Published online Jan 27, 2023. doi: 10.4254/wjh.v15.i1.41
Peer-review started: September 13, 2022
First decision: October 20, 2022
Revised: November 3, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: January 27, 2023
Processing time: 124 Days and 14.7 Hours
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a significant impact on the lives of millions of people, especially those with other concomitant di
Core Tip: The main aim of this brief review is to summarize current knowledge on the acute-on-chronic liver failure (ACLF) in patients with coronavirus disease 2019 (COVID-19). We also describe several mechanisms by which severe acute respiratory syndrome coronavirus 2 infection induces liver injury. Although several systematic reviews have already been published regarding liver impairment in COVID-19, there are few studies focusing on ACLF. We believe that this brief review has an informative value for clinicians and could contribute to better understanding of the disease and therefore improved management of this serious condition.
- Citation: Liptak P, Nosakova L, Rosolanka R, Skladany L, Banovcin P. Acute-on-chronic liver failure in patients with severe acute respiratory syndrome coronavirus 2 infection. World J Hepatol 2023; 15(1): 41-51
- URL: https://www.wjgnet.com/1948-5182/full/v15/i1/41.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i1.41
The coronavirus disease 2019 (COVID-19) pandemic caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a significant impact on the lives of millions of people, especially those with other concomitant diseases such as chronic liver diseases.
This minireview focused on acute-on-chronic liver failure (ACLF) in COVID-19-involved cases. ACLF is a relatively new syndrome that occurs in 10%-30% of all hospitalized patients with chronic liver disease[1]. Patients with ACLF are considered to be high-risk patients when they become infected with SARS-CoV-2 because of the increased morbidity and mortality in these cases. The etiology of chronic liver diseases varies substantially (e.g., viral hepatitis B and C, alcohol liver disease, non-alcohol steatohepatitis, automimune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, Wilsons’s disease). Even after more than two years of global pandemic, this is a rather underestimated topic with an uneven ratio of patients with chronic liver disease who have been infected with SARS-CoV-2.
Therefore, understanding the pathophysiology mechanisms of SARS-CoV-2 virus affecting the liver along with improved stratification of patients with chronic liver diseases can ultimately result in better management, and a significant reduction in mortality and morbidity in the case of COVID-19 infection.
To date, seven coronaviruses have been identified to infect humans. While human coronaviruses HCoV-229E, HCoV-NL63, HCoV-OC43 and HCoV-HKU1 cause a “common cold”, the other three, severe acute respiratory syndrome-related coronavirus (SARS-CoV) (2002-2003), Middle East respiratory syndrome-related coronavirus (MERS-CoV) (2012) and SARS-CoV-2 (from 2019), are highly pathogenic to humans and cause severe acute respiratory syndrome (SARS), with significant morbidity and mortality[2,3]. The main site of pathological action of these viruses is lung tissue. It has been widely hypothesized that the SARS-CoV-2 virus uses the angiotensin-converting enzyme 2 (ACE2) receptor to enter the respiratory tract cells[4]. The ACE2 receptor is expressed not only in the lungs, but also in other organs, such as the heart, intestine (ileum), pancreas, kidneys and endothelium, which may explain the multi-organ effect of virus infection[5]. A huge number of studies have proved that SARS-Co-V-2 shows affinity towards several organs, including those of gastrointestinal tract, such as the liver[6-9].
Evidence that coronaviruses could damage liver cells through the induction of apoptosis by act
Almost three years after the COVID-19 pandemic broke out, there is undoubtedly a large amount of scientific and clinical evidence that COVID-19 is in many cases directly connected with abnormal liver function to a varying extent. Right from the beginning of the pandemic there were indications of a similar mechanism of influence of SARS-CoV and SARS-CoV-2 on hepatocytes[16], although the strains bear approximately 79% structural similarity[17]. It was pointed out that recipients of liver transplant could be at higher risk for virus transmission through the transplanted organ[18]. The fact that non-alcoholic fatty liver disease (NAFLD) presents with a proinflammatory hypercoagulable state could be associated with a more severe course of the disease and thrombosis in these patients when infected with SARS-CoV-2[19]. The structural hepatic abnormalities could persist even after acute COVID-19, as was shown in a study using multiparametric ultrasound[20]. These changes include increased liver stiffness and increased viscosity and attenuation, which could be indicative of various types of parenchymal impairment, including fibrosis, inflammation and steatosis[20].
