grade.
Chronic hepatitis C (CHC) is an infectious disease caused by the hepatitis C virus, leading to liver issues like fibrosis, cirrhosis, cancer, and death. The accurate fibrosis stage identification is crucial for treatment decisions and predicting outcomes. Thus, blood markers are a source of relevant information on the staging of fibrosis, in a less invasive and representative way, compared to percutaneous biopsies.
Currently, approaches to staging fibrosis are invasive, subject to sampling errors and subjectivity between observers. In clinical routine, blood markers should be considered a relevant source of information. However, current approaches are limited to routine biochemical tests associated with clinical information, which is not very informative. Analyses based on liquid biopsy are less invasive, and blood plasma, since it circulates throughout the body, can provide information on pathologies that have not yet manifested themselves clinically, positively impacting on prognosis.
Analyze the plasmatic metabolome of CHC patients, looking for potential biomarkers to stratify these lesions.
Plasma metabolites from hepatitis C patients and 50 healthy volunteer participants were analyzed using the LTQ Mass Spectrometer. The sample and the control group were classified into Fibrosis grades was classified using the Metavir score. Liver samples were collected by percutaneous biopsy before any treatment and then analyzed histologically. The most relevant metabolites were categorized using the METLIN online metabolomics database. The molecules of interest were added to a list of candidates and subsequently fragmented in silico using the MassFrontier tool. Molecules compatible with those generated experimentally were then selected for functional analysis.
For each degree of fibrosis, six differential metabolites were identified that were able to establish an interesting grouping trend among patients with the same degree of fibrosis.
The results of this study suggest that liquid biopsy analyzes of plasma metabolites are a good source of molecular biomarkers capable of stratifying patients with CHC according to their fibrosis grade.
Some of the observed biomarkers, once validated, have the potential for application as prognostic biomarkers. This study has innovative potential regarding the detection of pre-clinical biomarkers in easily accessible plasma using minimally invasive methods.
| 1. | Peeling RW, Boeras DI, Marinucci F, Easterbrook P. The future of viral hepatitis testing: innovations in testing technologies and approaches. BMC Infect Dis. 2017;17:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (9)] |
| 2. | World Health Organization. Global hepatitis report 2017. World Health Organization. 2017;. [DOI] [Full Text] |
| 3. | Roudot-Thoraval F. Epidemiology of hepatitis C virus infection. Clin Res Hepatol Gastroenterol. 2021;45:101596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, Younossi Z. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (2)] |
| 5. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4219] [Article Influence: 200.9] [Reference Citation Analysis (11)] |
| 6. | Shay JES, Hamilton JP. Hepatic fibrosis: Avenues of investigation and clinical implications. Clin Liver Dis (Hoboken). 2018;11:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Mendes LC, Stucchi RS, Vigani AG. Diagnosis and staging of fibrosis in patients with chronic hepatitis C: comparison and critical overview of current strategies. Hepat Med. 2018;10:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Sebastiani G, Gkouvatsos K, Plebani M. Non-invasive assessment of liver fibrosis: it is time for laboratory medicine. Clin Chem Lab Med. 2011;49:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol. 1999;5:301-304. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 190] [Cited by in RCA: 213] [Article Influence: 7.9] [Reference Citation Analysis (4)] |
| 12. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 13. | Chindamo MC, Nunes-Pannain VL, Araújo-Neto JM, Moraes-Coelho HS, Luiz RR, Villela-Nogueira CA, Perez RM. Intermediate fibrosis staging in hepatitis C: a problem not overcome by optimal samples or pathologists' expertise. Ann Hepatol. 2015;14:652-657. [PubMed] |
| 14. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3130] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 15. | Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033-11053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Adams LA. Biomarkers of liver fibrosis. J Gastroenterol Hepatol. 2011;26:802-809. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Zamin JI, de Mattos AA, Perin C, Ramos GZ. [The importance of AST / ALT rate in nonalcoholic steatohepatitis diagnosis]. Arq Gastroenterol. 2002;39:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Manchia M, Comai S, Pinna M, Pinna F, Fanos V, Denovan-Wright E, Carpiniello B. Biomarkers in aggression. Adv Clin Chem. 2019;93:169-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Rector J, Lin JQ, Young JH, Sans M, Katta N, Giese N, Yu W, Nagi C, Suliburk J, Liu J, Bensussan A, DeHoog RJ, Garza KY, Ludolph B, Sorace AG, Syed A, Zahedivash A, Milner TE, Eberlin LS. