Published online Nov 27, 2023. doi: 10.4254/wjh.v15.i11.1210
Peer-review started: July 26, 2023
First decision: August 16, 2023
Revised: August 29, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: November 27, 2023
Processing time: 121 Days and 0.5 Hours
Nonalcoholic fatty liver disease (NAFLD) has become the leading cause of cirrhosis and other chronic liver diseases (COCLDs).
To conduct a comprehensive and comparable updated analysis of the global, regional, and national burden of COCLDs due to NAFLD in 204 countries and territories from 1990 and 2019 by age, sex, and sociodemographic index.
Data on COCLDs due to NAFLD were collected from the Global Burden of Disea
In 2019, the global age-standardized prevalence rate of COCLDs due to NAFLD was 15022.90 per 100000 population [95% uncertainty interval (UI): 13493.19-16764.24], which increased by 24.51% (22.63% to 26.08%) from 1990, with an estimated annual percentage change of 0.78 (95% confidence interval: 0.74-0.82). In the same year, however, the age-standardized death rate and age-standardized DALYs per 100000 population were 1.66 (95%UI: 1.20-2.17) and 43.69 (95%UI: 31.28-58.38), respectively. North Africa and the Middle East had the highest prevalence rates of COCLDs due to NAFLD. The death rate increased with age up to the 95+ age group for both sexes. Males had higher numbers of prevalence, death rate, and DALYs than females across all age groups before the 65-69 age group. The sociodemographic index was negatively correlated with the age-standardized DALYs.
Globally, the age-standardized prevalence rate has increased during the past three decades. However, the age-standardized death rate and age-standardized DALYs decreased. There is geographical variation in the burden of COCLDs due to NAFLD. It is strongly recommended to improve the data quality of COCLDs due to NAFLD across all countries and regions to facilitate better monitoring of the burden of COCLDs due to NAFLD.
Core Tip: Nonalcoholic fatty liver disease is the leading cause of cirrhosis and other chronic liver diseases. The global age-standardized prevalence rate of cirrhosis and other chronic liver diseases due to nonalcoholic fatty liver disease increased by 24.51% from 1990. The age-standardized death rate and age-standardized disability-adjusted life-years rate per 100000 population were 1.66 and 43.69, respectively. The highest prevalence rate was observed in North Africa and the Middle East. Males had a higher burden of prevalence, death, and disability-adjusted life-years lost than females before the 65-69 age group. Furthermore, there is a negative correlation between sociodemographic index and age-standardized death rate.
- Citation: Liu ZP, Ouyang GQ, Huang GZ, Wei J, Dai L, He SQ, Yuan GD. Global burden of cirrhosis and other chronic liver diseases due to nonalcoholic fatty liver disease, 1990-2019. World J Hepatol 2023; 15(11): 1210-1225
- URL: https://www.wjgnet.com/1948-5182/full/v15/i11/1210.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i11.1210
The incidence and prevalence of nonalcoholic fatty liver disease (NAFLD) have been rapidly increasing worldwide over the past few decades. Recent estimates suggest that approximately 25% of the world’s population is affected by NAFLD, with projections indicating a potential 56% surge in the prevalence of nonalcoholic steatohepatitis (NASH) within the coming decade[1]. NAFLD encompasses a spectrum of liver damage, ranging from simple steatosis to NASH, fibrosis, cirrhosis, and even hepatocellular carcinoma[2]. It is noteworthy that NAFLD now stands as the fifth leading cause of mortality among young adults within the category of metabolic diseases. Alarming forecasts predict a staggering 158.4% increase in its death rate by the year 2050[3]. Conversely, another separate study demonstrated divergent trends, finding that the age-standardized prevalence rate (ASPR) of NAFLD increased while the age-standardized death rate (ASDR) and age-standardized disability-adjusted life-year (DALY) rate (ASDAR) decreased from 1990 to 2019[4].
Cirrhosis is the leading cause of liver-related morbidity, contributing to more than 1 million deaths annually worldwide. The mortality increase escalates markedly for individuals grappling with decompensated cirrhosis[5], and the deaths from cirrhosis increased by 47.15% globally from 1990 to 2017[6]. Beyond mortality statistics, cirrhosis imposes a significant public health burden globally, substantially compromising quality of life[7,8]. The etiologies of cirrhosis include alcoholic liver disease, hepatitis B virus, hepatitis C virus, and NAFLD[9,10]. Over the past few decades, universal hepatitis B virus vaccination initiatives, coupled with rising obesity rates and the prevalence of type 2 diabetes, have positioned NAFLD as a major etiological factor contributing to cirrhosis[11-14].
Chronic liver disease (CLD) is a disease that is characterized by decreased liver function resulting from chronic inflammation or injury to the liver, leading to fibrosis and cirrhosis that progresses for more than 6 mo[15,16]. This spectrum encompasses an array of liver pathologies, encompassing inflammation, cirrhosis, portal hypertension, and hepatorenal syndrome. Notably, the incidence of CLD is increasing yearly, and it is now the fifth leading cause of death in the United Kingdom. In Western regions, NAFLD has become the leading cause of CLD[17]. Today, the United States has nearly 4.5 million adults afflicted by cirrhosis and CLD, resulting in an overall death toll of 414731[16]. However, no studies have focused on the epidemiology of cirrhosis and other CLDs (COCLDs) due to NAFLD across the globe.
In the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017, it was shown that the incidence of cases of liver cirrhosis caused by NASH increased by approximately 105.56%, and the age-standardized incidence rate increased by 1.35%. This study only included data from 195 countries, and data on prevalence, death, and DALYs were not provided[18]. Furthermore, no updated global studies on COCLDs due to NAFLD have been published since the 2017 estimates. Using the data from the GBD 2019, we conducted this comprehensive, updated analysis of the global, regional, and national levels of prevalence, death rate, and DALYs of COCLDs due to NAFLD with regard to age-standardized rates (ASRs) and raw numbers from 1990 to 2019, stratified by sex, age, and sociodemographic index (SDI).
The GBD 2019 was conducted by the Institute of Health Metrics and Evaluation and analyzed approximately 369 diseases and injuries, 282 causes of death, and 84 risk factors from 204 countries/territories, 21 regions, and 7 superregions from 1990 to 2019[19]. Detailed methods for GBD 2019 regarding date inputs, analytical processes, and outputs have been described in previous publications[19,20]. Additional information on fatal and nonfatal estimates can be found at https://vizhub.healthdata.org/gbd-compare/ and http://ghdx.healthdata.org/gbd-results-tool. Our study complied with the Guidelines for Accurate and Transparent Health Estimates Reporting statement[21].
NAFLD was defined as a range of liver conditions that mimic alcoholic liver disease but occur in people who drink little to no alcohol. It includes nonalcoholic fatty liver (characterized by fat deposition in liver cells), NASH (characterized by fat deposition and inflammation), and cirrhosis[19]. Cirrhosis is a CLD in which there is progressive destruction of functional hepatic cells and replacement with fibrosis (scarring) of the liver. In GBD 2019, COCLDs due to NAFLD were defined as COCLDs that were specifically caused by NAFLD, excluding all other potential etiologies[19]. All the GBD data used in this study are publicly available online at the Global Health Data Exchange.
The Bayesian meta-regression tool DisMod-MR 2.1 was used to assess and model estimates of the burden of COCLDs due to NAFLD by pooling all the available epidemiological data. Prior settings included remission of 0 before the age of 15 years in the DisMod-MR 2.1 model. No prevalence of COCLDs due to NAFLD before the age of 15 years was assumed. The age range was restricted to ≥ 15 years and was divided into 17 5-year age groups.
The estimated annual percentage change (EAPC) values were calculated to reflect the change in ASRs over a specified period. EAPC values above or below 0 indicate that the ASR is increasing or decreasing, respectively. If the EAPC range includes 0, this means the ASR is stable during this period. ASPR, ASDR, ASDAR, and EAPC were used to quantify global trends of COCLDs due to NAFLD.
The SDI was used as a composite indicator of the development status in each country and territory. It was calculated based on lag-distributed income, the total fertility rate for individuals younger than 25 years, and average years of education in people older than 15 years. SDI ranged from 0 to 1, with a higher score indicating a higher level of development. The 204 countries and territories were categorized into five groups: low SDI, low-middle SDI, middle SDI, high-middle SDI, and high SDI.
Smoothing spline models were employed to examine the shape of the correlation curve between the burden index of COCLDs due to NAFLD and SDI according to the GBD estimates across 204 countries and 21 regions from 1990 to 2019. The 95% uncertainty intervals (UIs) were defined as the 2.5 to the 97.5 percentile of the ordered draws. R software version 3.6.3 was used for all statistical analyses and figures. A P value of < 0.05 was considered statistically significant.