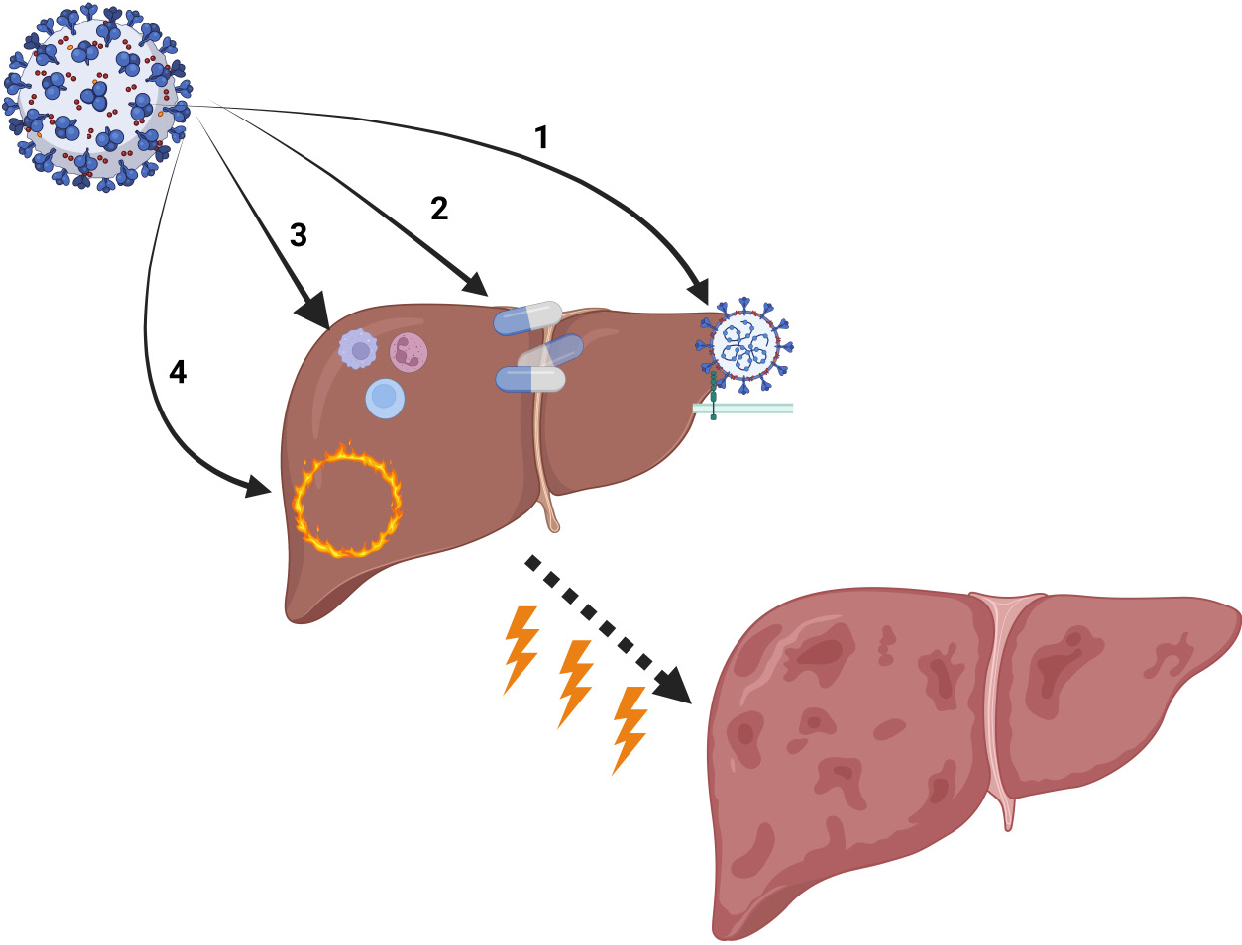
The current state of evidence points to several proposed mechanisms of liver injury in patients with COVID-19 (Figure 1). Liver impairment is considered to be the result of the direct effect of the virus on hepatic tissue cells, a systemic reaction consisting of inflammation, hypoxia and cytokine storm, and drug-induced liver injury[21-23] with the possible contribution of a perturbed gut-liver axis[24]. Reactivation of chronic hepatic disease could be another factor for liver impairment in patients with SARS-CoV-2 infection[25].
Moderate microvesicular steatosis and mild inflammation in the lobular and portal area was observed in the liver tissues obtained during autopsies of COVID-19 victims[26]. This is, however, not disease-specific, as it could also be detected in liver tissue samples in patients with sepsis or drug-induced liver injury (DILI)[26].