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 21. | Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr Protoc Bioinformatics. 2019;68:e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1567] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 22. | Menze BH, Kelm BM, Masuch R, Himmelreich U, Bachert P, Petrich W, Hamprecht FA. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinformatics. 2009;10:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 477] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 23. | European Association for the Study of the Liver. EASL Governing Board representative:; Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1308] [Article Influence: 261.6] [Reference Citation Analysis (1)] |
| 24. | Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (1)] |
| 25. | Wang W, Rong Z, Wang G, Hou Y, Yang F, Qiu M. Cancer metabolites: promising biomarkers for cancer liquid biopsy. Biomark Res. 2023;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 26. | Rattner JI, Kopciuk KA, Vogel HJ, Tang PA, Shapiro JD, Tu D, Jonker DJ, Siu LL, O'Callaghan CJ, Bathe OF. Early detection of treatment futility in patients with metastatic colorectal cancer. Oncotarget. 2022;13:61-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Shanmuganathan M, Sarfaraz MO, Kroezen Z, Philbrick H, Poon R, Don-Wauchope A, Puglia M, Wishart D, Britz-McKibbin P. A Cross-Platform Metabolomics Comparison Identifies Serum Metabolite Signatures of Liver Fibrosis Progression in Chronic Hepatitis C Patients. Front Mol Biosci. 2021;8:676349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Dai X, Hakizimana O, Zhang X, Kaushik AC, Zhang J. Orchestrated efforts on host network hijacking: Processes governing virus replication. Virulence. 2020;11:183-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Lavie M, Dubuisson J. Interplay between hepatitis C virus and lipid metabolism during virus entry and assembly. Biochimie. 2017;141:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Simon J, Ouro A, Ala-Ibanibo L, Presa N, Delgado TC, Martínez-Chantar ML. Sphingolipids in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma: Ceramide Turnover. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 31. | Paul B, Lewinska M, Andersen JB. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022;4:100479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 205] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 32. | Read SA, Tay E, Shahidi M, George J, Douglas MW. Hepatitis C virus infection mediates cholesteryl ester synthesis to facilitate infectious particle production. J Gen Virol. 2014;95:1900-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Woodhouse SD, Narayan R, Latham S, Lee S, Antrobus R, Gangadharan B, Luo S, Schroth GP, Klenerman P, Zitzmann N. Transcriptome sequencing, microarray, and proteomic analyses reveal cellular and metabolic impact of hepatitis C virus infection in vitro. Hepatology. 2010;52:443-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Tsugane K, Tamiya-Koizumi K, Nagino M, Nimura Y, Yoshida S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J Hepatol. 1999;31:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Senkal CE, Salama MF, Snider AJ, Allopenna JJ, Rana NA, Koller A, Hannun YA, Obeid LM. Ceramide Is Metabolized to Acylceramide and Stored in Lipid Droplets. Cell Metab. 2017;25:686-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 38. | Kaffe E, Magkrioti C, Aidinis V. Deregulated Lysophosphatidic Acid Metabolism and Signaling in Liver Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Liu CS, Schmezer P, Popanda O. Diacylglycerol Kinase Alpha in Radiation-Induced Fibrosis: Potential as a Predictive Marker or Therapeutic Target. Front Oncol. 2020;10:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Buchard B, Teilhet C, Abeywickrama Samarakoon N, Massoulier S, Joubert-Zakeyh J, Blouin C, Reynes C, Sabatier R, Biesse-Martin AS, Vasson MP, Abergel A, Demidem A. Two Metabolomics Phenotypes of Human Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease According to Fibrosis Severity. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Wu X, Wang Z, Luo L, Shu D, Wang K. Metabolomics in hepatocellular carcinoma: From biomarker discovery to precision medicine. Front Med Technol. 2022;4:1065506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 42. | Gong ZG, Zhao W, Zhang J, Wu X, Hu J, Yin GC, Xu YJ. Metabolomics and eicosanoid analysis identified serum biomarkers for distinguishing hepatocellular carcinoma from hepatitis B virus-related cirrhosis. Oncotarget. 2017;8:63890-63900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Alvarez ML, Lorenzetti F. Role of eicosanoids in liver repair, regeneration and cancer. Biochem Pharmacol. 2021;192:114732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Lu Y, Huang C, Gao L, Xu YJ, Chia SE, Chen S, Li N, Yu K, Ling Q, Cheng Q, Zhu M, Chen M, Ong CN. Identification of serum biomarkers associated with hepatitis B virus-related hepatocellular carcinoma and liver cirrhosis using mass-spectrometry-based metabolomics. Metabolomics. 2015;11:1526-1538. [DOI] [Full Text] |