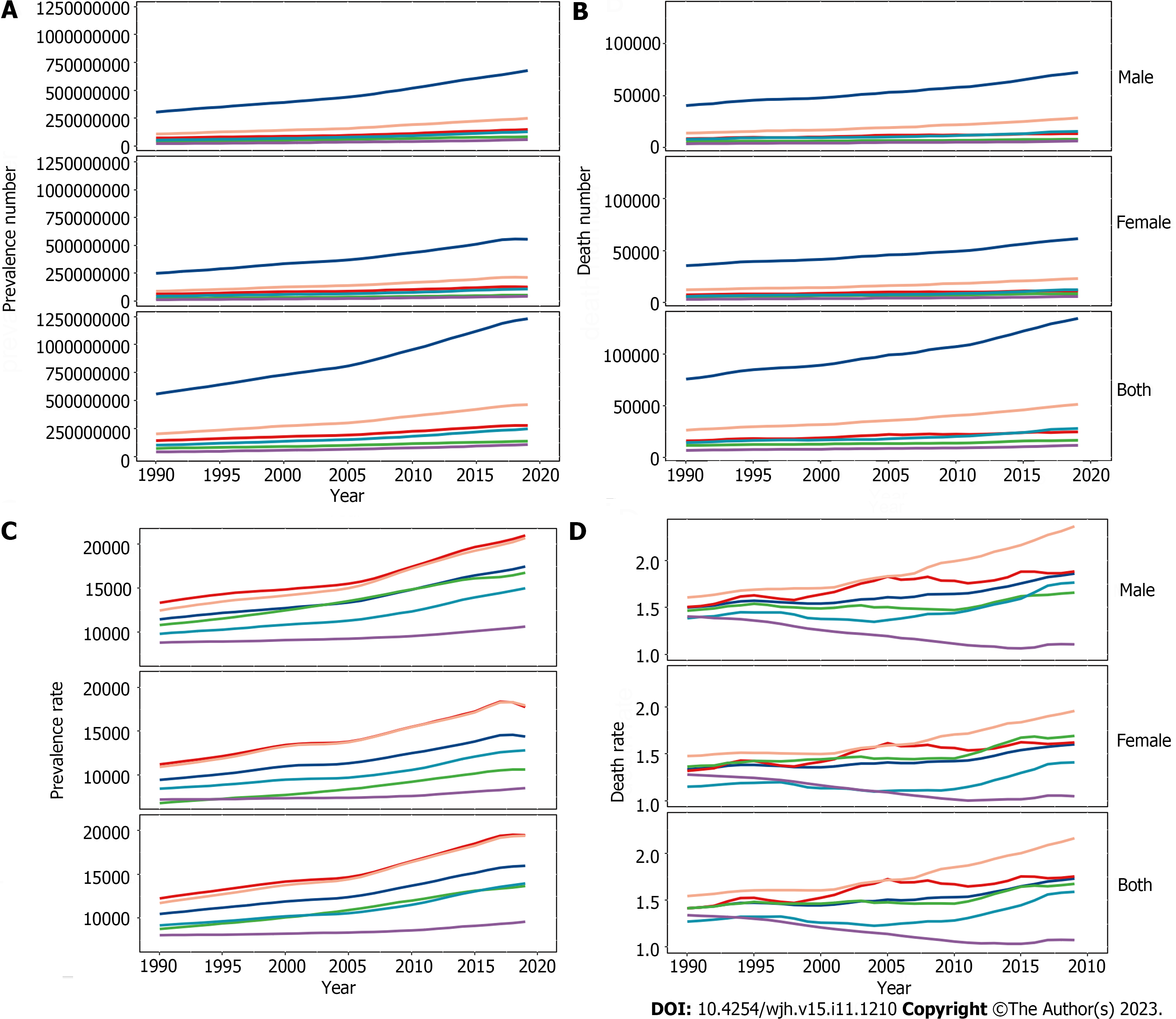
Globally in 2019 there were 1235652879 (95%UI, 1109501987-1378481210) prevalent cases of COCLDs due to NAFLD. From 1990 to 2019, the global ASPR increased from 12065.15 (10779.06-13536.49) to 15022.90 (13493.19-16764.24) per 100000 population, with an EAPC of 0.78 (0.74-0.82) (Table 1, Figure 1). COCLDs due to NAFLD accounted for 134240 (96483-176920) deaths globally in 2019, which was a substantial increase of 76.73% (61.23%-94.75%) over that in 1990. The ASDR of COCLDs due to NAFLD decreased from 1.94 per 100000 population (1.39-2.59 per 100000) in 1990 to 1.66 per 100000 population (1.20-2.17 per 100000) in 2019, with an EAPC of 0.65 (-3.68-2.48) (Table 1, Supplementary Figure 1). In the same year, COCLDs due to NAFLD accounted for 3621471.92 (2585375.27-4862918.36) DALY cases at the global level with an ASR of 43.69 per 100000 population. The global ASDAR was reduced from 51.92 per 100000 population (37.23-69.19 per 100000) in 1990 to 43.69 per 100000 population (95%UI: 31.28-58.38) in 2019, with an EAPC of -0.73 (-1.33 to -0.13) (Table 1, Supplementary Figure 2).
| Characteristics | Death (95%UI) | Prevalence (95%UI) | DALYs (95%UI) | ||||||
| 2019 | 2019 | 1990-2019 | 2019 | 2019 | 1990-2019 | 2019 | 2019 | 1990-2019 | |
| Death cases among COCLDs due to NAFLD | ASDR per 100000 population (95%UI) | EAPC (95%CI) | Prevalence cases among COCLDs due to NAFLD | ASPR per 100000 population (95%UI) | EAPC (95%CI) | DALY cases among COCLDs due to NAFLD | ASDAR per 100000 population (95%UI) | EAPC (95%CI) | |
| Global | 134240.56 (96483.10-176920.13) | 1.66 (1.20-2.17) | -0.65 (-3.68-2.48) | 1235652879.48 (1109501987.09-1378481210.81) | 15022.90 (13493.19-16764.24) | 0.78 (0.74-0.82) | 3621471.92 (2585375.27-4862918.36) | 43.69 (31.28-58.38) | -0.73 (-1.33 to -0.13) |
| Sex | |||||||||
| Male | 72379.97 (51296.70-98029.91) | 1.91 (1.36-2.54) | -0.62 (-3.46-2.31) | 679261655.31 (610650033.21-753716195.72) | 16789.38 (15136.45-18609.47) | 0.77 (0.74-0.81) | 2111194.29 (1490236.02-2905285.80) | 52.39 (37.17-71.63) | -0.65 (-1.20 to -0.10) |
| Female | 61860.58 (44641.50-81216.17) | 1.42 (1.03-1.86) | -0.70 (-3.95-2.66) | 556391224.17 (499050879.21-624989968.22) | 13278.47 (11900.05-14925.95) | 0.78 (0.74-0.82) | 1510277.64 (1087564.54-1984959.30) | 35.16 (25.35-46.24) | -0.84 (-1.49 to -0.18) |
| SDI | |||||||||
| Low SDI | 12175.43 (8552.07-16892.87) | 2.41 (1.65-3.38) | -1.04 (-3.48-1.45) | 108176159.27 (95419333.67-122650081.72) | 14278.53 (12741.85-16053.31) | 0.34 (0.30-0.38) | 367698.76 (255871.37-510346.73) | 59.56 (41.46-82.97) | -1.14 (-1.63 to -0.65) |
| Low-middle SDI | 28042.90 (20040.37-37459.68) | 2.09 (1.52-2.77) | -0.73 (-3.47-2.08) | 245742808.24 (219055120.10-276667118.34) | 15232.77 (13662.46-17078.41) | 0.56 (0.52-0.59) | 826708.77 (584688.97-1130359.54) | 54.77 (39.14-74.11) | -0.86 (-0.31 to -1.4) |
| Middle SDI | 51854.16 (37473.84-68073.39) | 2.23 (1.62-2.92) | -0.77 (-3.35-1.88) | 464259979.22 (417290788.31-516020477.89) | 17596.41 (15842.14-19525.24) | 0.66 (0.62-0.69) | 1347118.90 (956498.31-1772557.91) | 52.41 (38.16-68.22) | -0.98 (-1.52 to -0.44) |
| Middle-high SDI | 25074.30 (18164.41-33237.26) | 1.27 (0.93-1.67) | -0.75 (-4.08-2.69) | 278080918.57 (251203100.31-308471139.19) | 15336.20 (13798.84-17095.00) | 0.79 (0.75-0.83) | 676567.46 (480709.11-915257.33) | 34.81 (25.11-46.87) | -0.52 (-1.16-0.14) |
| High SDI | 16984.00 (12138.02-22893.89) | 0.95 (0.67-1.30) | -0.79 (-4.75-3.33) | 138619942.87 (125499384.96-153306375.41) | 10528.91 (9426.64-11724.43) | 1.22 (1.17-1.26) | 400520.00 (273854.75-550359.22) | 25.46 (17.47-35.02) | -0.84 (-1.61 to -0.05) |
| Region | |||||||||