There are several proposed mechanisms of SARS-CoV-2 influence on hepatocytes. One of the early histological and ultrastructural studies identified typical coronavirus particles in the hepatocytes’ cytoplasm, with mitochondrial swelling, endoplasmic reticulum dilatation and glycogen granule decrease with a general histological picture of massive hepatocyte apoptosis and binuclear hepatocytes[27].
One possible explanation is based on the binding of SARS-CoV-2 to the ACE2 receptors on the cholangiocytes, leading to their dysfunction and induction of a local and systemic inflammatory response, ultimately resulting in liver injury[28]. Although the ACE2 receptor is present on the biliary epithelial cells, it was repeatedly observed that the bilirubin level was normal in most of the cases[29], regardless of severity of the disease itself[30]. Although the effect of the virus is primary on the bile duct epithelial cells, some researchers have proposed that the compensatory hyperplasia of hepatic parenchymal cells induce the up-regulation of ACE2 receptor expression in liver tissue[22]. This could be one of the pathways by which SARS-CoV-2 is responsible for direct liver parenchyma injury.
A study by Zhao et al[31] showed a significant increase of viral loads in cholangiocytes 24-h post-infection with a substantial decrease 48 h after infection. Their data also indicated that the virus impairs the bile acid transporting function of cholangiocytes and impairs the luminal barrier by modulating the expression of genes involved in sustaining the tight junctions and transportation of bile acids[31]. The direct viral cytopathogenic effect is predominantly on target cells that express ACE2 and TMPRSS2[31]. ACE2 expression level is higher in cholangiocytes (59.7%) than in hepatocytes (2.6%)[32].
Stebbing et al[33] reported massive induction of ACE2 expression in hepatocytes after 16 h of exposure to Interferone-α2 (IFN-α2) and Interferone-β. Exposure to Interferone-γ, tumor necrosis factor-α and interleukins (IL-1, IL-6, IL-10, Il-18) does not have the same effect. They further pointed out that the effect was strongest with Interferone-α2. Therefore, it has been proposed that the increased levels of IFN-α2 predominantly in patients with severe inflammatory response to SARS-CoV-2 infection could lead to significant ACE2 expression in parenchymal liver cells, contributing to virulence and further damaging the cells by the virus[33].
Another study focused on the expression of ACE2, TMPRSS2 and FURIN (paired basic amino acid-cleaving enzyme) levels in various cells within the liver tissue. It was shown that these receptors are expressed across various cell types. ACE2 is mostly expressed in cholangiocytes and hepatocytes, TMPRSS2 in cholangiocytes, hepatocytes, periportal liver sinusoidal endothelial cells, erythroid cells, non-inflammatory macrophages and T cells, and FURIN is expressed through all cell lines within liver tissue[23].
A recent study by Wanner et al[9] has provided multilevel evidence of SARS-CoV-2 human liver tropism using a wide range of clinical, histopathological, virological, molecular and bioinformatic approaches. Their data showed strong upregulation of JFN responses, JAK-STAT signaling and liver-specific metabolic modulation. Mismatch of the expression of the ACE2 protein and the location of the SARS-CoV-2 spike protein in Kupffer cells was also observed in this study[9]. Also, the main pro-inflammatory cytokines, such as IL-6, which is responsible for cytokine storm, is regulated by JAK-STAT signaling. Due to this known pathophysiological mechanism, JAK inhibitors such as baricitinib have been used for treatment and have shown improvement in clinical outcomes in patients infected with SARS-COV-2. On the other hand, considering the potential adverse effects of this drug on the liver, more studies are needed to establish the proper dosage and timing, so the risk/benefit ratio can be determined in patients with high vulnerability for drug-induced liver injury[33,34]. Although several medications were used for treating COVID-19 with different outcomes, the “perfect” compound is still missing. However, results of the studies mentioned herein could facilitate the push of research towards targeting signaling pathways, receptors or even the virus itself.
Another study proposed high-density lipoprotein scavenger receptor class B member 1 (SRB1) as a facilitator for cell entry for the SARS-CoV-2 because of its strong protein expression in human liver cells[35]. This is based on the observation that SRB1 plays a crucial role in hepatitis virus C (HCV) cell entry[36]. SARS-CoV-2 shares some molecular features with HCV in the means of liver tropism[9]. Therefore, it is possible to assume that SRB1 could facilitate SARS-CoV-2 entry into liver cells along the well described ACE2 pathway.