| 45. | Caussy C, Chuang JC, Billin A, Hu T, Wang Y, Subramanian GM, Djedjos CS, Myers RP, Dennis EA, Loomba R. Plasma eicosanoids as noninvasive biomarkers of liver fibrosis in patients with nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2020;13:1756284820923904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Masoodi M, Gastaldelli A, Hyötyläinen T, Arretxe E, Alonso C, Gaggini M, Brosnan J, Anstee QM, Millet O, Ortiz P, Mato JM, Dufour JF, Orešič M. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18:835-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 47. | Borbath I, Stärkel P. Chemoprevention of hepatocellular carcinoma. Proof of concept in animal models. Acta Gastroenterol Belg. 2011;74:34-44. [PubMed] |
| 48. | Tsirulnikov K, Duarte S, Ray A, Datta N, Zarrinpar A, Hwang L, Faull K, Pushkin A, Kurtz I. Aminoacylase 3 Is a New Potential Marker and Therapeutic Target in Hepatocellular Carcinoma. J Cancer. 2018;9:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Roe B, Kensicki E, Mohney R, Hall WW. Metabolomic profile of hepatitis C virus-infected hepatocytes. PLoS One. 2011;6:e23641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Lu M, Zhu WW, Wang X, Tang JJ, Zhang KL, Yu GY, Shao WQ, Lin ZF, Wang SH, Lu L, Zhou J, Wang LX, Jia HL, Dong QZ, Chen JH, Lu JQ, Qin LX. ACOT12-Dependent Alteration of Acetyl-CoA Drives Hepatocellular Carcinoma Metastasis by Epigenetic Induction of Epithelial-Mesenchymal Transition. Cell Metab. 2019;29:886-900.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 51. | Lai KKY, Kweon SM, Chi F, Hwang E, Kabe Y, Higashiyama R, Qin L, Yan R, Wu RP, Lai K, Fujii N, French S, Xu J, Wang JY, Murali R, Mishra L, Lee JS, Ntambi JM, Tsukamoto H. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology. 2017;152:1477-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 52. | Ali A, Zhang Y, Fu M, Pei Y, Wu L, Wang R, Yang G. Cystathionine gamma-lyase/H(2)S system suppresses hepatic acetyl-CoA accumulation and nonalcoholic fatty liver disease in mice. Life Sci. 2020;252:117661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Muoio DM, Lewin TM, Wiedmer P, Coleman RA. Acyl-CoAs are functionally channeled in liver: potential role of acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2000;279:E1366-E1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 810] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 55. | Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 409] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 56. | Li S, Gao D, Jiang Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 57. | Hagenbuchner J, Scholl-Buergi S, Karall D, Ausserlechner MJ. Very long-/ and long Chain-3-Hydroxy Acyl CoA Dehydrogenase Deficiency correlates with deregulation of the mitochondrial fusion/fission machinery. Sci Rep. 2018;8:3254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Yugandhar VG, Clark MA. Angiotensin III: a physiological relevant peptide of the renin angiotensin system. Peptides. 2013;46:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Murphy AM, Wong AL, Bezuhly M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair. 2015;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 60. | Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2600] [Cited by in RCA: 3836] [Article Influence: 274.0] [Reference Citation Analysis (5)] |
| 61. | Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 839] [Article Influence: 69.9] [Reference Citation Analysis (4)] |
| 62. | Kim GW, Imam H, Khan M, Siddiqui A. N(6)-Methyladenosine modification of hepatitis B and C viral RNAs attenuates host innate immunity via RIG-I signaling. J Biol Chem. 2020;295:13123-13133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 63. | Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez C, Willer J, Ilkayeva OR, Law BA, Holley CL, Garcia-Blanco MA, Evans MJ, Suthar MS, Bradrick SS, Mason CE, Horner SM. N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe. 2016;20:654-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 64. | Dang W, Xie Y, Cao P, Xin S, Wang J, Li S, Li Y, Lu J. N6-Methyladenosine and Viral Infection. Front Microbiol. 2019;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Welbourn S, Jirasko V, Breton V, Reiss S, Penin F, Bartenschlager R, Pause A. Investigation of a role for lysine residues in non-structural proteins 2 and 2/3 of the hepatitis C virus for their degradation and virus assembly. J Gen Virol. 2009;90:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Association for Cancer Research (EACR), EACR34184.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kishida Y, Japan; Morozov S, Russia; Sholkamy A, Egypt S-Editor: Qu XL L-Editor: A P-Editor: Cai YX