| Andean Latin America | 2935.06 (2012.67-4051.53) | 5.31 (3.62-7.30) | -0.23 (-1.96-1.54) | 8442278.35 (7603147.20-9329779.19) | 13689.71 (12376.75-15086.12) | 0.50 (0.47-0.54) | 70058.17 (47362.01-98970.21) | 121.70 (82.37-172.61) | -0.69 (-1.05 to -0.34) |
| Australasia | 413.12 (297.05-538.48) | 0.87 (0.63-1.13) | -0.22 (-4.56-4.32) | 3459804.59 (3121214.49-3821041.92) | 9444.14 (8455.34-10478.02) | 0.91 (0.86-0.95) | 9514.38 (6661.05-12694.41) | 22.52 (15.80-30.52) | -0.22 (-1.09-0.65) |
| Caribbean | 1607.95 (1113.98-2236.69) | 3.11(2.15-4.30) | -0.71 (-3.01-1.65) | 8176172.43 (7382389.69-9017056.63) | 16169.48 (14591.11-17866.53) | 0.40 (0.37-0.43) | 41576.95 (28066.48-60045.06) | 80.64 (54.52-115.96) | -0.75 (-1.21 to -0.29) |
| Central Asia | 2633.58 (1802.74-3693.53) | 3.56 (2.51-4.95) | 2.00 (-0.39-4.45) | 12814865.72 (11452914.10-14330325.35) | 14150.39 (12737.16-15748.23) | 0.50 (0.46-0.54) | 84039.15 (57215.03-120570.79) | 97.76 (67.81-137.00) | 1.97 (1.51-2.43) |
| Central Europe | 2061.07 (1378.12-2972.76) | 1.05 (0.71-1.49) | -0.36 (-4.12-3.56) | 18627848.59 (16912570.59-20480313.45) | 11894.85 (10754.82-13109.51) | 0.34 (0.30-0.38) | 54731.91 (36055.44-79057.76) | 30.45 (19.95-43.92) | -0.35 (-1.06-0.37) |
| Central Latin America | 13972.52 (10310.77-18101.39) | 5.90 (4.32-7.66) | -0.61 (-2.24-1.06) | 42166216.88 (37817332.61-46840542.82) | 16617.99 (14959.55-18388.64) | 0.43 (0.40-0.46) | 379421.91 (275350.09-504051.39) | 154.08 (112.26-204.63) | -0.84 (-1.16 to -0.52) |
| Central Sub-Saharan Africa | 1464.95 (913.12-2215.29) | 2.71 (1.74-4.05) | -0.79 (-3.19-1.67) | 11343938.83 (9886866.10-12995919.12) | 13331.21 (11795.46-15097.36) | 0.16 (0.13-0.20) | 47553.59 (29400.33-74006.26) | 70.04 (43.82-106.28) | -0.78 (-1.26 to -0.30) |
| East Asia | 15620.62 (11027.12-21039.77) | 0.80 (0.58-1.07) | -2.35 (-6.22-1.68) | 303112259.13 (272363603.29-339481359.63) | 15680.86 (14022.56-17552.19) | 0.85 (0.81-0.89) | 391872.94 (268774.96-535743.63) | 18.91 (13.28-25.56) | -2.71 (-3.50 to -1.91) |
| Eastern Europe | 7354.24 (5071.40-10094.04) | 2.41 (1.67-3.30) | 3.04 (0.05-6.12) | 34110583.23 (30980982.64-37557624.89) | 12295.07 (11098.49-13601.74) | 0.39 (0.35-0.42) | 245483.77 (166670.25-344145.21) | 85.82 (58.40-120.98) | 3.58 (3.06-4.11) |
| Eastern Sub-Saharan Africa | 6094.93 (4211.94-8428.57) | 3.98 (2.74-5.49) | -0.66 (-2.62-1.34) | 36093305.19 (31609582.82-41132257.66) | 13709.06 (12222.30-15370.26) | 0.29 (0.26-0.33) | 175459.64 (119576.20-250541.44) | 91.96 (62.83-129.95) | -0.82 (-1.22-0.41) |
| High-income Asia Pacific | 1462.94 (990.41-2089.17) | 0.31 (0.21-0.42) | -3.18 (-8.97-2.97) | 20933934.70 (18773194.85-23278277.91) | 7671.66 (6829.94-8629.15) | 0.56 (0.51-0.61) | 26564.50 (18456.53-35825.79) | 7.20 (4.90-9.83) | -3.36 (-4.57 to -2.14) |
| High-income North America | 7014.45 (4867.08-9682.42) | 1.20 (0.83-1.63) | 0.34 (-3.56-4.39) | 44312078.16 (39693504.66-49544059.79) | 9395.90 (8403.86-10542.43) | 0.98 (0.94-1.03) | 178065.34 (120160.62-251423.01) | 33.23 (22.97-46.98) | 0.36 (-0.40-1.12) |
| North Africa and Middle East | 11756.00 (7699.85-17161.92) | 3.18 (2.07-4.60) | -0.35 (-2.59-1.94) | 161456578.66 (146407605.69-177659074.34) | 27748.49 (25409.87-30283.48) | 0.47 (0.45-0.50) | 265596.53 (171336.92-375177.75) | 61.14 (39.58-87.12) | -0.31 (-0.83-0.21) |
| Oceania | 87.79 (55.51-129.24) | 1.13 (0.77-1.60) | -0.17 (-3.94-3.75) | 1744101.91 (1541892.36-1965419.91) | 16869.18 (15133.44-18726.79) | 0.18 (0.15-0.21) | 3194.63 (1975.61-4878.17) | 33.00 (20.97-48.54) | -0.21 (-0.91-0.50) |
| South Asia | 17739.51 (12596.43-24044.26) | 1.28 (0.90-1.73) | -1.55 (-4.85-1.87) | 241838470.86 (214848138.41-273682478.00) | 14513.87 (12969.20-16400.60) | 0.55 (0.51-0.58) | 546376.19 (385933.87-757708.62) | 34.54 (24.64-47.48) | -1.76 (-2.41 to -1.11) |