It is interesting to compare the mechanism of action of SARS-CoV-2 with other coronaviruses. An indirect mechanism that resulted in hepatic damage through a complex inflammatory cascade was proposed in case of the SARS-CoV virus infection[13,37]. On the other hand, MERS-CoV requires dipeptidyl peptidase-4 (DPP-4) receptor for cell entry, which is different from SARS-CoV-1 and SARS-CoV-2 adherence mechanisms. Thus, the pathophysiology of the disease is different to some extent. The liver damage observed in MERS-CoV-infected cases was mostly mild. It is difficult to determine whether this is the result of a direct action of the virus or the inflammation-mediated reaction due to a lack of sufficient data[37]. An interesting fact is the comparison with hepatitis viruses, which, from a phylogenetic point of view, have developed a natural affinity for the liver tissue and whose infections are of a stealthy nature. Despite the fact that the mechanism of infection is not fully understood, it is assumed that hepatitis viruses do not have a direct cytopathic effect on hepatocytes but rather to trigger immune mechanisms that result in liver damage[38-40].
Lopinavir and ritonavir are widely used antiviral drugs that are predominantly metabolized by the liver. These drugs were shown to have a potentially damaging effect on the liver by inducing inflammation and lipid metabolism disorders via the endoplasmic reticulum stress pathway and also could cause apoptosis of hepatocytes via the caspase system[22].
Integration of drug cytochrome P-450 could contribute to the secondary toxicity of several drugs commonly and widely used in the treatment of COVID-19 such as paracetamol (acetaminophen), lopinavir/ritonavir or azithromycin[41]. The meta-analysis by Yadav et al[42] showed that treatment by lopinavir/ritonavir is strongly correlated with liver injury, while other commonly used medications are not significantly connected with hepatic impairment.
Another commonly used drug in COVID-19 treatment is the antiviral drug favipiravir. It was reported that favipiravir used with interferon alpha resulted in liver injury in 2.9% of these patients[22].
One of the factors contributing secondarily to the hypoxic damage of hepatocytes could be hepatic congestion due to high positive end respiratory pressure in critically ill, mechanically ventilated patients[32]. Platelet activation is well described in patients with a serious course of COVID-19, and it has been proposed that vascular dysfunction due to endotheliopathy and platelet activation in response to a systemic inflammatory response could contribute to impaired liver function, predominantly in patients with a pre-existing chronic liver disease[43].
Systemic inflammatory response generally leads to cellular ischemia and abnormal coagulation with micro-thrombotic events. Inflammatory response in COVID-19 is characterized by high lymphocyte activation, neutrophilia with significantly elevated levels of serum interleukins, tumor necrosis factor, granulocyte-macrophage colony stimulating factor (GM-CSF), interferon inducible protein 10, monocyte chemotactic protein 1 and macrophage inflammatory protein 1 alpha[44]. Accumulation of T cells in the post-mortem liver histological findings further supports the theory of immune-mediated response related to liver damage[44].
A well-functioning immune system is essential in the fight against infections. The liver is known to play an important role in the body’s immune response to an infectious stimulus. Many factors are involved in the physiological immune response of the host, such as immune cells, antimicrobial peptides and so-called pattern recognition receptors (PRRs), which can detect dangerous microbial signals through molecular patterns[45]. The liver is the major source for the production of PRRs, which have two main functions: complement activation and opsonization, which is an important step of phagocytosis[46]. An important subgroup of PRRs is the toll-like receptors, which play a crucial role in several liver disorders, such as alcoholic liver disease, non-alcoholic steatohepatitis, viral hepatitis, hepatic fibrosis, autoimmune hepatitis and liver cancer. Thus, the liver plays an important role in the adaptive immunity of the body, which is essential against infections and not only bacterial ones. Liver cirrhosis interferes and damages the proper functioning of adaptive immunity by impairing the synthesis of PRRs and various proteins, which can result not only in immune dysfunction but also in immunodeficiency[47,48]. The association between SARS-CoV-2 and the activation of the pro-inflammatory cascade results in excessive overproduction of pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-alpha, and attenuation of the body’s anti-inflammatory response, resulting in the development of the so-called cytokine storm, as it has been repeatedly described in the case of COVID-19 infection. The cytokine storm possibly reflects the severity of the disease[49]. Cirrhotic patients are at a higher risk of developing a systemic inflammatory response syndrome with overproduction of the above-mentioned cytokines, which, together with deregulation of the immune response and ongoing acute infection, may have fatal consequences. ACLF is a relatively novel umbrella term where acute and chronic liver insults exist along with an imbalance between systemic pro-inflammatory and anti-inflammatory responses. All the above-mentioned could then trigger an uncontrolled and complex sequence of events, which may result in ACLF with fatal consequences to patients with acute COVID-19[47,50].
A study based on histological findings from COVID-19 victim biopsies showed a 10-fold increase in the number of ACE-2 positive cells in the liver (predominantly in the form of activated hepatic stellar cells) in patients with pre-existing alcohol use disorder compared to patients with normal liver function who died before the pandemic[51]. As chronic alcohol abuse is related to chronic liver damage, these findings may have potential clinical implications. These are further supported by evidence of massive up-regulation of ACE2 (a 97-fold increase in a widespread parenchymal pattern) in cirrhotic liver and NASH induced by high-fat diet [52]. The significant ACE2 upregulation in liver cells was also observed in animal models with high-fat-diet-induced non-alcoholic steatohepatitis, with concomitant treatment with pioglitazone[24]. Therefore, diabetic patients who are treated with PPARγ agonist and present with chronic liver impairment have a higher susceptibility to SARS-CoV-2 infection, and possibly with more severe consequences. There is also evidence that the level of hepatokines is disturbed in patients with COVID-19, and these are associated with disease severity and outcomes[53]. A relationship between hepatokines, liver steatosis and metabolic diseases, such as diabetes mellitus[54], has been suggested. ACE2, as a main receptor for viral entry and a modulator of inflammatory responses, is also considered a potential target for treatment strategies. There are only a few ACE2-related molecules (e.g. DIZE, Ang 1-7) that are tested in humans. Some of these molecules can, for example, reduce tissue ACE2 activity. Many of them have already been tested on animal models; however extensive research in humans is still needed[55].
In patients with viral hepatis B (HBsAg-positive and hepatitis B core antibody positive patients) a higher risk of HBV reactivation with liver injury and fatal course of the COVID-19 was observed[56]. This could be considered a secondary result of SARS-CoV-2 infection on the liver in patients with chronic hepatic disease.
ACLF is a relatively new syndrome that occurs in 10%-30% of all hospitalized patients with chronic liver disease[1]. It is crucial to recognize high-risk patients due to the increased morbidity and mortality in these cases. The main hepatological societies (APASL, EASL and AASLD) have proposed their own definitions of ACLF, each of which differs from the others[57]. However, despite several differences, the main criteria are roughly the same. These are dominantly the presence of liver disease, precipitant factors of ACLF and hepatic or extrahepatic failure[58-60]. One definition was proposed by the World Gastroenterology Organization in 2014 to unify and simplify the diagnosis. It defined ACLF as a syndrome with very a high short-term mortality in patients with chronic liver disease with known or unknown cirrhosis characterized by acute hepatic decompensation, resulting in liver failure and at least one extrahepatic failure[61,62].
Activated pathogen-associated molecular patterns and damage-associated molecular patterns as drivers of systemic inflammation are proposed as the main etiopathological factors[63]. Activation of this systemic inflammatory response can be triggered by various conditions. Identification of precipitating factors can predict the course of the disease. The trigger of ACLF depends on the region. While in Asian populations this is usually reactivation of hepatitis B, in Western countries it is usually alcohol hepatitis, gastrointestinal bleeding or another infection[57,62].
Several published studies have reported virus infection as a trigger factor for ACLF. Infection with hepatitis B virus could lead to occurrence of a specific syndrome – hepatitis B virus-related ACLF with a wide variety of disease course[64,65]. Hepatitis A and hepatitis E viruses lead significantly less often to the development of ACLF[66,67]. The ability of the SARS-CoV-2 virus to adhere to ACE2 on the hepatocyte and cholangiocyte membrane is known[68]. However, the data describing the prevalence of ACLF in patients with chronic liver diseases suffering SARS-CoV-2 infection are scarce. Iavarone et al[69] carried out a retrospective study on a cohort of 50 cirrhotic patients infected with SARS-CoV-2 with an observed high mortality rate of > 34%. ACLF was present in 28% of patients, and death related to liver impairment was present in 29% of the cases. An independent factor for worse prognosis of COVID-19 in patients with concomitant chronic liver disease is the presence of an alcohol-related liver disease and ongoing drinking[69]. Reports of a predictive role of the CLIF and MELD scores in the setting of ACLF influenced by acute SARS-CoV-2 infection are emerging[70]. Sarin et al[71] investigated a population of 228 patients with liver disease (185 patients with chronic liver disease and 43 patients with cirrhosis) and found that 43% of patients with chronic liver disease infected with SARS-CoV-2 also presented with acute liver injury. Almost 12% (11.9%) of cirrhotic patients in this patient group developed ACLF. Complications related to liver function deterioration were present in half of the patients with decompensated cirrhosis, with higher mortality. Obesity was identified as a predictor of worse prognosis. In a multicenter study, Bajaj et al[60] reported the incidence of ACLF within a group of cirrhotic patients infected with SARS-CoV-2 as high as 36%. Interestingly there was no significant difference in mortality rate compared to patients with cirrhosis and negative for acute SARS-CoV-2. Another study from Shalimar et al[72] recorded the presence of ACLF in 9 of 28 patients from their study cohort. Mortality in these patients reached 100%[72], and mechanical ventilation was associated with poor prognosis. Besides a scarce number of prospective or retrospective cohort studies, there are also several individual case reports describing the occurrence of ACLF in a patient with chronic liver disease[68].