| Southeast Asia | 19624.56 (13971.70-26356.88) | 3.43 (2.46-4.62) | -0.23 (-2.40-1.99) | 128065513.81 (114729541.70-142457456.46) | 18299.21 (16508.63-20309.58) | 0.46 (0.42-0.49) | 539043.89 (379432.49-734864.03) | 82.56 (58.82-110.61) | -0.58 (-1.01 to -0.14) |
| Southern Latin America | 1429.48 (982.16-1989.97) | 1.73 (1.18-2.41) | -0.18 (-3.30-3.02) | 6483740.60 (5831421.14-7191963.86) | 8602.52 (7719.35-9564.23) | 0.90 (0.85-0.95) | 34294.28 (22901.01-48792.78) | 42.86 (28.61-61.04) | -0.52 (-1.14-0.11) |
| Southern Sub-Saharan Africa | 888.05 (632.75-1213.53) | 1.61 (1.14-2.18) | -0.53 (-3.41-2.44) | 13161620.39 (11810250.76-14681736.21) | 18075.91 (16347.92-19997.66) | 0.41 (0.37-0.44) | 26198.48 (18261.46-36311.83) | 40.87 (28.84-55.60) | -0.69 (-1.26 to -0.12) |
| Tropical Latin America | 4447.37 (3232.26-5827.27) | 1.84 (1.35-2.40) | -0.44 (-3.42-2.63) | 37986300.10 (34231442.55-41945927.58) | 15241.19 (13723.17-16818.90) | 0.49 (0.45-0.52) | 120114.10 (85232.80-161026.13) | 48.00 (34.37-63.83) | -0.60 (-1.18 to -0.02) |
| Western Europe | 9604.81 (6833.82-12825.54) | 1.09 (0.78-1.46) | -1.81 (-5.22-1.72) | 59002155.13 (53307928.02-65208561.44) | 9932.86 (8931.95-11044.15) | 0.80 (0.76-0.85) | 201120.00 (140612.13-273526.30) | 26.97 (18.77-37.08) | -2.03 (-2.72 to -1.35) |
| Western Sub-Saharan Africa | 6027.57 (4018.12-8572.80) | 3.31 (2.21-4.67) | -0.67 (-2.78-1.48) | 42321112.21 (37193511.05-48136808.11) | 14283.82 (12756.86-15952.84) | 0.22 (0.18-0.25) | 181191.57 (116196.70-270466.76) | 79.06 (51.99-113.80) | -0.70 (-1.13 to -0.26) |
The number of prevalence, deaths, and DALYs of COCLDs due to NAFLD increased in all five SDI regions from 1990 to 2019. Among them, the greatest increases in prevalence (1.54-fold), deaths (0.96-fold), and DALY (0.77-fold) cases were observed in low-SDI, middle-SDI, and low-middle-SDI regions, respectively (Figure 2, Supplementary Figure 3). All-age prevalence rates of COCLDs due to NAFLD increased across all SDI quintiles, with the most significant increase observed in the middle-SDI region (0.65-fold). Outside of the low-SDI quintile, all-age death rates and DALYs showed an increasing trend. The ASDR and ASDAR of COCLDs due to NAFLD exhibited a decreasing trend across all five SDI quintiles. The low-SDI quintile had the greatest absolute decreases in the ASDR (EAPC = -1.04; -3.48-1.45) and the ASDAR (EAPC = -1.14; -1.63 to -0.65). The ASPR of COCLDs due to NAFLD increased in all SDI regions from 1990 to 2019, with the highest increase observed in the high-SDI region (EAPC = 1.22; 1.17-1.26) (Table 1, Supplemen
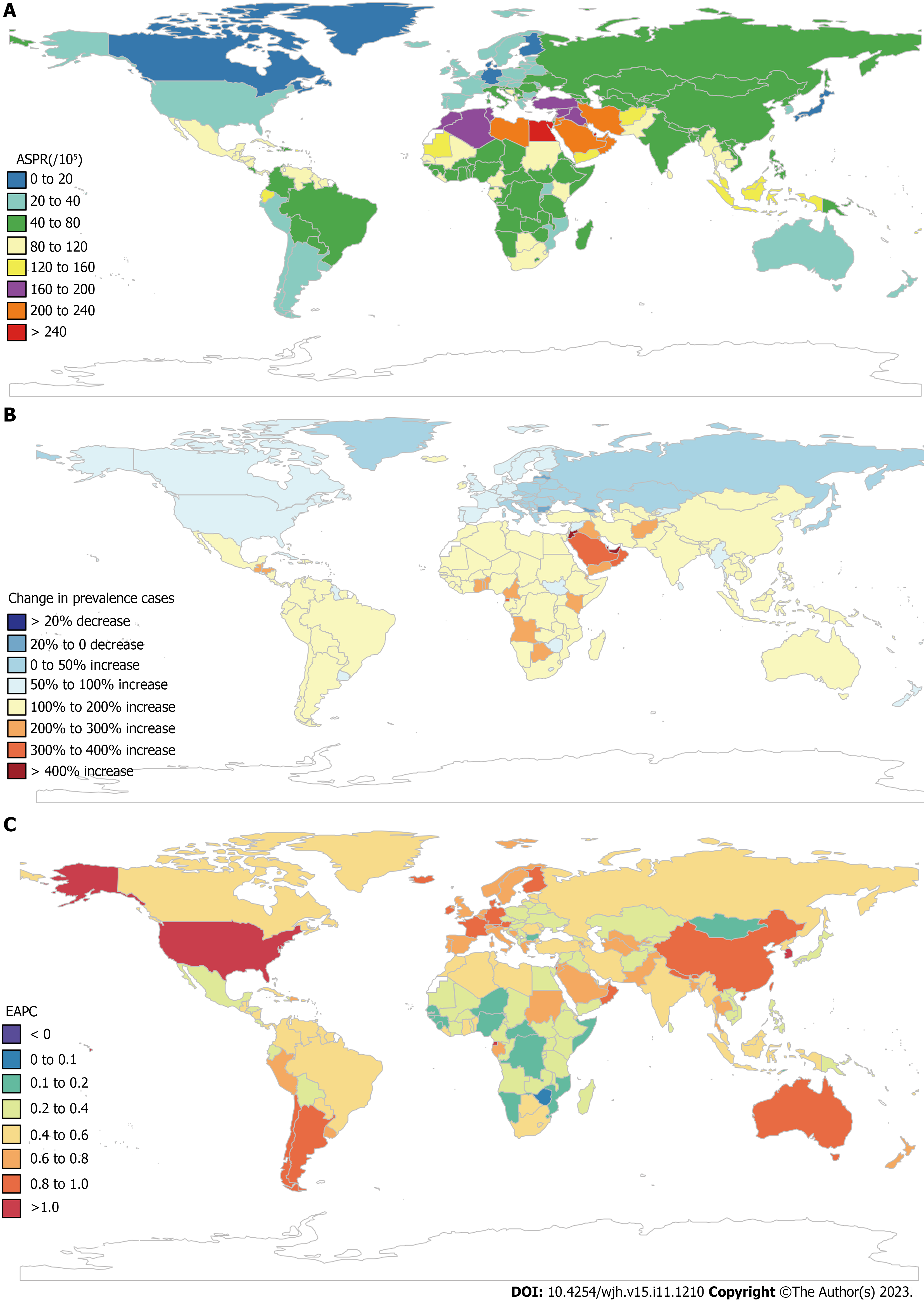
Among the 21 GBD regions, both the highest ASDR and ASDAR were observed in Central Latin America in 2019. Both the lowest ASDR and ASDAR were observed in high-income Asia Pacific in 2019 (Supplementary Tables 1 and 2, Supplementary Figures 5 and 6). The highest increases in the ASDR (EAPC = 3.04; 0.05-6.12) and ASDAR (EAPC = 3.58; 3.06-4.11) were found in Eastern Europe, followed by Central Asia and high-income North America. In contrast, high-income Asia Pacific exhibited the most pronounced decreases in the ASDR (EAPC= -3.18; -8.97-2.97) and ASDAR (EAPC= -3.36; -4.57 to -2.14) (Table 1).
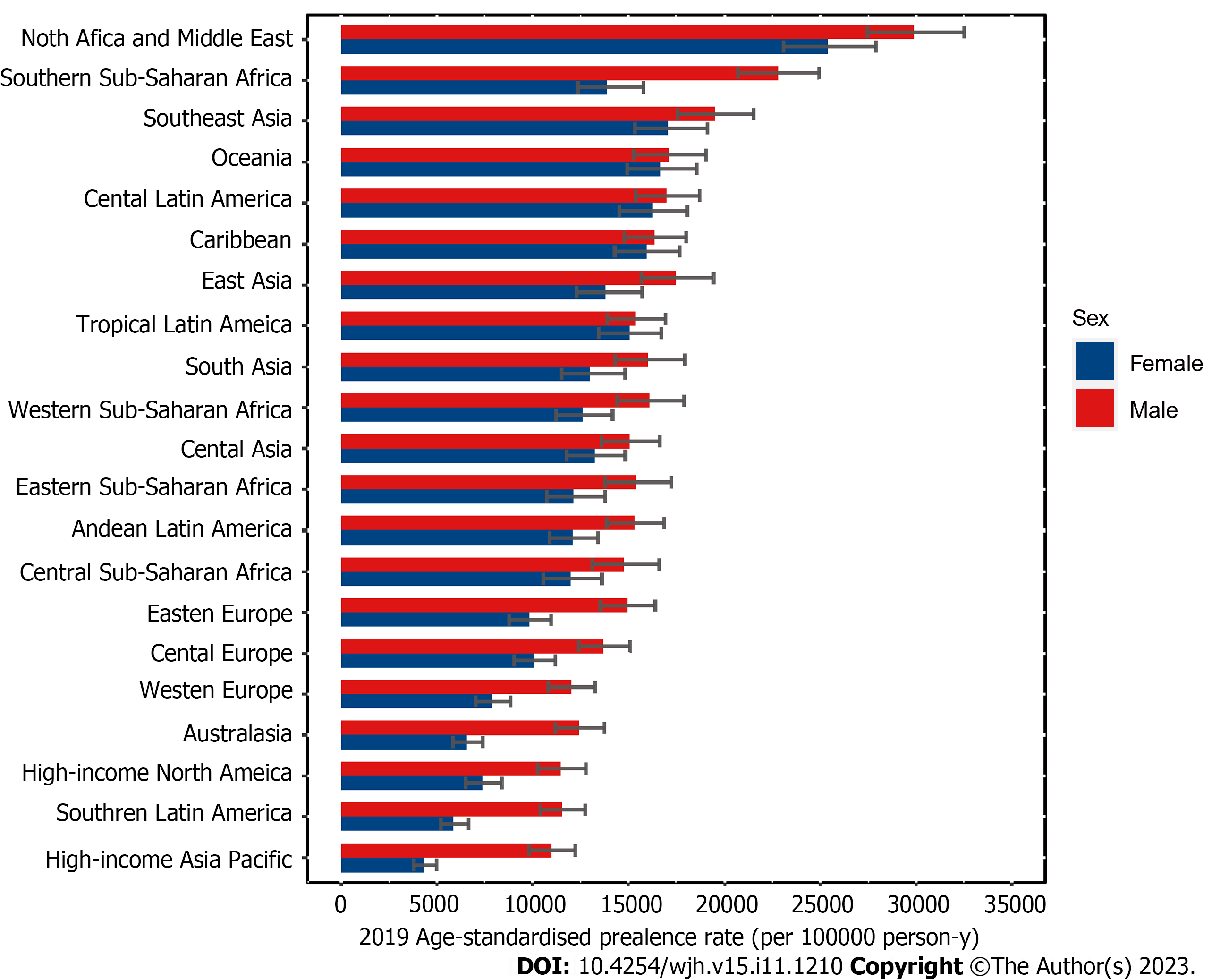
The highest ASPR of COCLDs due to NAFLD was found in North Africa and the Middle East regions, followed by Southeast Asia and Southern Sub-Saharan Africa (Figure 3, Supplementary Table 3). The highest increase in the ASPR was observed in high-income North America, followed by Australasia and Southern Latin America. Central Sub-Saharan Africa showed the lowest increases from 1990 to 2019 (Table 1).
At the national level, the ASPR of COCLDs due to NAFLD ranged from 6680.34 to 34515.88 per 100000 population in 2019. In that year, Egypt [34515.89 (31796.95-37253.06) per 100000] had the highest ASPR in 2019, followed by Qatar and Kuwait. Conversely, Finland, Canada, and Greenland had the lowest ASPR in 2019. The most pronounced changes in prevalent cases from 1990 to 2019 were seen in Qatar and Georgia. The largest increase in ASPRs was observed in the Republic of Korea [EAPC = 1.08; 95% confidence interval (CI): 1.03-1.13] and Equatorial Guinea between 1990 and 2019. Only Zimbabwe (EAPC = -0.06; 95%CI: -0.10 to -0.03) showed a decreasing trend during this period (Supplemen
The highest ASDR of COCLDs due to NAFLD was observed in Egypt [14.02 (8.42-22.43)] in 2019, followed by Honduras and Guatemala. In contrast, Montenegro, Japan, and Singapore had the lowest ASDRs. Between 1990 and 2019, the United Arab Emirates [828.78% (368.37%-1461.06%)] showed the most significant increase in the number of deaths caused by COCLDs due to NAFLD, whereas Hungary showed a decrease of 39.94%. The largest increases in the ASDRs of COCLDs due to NAFLD were observed in Armenia (EAPC = 4.21; 95%CI: 0.98-7.54) and Kazakhstan (EAPC = 4.11; 95%CI: 1.33-6.97) from 1990 to 2019. In contrast, 143 countries or territories experienced decreasing trends, with the Republic of Korea presenting the largest decrease in ASDR during this period (EAPC = -4.19; 95%CI: -8.84 to -0.70) (Supplementary Table 5, Supplementary Figure 2).
The highest ASDAR of COCLDs due to NAFLD was observed in Guatemala [258.61 (170.42-329.98)] in 2019, followed by Honduras and Egypt. In contrast, Montenegro, Japan, and Singapore had the lowest ASDAR. Between 1990 and 2019, the United Arab Emirates [905.78% (402.13%-1627.41%)] showed the most significant increase in the number of DALYs from COCLDs due to NAFLD, whereas Hungary exhibited a decrease of 49.82% (-61.59% to -35.81%). The countries with the largest increases in ASDR during this period were Kazakhstan (EAPC = 4.02; 95%UI: 3.50-4.55) and Belarus. In contrast, the Republic of Korea experienced the largest decrease in ASDR, with the greatest reduction in ASDAR over the same period (Supplementary Table 6, Supplementary Figure 3).
Globally, the prevalent number of COCLDs due to NAFLD exhibited an age-dependent pattern, reaching its peak at 45-49 years for males and 50-54 years for females. There was a declining trend in prevalence as age increased (Supplementary Figure 7). Similarly, the disease prevalence rate showed increasing and then decreasing trends with age in both sexes, with the highest prevalence rate observed in people aged 70-74 years, decreasing after this age group.
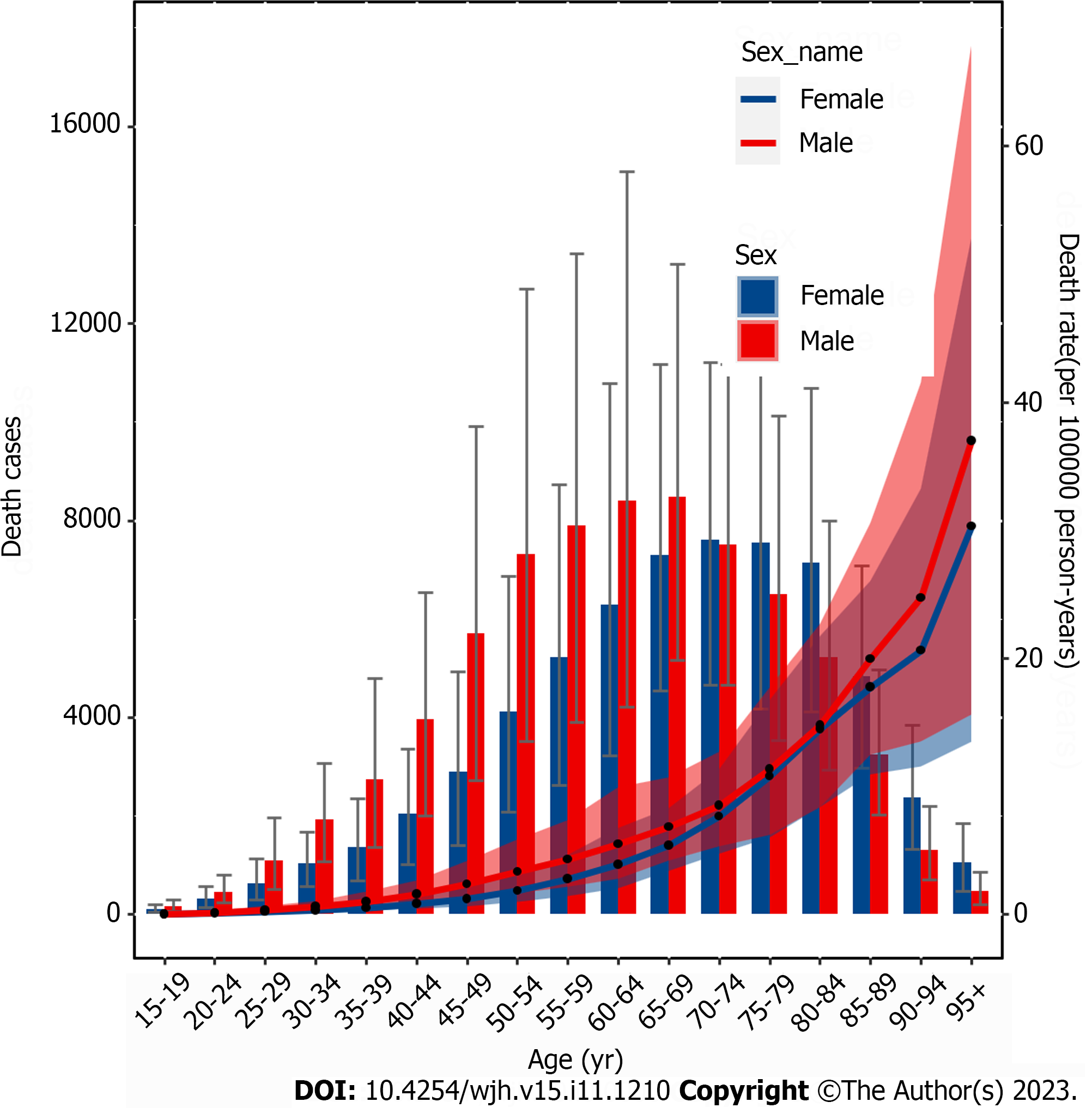
Globally, mortality rates increased with age and peaked at 65-69 years for males and 70-74 years for females before declining. The mortality rate steadily increased with age up to the 95+ age group for both sexes in 2019 (Figure 4). In the same year, the 50-54 age group for males and the 60-64 age group for females had the highest number of DALY cases, which decreased as age increased. The rate of DALYs peaked in the 80-84 age group, decreased in the 85-94 age group, and subsequently increased in the 95+ age group (Supplementary Figure 8). Among individuals under 70 years old, the numbers of prevalent cases, deaths, and DALYs lost was higher among males than females. However, among those aged 70 years and older, all three numbers were lower among males than among females (Figure 4, Supplemen
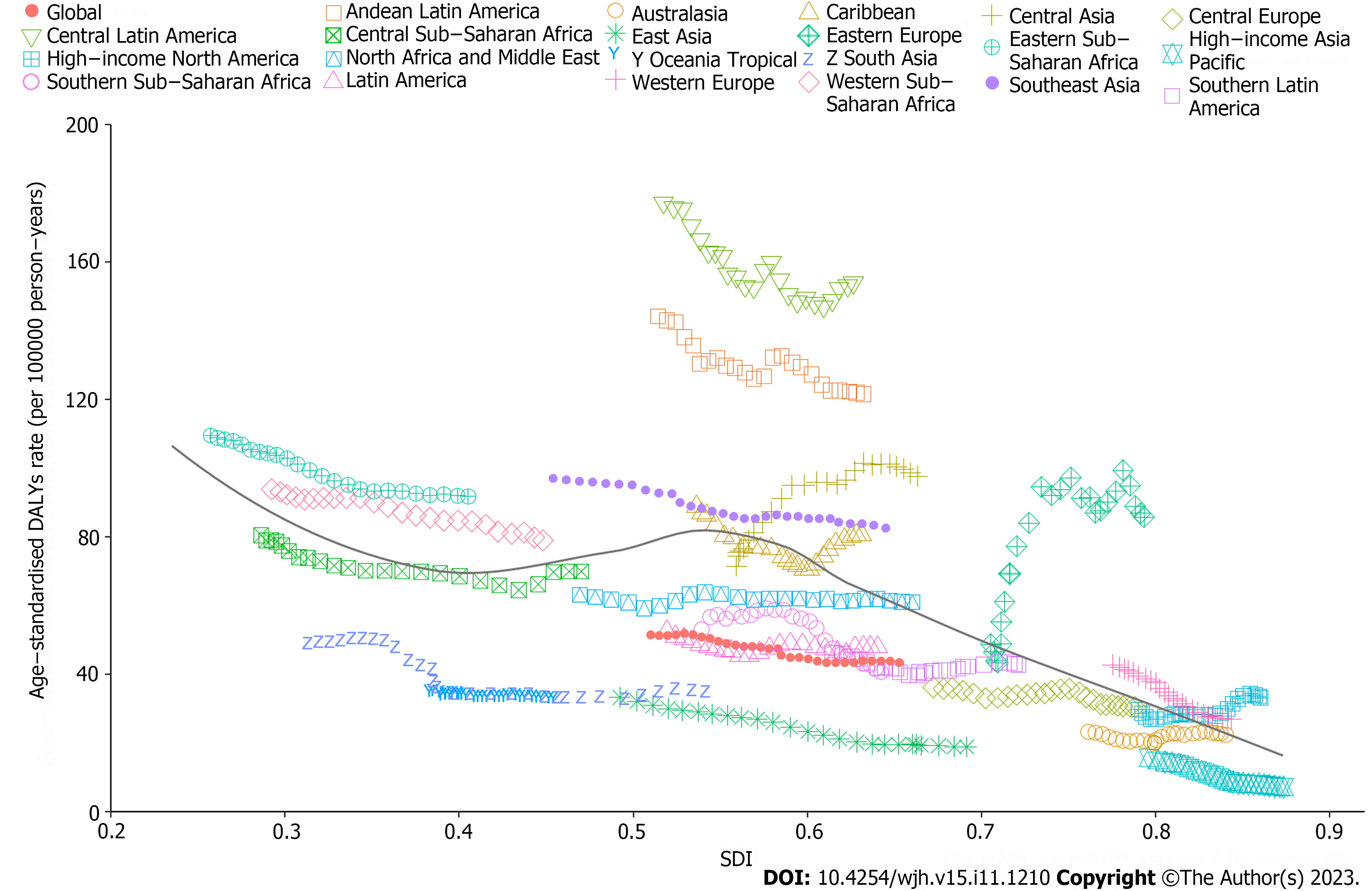