SARS-CoV-2 is a virus with multiorgan affinity. A substantial percentage of patients with COVID-19 could be simultaneously diagnosed with liver impairment to a varying degree, with different prognosis and duration. The virus affects the liver via different pathways (Table 1). Patients with chronic liver disease are at a higher risk for poor disease outcome when infected with the novel coronavirus. One of the lesser reported and described subgroups of these patients are those who developed ACLF. Patients with chronic liver disease and cirrhosis simultaneously infected with SARS-CoV-2 are at a risk of developing ACLF, with poor prognosis of survival. Available data are heterogenous, and the incidence of ACLF varies from 11.9% to 36%.
| Pathophysiologic mechanism of virus | Clinical impact | Considerations for clinical management |
| Direct influence of the virus on the liver cells | Significant ACE2 expression in parenchymal liver cells contributing to virulence and further damaging effect of the virus on the cells | Several antiviral agents are approved for treatment of SARS-CoV-2 infection e.g. remdesivir and ritonavir-boosted nirmatrelvir, which can inhibit viral replication. Also, monoclonal antibodies reduce the binding ability of SARS-CoV-2 to the ACE2 receptor |
| Drug-induced liver injury | Drug metabolized by cytochrome P-450 could contribute to secondary toxicity of several drugs (paracetamol, antibiotics) | Following the strict rules for avoidance of hepatotoxic drugs if possible. Standard use of hepatoprotective medications |
| Results of systemic inflammation response and general hypoxia | The hypoxic damage of hepatocytes, platelet activation, endotheliopathy, immune-mediated response related to liver damage | Administration of corticosteroids and other immunomodulators can reduce or modulate the adverse impact of immune over-response |
| Role of immunity | Impaired synthesis of PRRs are toll-like receptors, activation of the pro-inflammatory cytokines such as IL-1, IL-6, TNF-alpha | Use of IL-1 and IL-6 inhibitors, such as anakinra or tocilizumab, as well as Janus kinase inhibitors, such as baricitinib, can decrease the excessive effect of pro-inflammatory cytokines |
Although the clinical management of patients with liver diseases who contracted SARS-CoV-2 infection is still evolving, several consensus guidelines have been developed[73-75]. These guidelines were created based on multicenter and international studies, which can provide guidance for better clinical management. Several steps should be followed by clinicians to identify patients with higher risk of liver disease progression according to these recommendations. Thorough history taking and physical examination should be a cornerstone in the diagnosis process. It is also crucial to further investigate the possible presence of underlying chronic liver diseases. For doing this, a serological test for hepatitis viruses, frequent monitoring of liver enzymes or imaging examinations, such as ultrasound, could be used. It is also important to thoroughly review patients’ chronic and currently administered medications due to the possibility of liver damage related to specific drugs (e.g., antivirals, antibiotics, anti-inflammatory medications, etc.).
To summarize, it is important to consider patients with ACLF as a distinct patient population with a high risk for a severe course of SARS-CoV-2 infection and to manage them appropriately.
| 1. | Testino G, Pellicano R. Acute-on-chronic liver failure by SARS-CoV-2 in active alcohol use disorder cirrhotic patient. Minerva Gastroenterol (Torino). 2021;67:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Chen B, Tian EK, He B, Tian L, Han R, Wang S, Xiang Q, Zhang S, El Arnaout T, Cheng W. Overview of lethal human coronaviruses. Signal Transduct Target Ther. 2020;5:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 3. | Millán-Oñate J, Rodriguez-Morales AJ, Camacho-Moreno G, Mendoza-Ramírez H, Rodríguez-Sabogal IA, Álvarez-Moreno C. A new emerging zoonotic virus of concern: the 2019 novel Coronavirus (COVID-19). Infectio. 2020;24:187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, Castaldo G, Bianco A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung. 2020;198:867-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 352] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 5. | Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1213] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 6. | Singhai A, Pavan GS, Panda S. Evaluation of liver function in symptomatic COVID-19 patients. J Family Med Prim Care. 2021;10:3252-3256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Wang X, Lei J, Li Z, Yan L. Potential Effects of Coronaviruses on the Liver: An Update. Front Med (Lausanne). 2021;8:651658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Zhao W, Zhang X, Zhu F, Jiang X. Dynamic Changes of Liver Function Indexes in Patients with Different Clinical Types of COVID-19. Int J Gen Med. 2022;15:877-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 10. | Eléouët JF, Slee EA, Saurini F, Castagné N, Poncet D, Garrido C, Solary E, Martin SJ. The viral nucleocapsid protein of transmissible gastroenteritis coronavirus (TGEV) is cleaved by caspase-6 and -7 during TGEV-induced apoptosis. J Virol. 2000;74:3975-3983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Liu C, Xu HY, Liu DX. Induction of caspase-dependent apoptosis in cultured cells by the avian coronavirus infectious bronchitis virus. J Virol. 2001;75:6402-6409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Duan ZP, Chen Y, Zhang J, Zhao J, Lang ZW, Meng FK, Bao XL. [Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome]. Zhonghua Gan Zang Bing Za Zhi. 2003;11:493-496. [PubMed] |
| 13. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 14. | Farcas GA, Poutanen SM, Mazzulli T, Willey BM, Butany J, Asa SL, Faure P, Akhavan P, Low DE, Kain KC. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1308] [Article Influence: 218.0] [Reference Citation Analysis (8)] |
| 16. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14581] [Article Influence: 2430.2] [Reference Citation Analysis (3)] |
| 17. | Gholizadeh P, Safari R, Marofi P, Zeinalzadeh E, Pagliano P, Ganbarov K, Esposito S, Khodadadi E, Yousefi M, Samadi Kafil H. Alteration of Liver Biomarkers in Patients with SARS-CoV-2 (COVID-19). J Inflamm Res. 2020;13:285-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, Chen MH; Chinese Society of IBD, Chinese Elite IBD Union; Chinese IBD Quality Care Evaluation Center Committee. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 19. | Ji D, Zhang M, Qin E, Zhang L, Xu J, Wang Y, Cheng G, Wang F, Lau G. Letter to the Editor: Obesity, diabetes, non-alcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease are proinflammatory hypercoagulable states associated with severe disease and thrombosis in Covid-19. Metabolism. 2021;115:154437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Radzina M, Putrins DS, Micena A, Vanaga I, Kolesova O, Platkajis A, Viksna L. Post-COVID-19 Liver Injury: Comprehensive Imaging With Multiparametric Ultrasound. J Ultrasound Med. 2022;41:935-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 22. | Du M, Yang S, Liu M, Liu J. COVID-19 and liver dysfunction: Epidemiology, association and potential mechanisms. Clin Res Hepatol Gastroenterol. 2022;46:101793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Zhang W, Xu YZ, Liu B, Wu R, Yang YY, Xiao XQ, Zhang X. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. ScientificWorldJournal. 2014;2014:603409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Lozano-Sepulveda SA, Galan-Huerta K, Martínez-Acuña N, Arellanos-Soto D, Rivas-Estilla AM. SARS-CoV-2 another kind of liver aggressor, how does it do that? Ann Hepatol. 2020;19:592-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5831] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 27. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 28. | Siddiqui MA, Suresh S, Simmer S, Abu-Ghanimeh M, Karrick M, Nimri F, Musleh M, Mediratta V, Al-Shammari M, Russell S, Jou J, Dang D, Salgia R, Zuchelli T. Increased Morbidity and Mortality in COVID-19 Patients with Liver Injury. Dig Dis Sci. 2022;67:2577-2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (3)] |
| 30. | Lipták P, Banovcin P, Rosoľanka R, Prokopič M, Kocan I, Žiačiková I, Uhrik P, Grendar M, Hyrdel R. A machine learning approach for identification of gastrointestinal predictors for the risk of COVID-19 related hospitalization. PeerJ. 2022;10:e13124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (2)] |
| 32. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (2)] |