From 1990 to 2019, there was generally a negative correlation between SDI and global and regional ASDARs of COCLDs due to NAFLD. Globally, the observed burden of COCLDs due to NAFLD was lower than expected. In most regions, higher SDI values were associated with decreased ASDAR, except in Central Asia, high-income North America, and Eastern Europe, which showed an increasing trend during the study period. At the regional level, the observed burden of ASDAR of COCLDs due to NAFLD in Central Latin America, Andean Latin America, Eastern Sub-Saharan Africa, Western Sub-Saharan Africa, Southeast Asia, and Western Europe was found to be higher than the expected level based on the SDI from 1990 to 2019 (Figure 5). The link between the SDI and ASDR of COCLDs due to NAFLD had a similar pattern from 1990 to 2019 (Supplementary Figure 9). The predicted relationship between SDI and ASPR of COCLDs due to NAFLD exhibited an initial increasing trend, followed by a decreasing trend at an SDI value of 0.58 (Supplemen
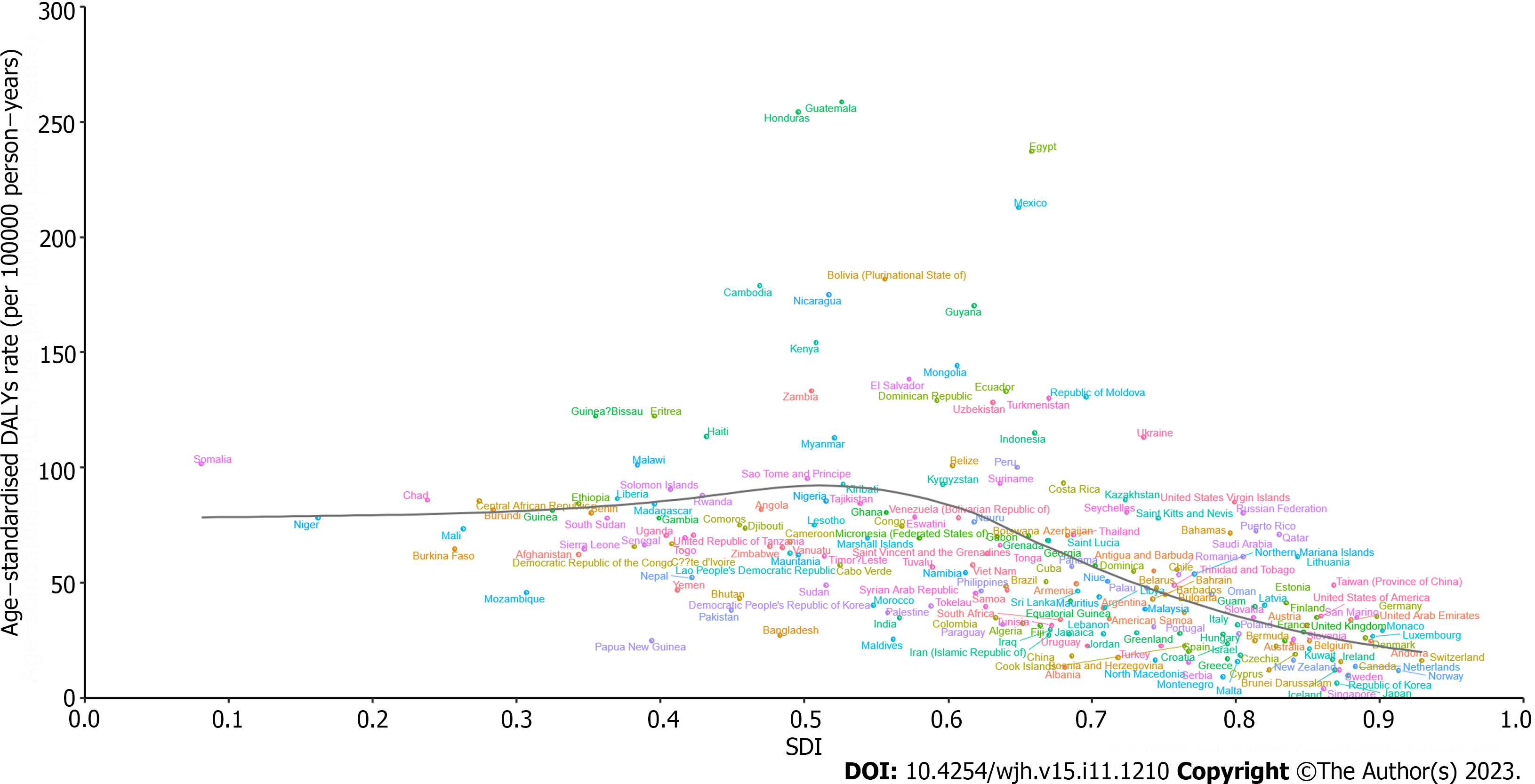
At the national level, the ASDAR of COCLDs due to NAFLD in 2019 generally displayed a negative correlation with SDI. In numerous countries/territories, including Egypt, Guatemala, Honduras, and Mexico, the ASDAR was higher than the expected level based on SDI in 2019; conversely, in countries such as the Maldives, Bangladesh, Papua New Guinea, and Mozambique, the burden was lower than expected (Figure 6). Negative correlations between the national-level ASDR and ASPR of COCLDs due to NAFLD and SDI in 2019 were also found (Supplementary Figures 11 and 12).
This study comprehensively described the trends and patterns in prevalence, DALYs, and deaths caused by COCLDs due to NAFLD at the global, regional, and national levels over the past three decades. Globally, there were an estimated 123.56 million prevalent cases, 0.13 million deaths, and 3.62 million DALYs lost in 2019. Our findings indicated a substantial increase in the number of all-age deaths, prevalent cases, and DALYs. The global prevalence and ASPR of COCLDs due to NAFLD both showed increasing trends from 1990 to 2019. Although the ASDR and ASDAR of COCLDs due to NAFLD decreased between 1990 and 2019, the total number of deaths and DALYs experienced an increasing trend, which can be partly explained by population growth, longer life expectancy, and higher prevalence in older age groups. COCLDs due to NAFLD are an increasing threat to our population and place a strain on valuable health resources. As there is currently no effective treatment for NAFLD, this trend is likely to continue, driven by its increasing prevalence.
To highlight the vital role of metabolic dysfunction in the pathogenesis of fatty liver disease, the Asian Pacific Association for the study of the Liver proposed that NAFLD be renamed metabolic- or metabolic dysfunction-associated fatty liver disease (MAFLD) in 2020[22,23]. However, there exist slight discrepancies in the definitions of MAFLD and NAFLD among different populations. The key distinctions between NAFLD and MAFLD lie in the requirement for NAFLD to exclude alcohol consumption and other risk factors, such as chronic viral hepatitis, whereas the MAFLD diagnosis focuses on detecting fatty liver in conjunction with metabolic risk factors, without necessitating the exclusion of other liver disorders[24]. In addition, GBD 2019 only estimated the burden of COCLDs due to NAFLD, and the MAFLD burden was not estimated. Therefore, in this study, we focused on the burden of COCLDs due to NAFLD.