| 33. | Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, Shen JX, Sommerauer C, Tiseo G, Ghiadoni L, Virdis A, Monzani F, Rizos LR, Forfori F, Avendaño Céspedes A, De Marco S, Carrozzi L, Lena F, Sánchez-Jurado PM, Lacerenza LG, Cesira N, Caldevilla Bernardo D, Perrella A, Niccoli L, Méndez LS, Matarrese D, Goletti D, Tan YJ, Monteil V, Dranitsaris G, Cantini F, Farcomeni A, Dutta S, Burley SK, Zhang H, Pistello M, Li W, Romero MM, Andrés Pretel F, Simón-Talero RS, García-Molina R, Kutter C, Felce JH, Nizami ZF, Miklosi AG, Penninger JM, Menichetti F, Mirazimi A, Abizanda P, Lauschke VM. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 34. | Levy G, Guglielmelli P, Langmuir P, Constantinescu SN. JAK inhibitors and COVID-19. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, Zhang Y, Fan C, Li D, Deng Y, Sun J, Gong J, Wang Y, Li J, Yang H, Li H, Zhang Z, Wang R, Du P, Zong Y, Yin F, Zhang W, Wang N, Peng Y, Lin H, Feng J, Qin C, Chen W, Gao Q, Zhang R, Cao Y, Zhong H. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 36. | Catanese MT, Ansuini H, Graziani R, Huby T, Moreau M, Ball JK, Paonessa G, Rice CM, Cortese R, Vitelli A, Nicosia A. Role of scavenger receptor class B type I in hepatitis C virus entry: kinetics and molecular determinants. J Virol. 2010;84:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Kukla M, Skonieczna-Żydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, Pazgan-Simon M, Stachowska E, Kaczmarczyk M, Koulaouzidis A, Marlicz W. COVID-19, MERS and SARS with Concomitant Liver Injury-Systematic Review of the Existing Literature. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Chan ST, Ou JJ. Hepatitis C Virus-Induced Autophagy and Host Innate Immune Response. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Wang M, Feng Z. Mechanisms of Hepatocellular Injury in Hepatitis A. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 43. | McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol Commun. 2022;6:255-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 44. | Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World J Gastroenterol. 2014;20:2564-2577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 138] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 46. | Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 737] [Article Influence: 40.9] [Reference Citation Analysis (1)] |
| 47. | Noor MT, Manoria P. Immune Dysfunction in Cirrhosis. J Clin Transl Hepatol. 2017;5:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Tuchendler E, Tuchendler PK, Madej G. Immunodeficiency caused by cirrhosis. Clin Exp Hepatol. 2018;4:158-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 1078] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 50. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 51. | Nuovo GJ, Suster D, Awad H, Michaille JJ, Tili E. The histologic and molecular correlates of liver disease in fatal COVID-19 including with alcohol use disorder. Ann Diagn Pathol. 2022;57:151881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, Delaye-Martinez M, Rodriguez-Alvarez F, Romero-Morales B, Liu WH, Huang CA, Kershenobich D, Navarro-Alvarez N. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis. 2020;40:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Pazgan-Simon M, Serafińska S, Kukla M, Kucharska M, Zuwała-Jagiełło J, Buczyńska I, Zielińska K, Simon K. Liver Injury in Patients with COVID-19 without Underlying Liver Disease. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 472] [Article Influence: 52.4] [Reference Citation Analysis (2)] |
| 55. | Jia H, Neptune E, Cui H. Targeting ACE2 for COVID-19 Therapy: Opportunities and Challenges. Am J Respir Cell Mol Biol. 2021;64:416-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 56. | Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu, Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (5)] |
| 57. | Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 58. | Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P, Moreau R, Ginès P, Durand F, Angeli P, Alessandria C, Laleman W, Trebicka J, Samuel D, Zeuzem S, Gustot T, Gerbes AL, Wendon J, Bernardi M, Arroyo V; CANONIC Study Investigators; EASL-CLIF Consortium. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 59. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 644] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 60. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS; NACSELD. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 61. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2278] [Article Influence: 175.2] [Reference Citation Analysis (6)] |
| 62. | Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2021;3:100176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 63. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 583] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 64. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (2)] |
| 65. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 66. | Fantilli A, López Villa SD, Zerega A, Di Cola G, López L, Wassaf Martínez M, Pisano MB, Ré VE. Hepatitis E virus infection in a patient with alcohol related chronic liver disease: a case report of acute-on-chronic liver failure. Virol J. 2021;18:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 67. | Kanda T, Sasaki-Tanaka R, Nakamoto S. Hepatitis A Virus Infection and Molecular Research. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, Li C, Cheng G, Wang Y, Chen Z, Qin E, Lau G. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 69. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (2)] |
| 70. | Kumar P, Sharma M, Sulthana SF, Kulkarni A, Rao PN, Reddy DN. Severe Acute Respiratory Syndrome Coronavirus 2-related Acute-on-chronic Liver Failure. J Clin Exp Hepatol. 2021;11:404-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 72. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | APASL Covid-19 Task Force, Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 74. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 75. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovakia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ma C, China; Teng TZJ, Singapore S-Editor: Liu JH L-Editor: Ma JY-MedE P-Editor: Liu JH