NAFLD is closely associated with overweight and obesity, affecting an estimated 25% of the general adult population. Obesity significantly elevates the risk of NAFLD development. In a previous study, obesity was found in approximately 81% of patients with NASH and 50% of patients with NAFLD[25]. Lifestyle modifications and bariatric surgery have significantly improved NAFLD activity scores[26,27]. Earlier epidemiological investigations projected that the worldwide count of obese or overweight individuals surpassed 2.1 billion, constituting a pervasive global health concern[28]. For 2019, we identified the prevalent count of COCLDs attributed to NAFLD as 1.2 billion individuals, which was slightly less than the number of obese individuals.
Interestingly, reports indicate that around 20% of Asian individuals diagnosed with NAFLD have body mass index and waist circumference measurements falling within the range designated as lean, despite the prevailing association of NAFLD with overweight or obesity[29]. Notably, regions with higher incomes, such as the United States, France, and Japan, tend to exhibit an elevated prevalence of lean MAFLD[30]. However, certain developing countries, such as India and Sri Lanka, have an even higher prevalence rate of lean MAFLD, which may be attributed to racial and dietary factors. United States-based studies also indicate that people with ancestry from Latin America have a higher prevalence rate of lean MAFLD, whereas African Americans have a lower prevalence rate. The suggested racial and ethnic variations are important risk factors for the prevalence of lean MAFLD[30,31]. In addition, lean MAFLD patients have worse long-term outcomes than healthy people and have a similar prognosis to overweight or obese MAFLD patients. Therefore, more attention should be focused on normal-weight MAFLD patients, and a 3%-5% weight reduction and improvement in diet quality are strongly recommended to improve lean MAFLD[30]. Beyond body weight, additional factors such as diabetes, ethnic disparities, and genetic variations could also contribute to the prevalence and outcomes of lean NAFLD[32]. Given the ongoing global epidemic of obesity and diabetes, the burden of COCLDs attributed to NAFLD is expected to rise in the coming years.
According to previous research, the number of deaths due to cirrhosis caused by NAFLD/NASH was 102615 in 2012 and 118030 in 2017. The annual percent change in the ASDR of cirrhosis due to NAFLD/NASH from 2012 to 2019 was 0.29[33]. In contrast, our study found that the EAPC of the ASDR of COCLDs due to NAFLD was -0.65 from 1990 to 2019, indicating a decreasing trend. This variation in findings can be ascribed to disparities in the time frames of participant enrolment: Our study encompassed data from 1990 to 2019, while the study by Paik et al[33] included data from 2012 to 2017. Notably, we also calculated the EAPC of the ASDR of COCLDs due to NAFLD from 2012 to 2019 and found a decreasing trend with a value of -0.05. This indicates that there may have been a decreasing trend from 2017 to 2019.
The global upsurge in type 2 diabetes and obesity has raised the prevalence of NAFLD in both developed and developing nations[4]. Our study found that the highest ASPRs of COCLDs due to NAFLD were in some developing regions, such as North Africa, the Middle East regions, and Southeast Asia, and in specific countries such as Egypt, Qatar, and Kuwait. Previous research reported an NAFLD prevalence of 42.04% in South Asia and 31.79% in the Middle East, which is consistent with our finding that these regions had the highest prevalence rates[25,34].
According to previous research, approximately half of the global burden of liver complications associated with NAFLD is concentrated in the Middle East, North Africa, and Asia[35]. The escalated prevalence in these geographical areas can be attributed to intricate interplays of lifestyle choices, economic conditions, and ethnic factors. The tandem epidemics of obesity and NAFLD are predominantly propelled by unhealthy dietary practices and sedentary habits characterized by consumption of calorie-dense foods and insufficient physical activity[34]. Studies have indicated that Middle East and North African countries have a high prevalence of overweight and obesity, with more than 30% of females and more than 20% of males being obese in most countries in the region. This trend is attributed to unhealthy diets and the lowest levels of physical activity worldwide[36]. Concurrently, the Middle East and North Africa register the highest prevalence rates of cirrhosis of the liver attributable to NAFLD. This underscores the critical need for effective interventions aimed at mitigating the escalating prevalence of obesity and diabetes to ameliorate the burden of COCLDs attributed to NAFLD[37].
According to our study, the prevalence, deaths, and DALYs lost due to COCLDs stemming from NAFLD were higher in males than in females across all age groups before the age of 65-69 years. Similarly, the rates of these occurrences were comparable when compared with their female counterparts across all age groups. This sex disparity is consistent with previous research demonstrating that from 1990 to 2017, the burden of cirrhosis in males was universally higher in males than in females[38]. Hormonal factors could underlie this pattern, where estrogen, acting as an antioxidant, mitigates the activity of stellate cells and the advancement of liver fibrosis[39]. After menopause, women lose the protective effect of estrogen[40], and physiological changes associated with hypoestrogenism, such as insulin resistance, dysglycemia, dyslipidemia, and visceral fat accumulation, may be associated with the higher prevalence of COCLDs due to NAFLD in postmenopausal women[41,42]. This may partly elucidate the higher prevalence in males older than 70 years in our study.
We found that the number of prevalent cases, deaths, and DALYs lost due to COCLDs due to NAFLD was the highest among middle-aged groups (approximately 45-69 years). Within this range, the 75-79 age group presented the highest prevalence rate, while the 95+ age group exhibited the highest mortality rate. The underlying reasons for these findings are multifactorial. One potential explanation involves the metabolic alterations that transpire in older age groups[43]. Another possible reason is the natural history of liver cirrhosis, which is characterized by a compensated phase that lasts significantly longer than the rapidly progressive decompensated phase, with a median survival time of more than 12 years. A large proportion of patients with cirrhosis die after transitioning from the compensated phase to the decompensated phase[5]. Given this context, the imperative need for accurate, noninvasive methodologies to enable early identification of COCLDs stemming from NAFLD becomes evident.
We found a negative correlation between the SDI and the ASDAR of COCLDs due to NAFLD in the 21 GBD regions from 1990 to 2019 and in 204 countries in 2019. Generally, regions with higher SDI had a lower burden of cirrhosis due to NAFLD, which may be attributed to accessible high-quality health care and enough safe spaces to exercise[44,45]. Conversely, low-SDI and low-middle-SDI regions tended to bear a higher burden. The Sub-Saharan African region showed a high ASDAR of COCLDs due to NAFLD in 2019. Some regions such as Western Sub-Saharan Africa, Southeast Asia, Central Latin America, Andean Latin America, and Eastern Sub-Saharan Africa, as well as some countries and territories, such as Egypt, Honduras, and Guatemala, had burdens higher than expected based on their SDIs, indicating that these regions and countries should receive more investment and public health programs.
The escalating prominence of COCLDs emanating from NAFLD has positioned it as a preeminent public health challenge. Notably, an absence of effective pharmaceutical interventions to fully eradicate NAFLD persists. Consequently, interventions targeting weight loss could be efficacious and cost-effective strategies to avert the progression of NAFLD to COCLDs. In addition, exercise interventions without significant weight loss have also had a beneficial effect on alleviating NAFLD[46]. Thus, it is imperative to emphasize the critical role of weight management and exercise within public health programs. Furthermore, an enhancement of noninvasive diagnostic methods and the development of effective treatment strategies will be pivotal in alleviating the burden of COCLDs stemming from NAFLD. Importantly, public awareness regarding NAFLD and its associated complications remains inadequate. A concerted effort to raise population awareness about the implications of NAFLD is paramount.
In this study, we pioneered a comprehensive analysis of the relative burden of COCLDs attributed to NAFLD on global, regional, and national scales spanning the period from 1990 to 2019. Nevertheless, several limitations warrant consideration. First, the data from GBD 2019 have the general limitations of the GBD approach that have been described. The GBD estimates depended on robust statistical methods and trends from neighboring countries to overcome data scarcity and low data quality in some countries. Second, liver biopsy remains the gold-standard diagnostic test for patients with COCLDs due to NAFLD, but its poor acceptability during compensation and sampling variability may lead to misdiagnosis and underdiagnosis[47]. A dearth of diagnostic techniques may cause an underestimation of COCLDs due to NAFLD, which may be more severe in regions with low SDIs. Third, patients admitted to hospitals mostly had decompensated cirrhosis. Therefore, the number of compensated cirrhosis cases may be underestimated. The underreporting of cirrhosis can bias the estimates. Fourth, GBD 2019 failed to adopt the new term MAFLD to replace NAFLD. NAFLD was defined only after the exclusion of other causes of hepatic steatosis, and there is an unclear differentiation between NAFLD and alcoholic liver disease owing to different adjustments for alcohol use. Finally, the exclusion of patients with cirrhosis with hepatocellular carcinoma from our study could result in an underestimation of the true mortality rate among individuals with liver cirrhosis due to NAFLD.
This study described the burden of COCLDs due to NAFLD in 204 countries and territories from 1990 to 2019 by age, sex, and SDI. COCLDs due to NAFLD are becoming a significant global public health concern. Over the past three decades, there has been a notable increase in the ASPR, while the ASDR and ASDAR have exhibited downward trends. Notably, substantial geographic disparities exist in the burden of COCLDs due to NAFLD, with the highest prevalence rates observed in North Africa and the Middle East. In 2019, males had a higher burden of prevalence, deaths, and DALYs lost than females before the 65-69 age group. Furthermore, there is a negative correlation between SDI values and ASDAR. We hope this study raises public awareness of COCLDs due to NAFLD and broadcasts the need for more effective prevention strategies to minimize the future health care burden.
The incidence and prevalence of nonalcoholic fatty liver disease (NAFLD) have been rapidly increasing worldwide over the past few decades, leading to cirrhosis and other chronic liver diseases (COCLDs). Cirrhosis is the leading cause of liver-related morbidity and contributes to more than 1 million deaths annually worldwide. NAFLD has become the leading cause of COCLDs.
A previous study reported the burden of liver cirrhosis caused by nonalcoholic steatohepatitis. However, no studies have focused on the epidemiology of COCLDs due to NAFLD across the globe.
We conducted a comprehensive and comparable updated analysis of the global, regional, and national levels of prevalence, death, and disability-adjusted life-years (DALYs) of COCLDs due to NAFLD in regards to age-standardized rates and numbers from 1990 to 2019, stratified by sex, age, and sociodemographic index.
Data on COCLDs due to NAFLD were collected from the Global Burden of Diseases, Injuries, and Risk Factors Study 2019. Numbers and age-standardized prevalence, death, and DALYs were estimated through a systematic analysis of modeled data from the Global Burden of Diseases, Injuries, and Risk Factors Study 2019. Estimated annual percentage change was used to determine the burden trend.
We found that the global age-standardized prevalence rate of COCLDs due to NAFLD was 15022.90 per 100000 population in 2019, with an estimated annual percentage change of 0.78. The age-standardized death rate and age-standardized DALYs rate per 100000 population were 1.66 and 43.69 in 2019, respectively. The highest prevalence rate was observed in North Africa and the Middle East. The numbers of prevalent cases, deaths, and DALYs cases of COCLDs due to NAFLD were higher in males than in females across all age groups before the age of 65-69 years. There was a negative correlation between sociodemographic index and age-standardized DALYs rate.
COCLDs due to NAFLD have emerged as a large and growing public health burden worldwide. Globally, the ASPR has increased during the past three decades, whereas the ASDR and age-standardized DALY rate have decreased. There is geographical variation in the burden of COCLDs due to NAFLD. It is strongly recommended to improve the quality of COCLDs due to NAFLD health data across all countries and regions to facilitate better monitoring of the burden of COCLDs due to NAFLD.
We believe that the findings of this study will provide insight into the global disease burden of COCLDs due to NAFLD and assist policymakers in formulating effective policies to mitigate modifiable risk factors.
We thank the work of the Institute for Health Metrics and Evaluation staff and its collaborators.
| 1. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1370] [Article Influence: 274.0] [Reference Citation Analysis (0)] |
| 2. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8582] [Article Influence: 408.7] [Reference Citation Analysis (9)] |
| 3. | Chong B, Kong G, Shankar K, Chew HSJ, Lin C, Goh R, Chin YH, Tan DJH, Chan KE, Lim WH, Syn N, Chan SP, Wang JW, Khoo CM, Dimitriadis GK, Wijarnpreecha K, Sanyal A, Noureddin M, Siddiqui MS, Foo R, Mehta A, Figtree GA, Hausenloy DJ, Chan MY, Ng CH, Muthiah M, Mamas MA, Chew NWS. The global syndemic of metabolic diseases in the young adult population: A consortium of trends and projections from the Global Burden of Disease 2000-2019. Metabolism. 2023;141:155402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 4. | Wang D, Xu Y, Zhu Z, Li Y, Li X, Shen H, Wu W, Liu Y, Han C. Changes in the global, regional, and national burdens of NAFLD from 1990 to 2019: A systematic analysis of the global burden of disease study 2019. Front Nutr. 2022;9:1047129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 5. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2216] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 6. | Ye F, Zhai M, Long J, Gong Y, Ren C, Zhang D, Lin X, Liu S. The burden of liver cirrhosis in mortality: Results from the global burden of disease study. Front Public Health. 2022;10:909455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Patel AA, Walling AM, Ricks-Oddie J, May FP, Saab S, Wenger N. Palliative Care and Health Care Utilization for Patients With End-Stage Liver Disease at the End of Life. Clin Gastroenterol Hepatol. 2017;15:1612-1619.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB, Bell DE, Gilles H, Morton K, Noble N, Puri P, Sanyal AJ. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, Sheron N; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 502] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 10. | Bosetti C, Levi F, Lucchini F, Zatonski WA, Negri E, La Vecchia C. Worldwide mortality from cirrhosis: an update to 2002. J Hepatol. 2007;46:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142:1360-1368.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther. 2016;43:674-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1377] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 14. | Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, Ahmed A. Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016. Gastroenterology. 2018;155:1154-1163.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (2)] |
| 15. | Hope AA, Morrison RS. What Is the Clinical Course of Advanced Liver Disease and What Symptoms Are Associated With It? In Evidence-Based Practice of Palliative Medicine. Elsevier Inc. 2012;300-307. [DOI] [Full Text] |
| 16. | Sharma A, Nagalli S. Chronic Liver Disease. StatPearls. 2023 Available from: https://www.ncbi.nlm.nih.gov/books/NBK554597/. |
| 17. | Mann J. Liver Pathophysiol Ther Antioxidants. Boston: Academic Press. 2017;199-211. [DOI] [Full Text] |
| 18. | Zhai M, Liu Z, Long J, Zhou Q, Yang L, Liu S, Dai Y. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11:5195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13226] [Cited by in RCA: 12082] [Article Influence: 2013.7] [Reference Citation Analysis (42)] |
| 20. | GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1160-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1222] [Article Influence: 203.7] [Reference Citation Analysis (1)] |
| 21. | Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, Grove JT, Hogan DR, Hogan MC, Horton R, Lawn JE, Marušić A, Mathers CD, Murray CJ, Rudan I, Salomon JA, Simpson PJ, Vos T, Welch V; (The GATHER Working Group). Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388:e19-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 1135] [Article Influence: 113.5] [Reference Citation Analysis (1)] |
| 22. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 618] [Article Influence: 103.0] [Reference Citation Analysis (3)] |
| 23. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2427] [Article Influence: 404.5] [Reference Citation Analysis (4)] |
| 24. | Chan WK, Wong VW. Meaning of non-overlapping patients between the MAFLD and NAFLD definitions. Liver Int. 2022;42:271-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7952] [Article Influence: 795.2] [Reference Citation Analysis (8)] |
| 26. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1786] [Article Influence: 162.4] [Reference Citation Analysis (3)] |
| 27. | Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, Romon M, Pattou F. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 28. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8182] [Article Influence: 681.8] [Reference Citation Analysis (0)] |
| 29. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 636] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 30. | Eslam M, El-Serag HB, Francque S, Sarin SK, Wei L, Bugianesi E, George J. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 31. | Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, Watkins S, Reddy KR, Weiss M, Zink RC, Lok AS. Lean Americans With Nonalcoholic Fatty Liver Disease Have Lower Rates of Cirrhosis and Comorbid Diseases. Clin Gastroenterol Hepatol. 2021;19:996-1008.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, Bayoumi A, Metwally M, Azardaryany MK, Coulter S, Choo JM, Younes R, Rosso C, Liddle C, Adams LA, Craxì A, George J, Eslam M. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology. 2020;71:1213-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 33. | Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology. 2020;72:1605-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 564] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 34. | Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 759] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 35. | Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009-2019. J Hepatol. 2021;75:795-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 36. | Azizi F, Hadaegh F, Hosseinpanah F, Mirmiran P, Amouzegar A, Abdi H, Asghari G, Parizadeh D, Montazeri SA, Lotfaliany M, Takyar F, Khalili D. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol. 2019;7:866-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 37. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1839] [Article Influence: 229.9] [Reference Citation Analysis (0)] |
| 38. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1123] [Article Influence: 187.2] [Reference Citation Analysis (5)] |
| 39. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 40. | Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 41. | Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 693] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 42. | Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, Yosef M, Symons J. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 43. | Churilla JR, Fitzhugh EC, Thompson DL. The Metabolic Syndrome: How Definition Impacts the Prevalence and Risk in U.S. Adults: 1999-2004 NHANES. Metab Syndr Relat Disord. 2007;5:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Talens M, Tumas N, Lazarus JV, Benach J, Pericàs JM. What Do We Know about Inequalities in NAFLD Distribution and Outcomes? A Scoping Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Niessen LW, Mohan D, Akuoku JK, Mirelman AJ, Ahmed S, Koehlmoos TP, Trujillo A, Khan J, Peters DH. Tackling socioeconomic inequalities and non-communicable diseases in low-income and middle-income countries under the Sustainable Development agenda. Lancet. 2018;391:2036-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 46. | Babu AF, Csader S, Lok J, Gómez-Gallego C, Hanhineva K, El-Nezami H, Schwab U. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 47. | Lambrecht J, Verhulst S, Mannaerts I, Reynaert H, van Grunsven LA. Prospects in non-invasive assessment of liver fibrosis: Liquid biopsy as the future gold standard? Biochim Biophys Acta Mol Basis Dis. 2018;1864:1024-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Tai DI, Taiwan S-Editor: Qu XL L-Editor: Filipodia P-Editor: Cai YX