Published online Sep 27, 2022. doi: 10.4254/wjh.v14.i9.1730
Peer-review started: May 30, 2022
First decision: July 13, 2022
Revised: July 18, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 27, 2022
Processing time: 115 Days and 10.9 Hours
The prevalence of hepatocellular carcinoma (HCC) is rapidly increasing, driven not least in part by the escalating prevalence of non-alcoholic fatty liver disease. Bile acid (BA) profiles are altered in patients with HCC and there is a developing body of evidence from in vitro human cellular models as well as rodent data suggesting that BA are able to modulate fundamental processes that impact on cellular phenotype predisposing to the development of HCC including senes
Core Tip: The rapidly increasing prevalence of hepatocellular carcinoma (HCC) highlights the unmet clinical need to develop and enhance early diagnostic strategies. Evidence from rodent and in vitro models suggests that bile acids may have a crucial role in the pathogenesis of HCC. Changes in circulating bile acid profiles are observed in patients with HCC. Serum and urine bile acid profiles may predict HCC risk and may have potential as a non-invasive screening tool.
- Citation: Colosimo S, Tomlinson JW. Bile acids as drivers and biomarkers of hepatocellular carcinoma. World J Hepatol 2022; 14(9): 1730-1738
- URL: https://www.wjgnet.com/1948-5182/full/v14/i9/1730.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i9.1730
The increasing prevalence of hepatocellular carcinoma (HCC) in recent years has focused the need not only to develop better treatment strategies, but also to identify and validate accurate, non-invasive early detection strategies with the aim of improving clinical outcome. In 2018, HCC was ranked as the sixth most common incident cancer and fourth highest for mortality outcome by the World Health Orga
Currently, screening strategies for HCC detection are based on regular interval ultrasound scanning (US) (usually 6 moly) while diagnosis is often confirmed by computerized tomography (CT) scan or magnetic resonance imaging (MRI). The performance of US in early detection of HCC is dependent upon both the expertise of the operator and the quality of the equipment[3]. CT scanning is not recommended for surveillance due to radiation exposure and MRI is expensive and not as widely available. Alpha-fetoprotein (AFP) is a the most widely available serum biomarker test for HCC. Despite being tested as a diagnostic tool, only two studies have investigated its performance as a stand-alone screening method for early-stage HCC[4,5]. The data are mixed and to some extent conflicting; whilst one study suggested that AFP screening can miss many incident HCCs, the other reported improved early detection, although there was no impact on clinical outcome. Importantly, in both studies, the major underlying chronic liver disease was either hepatitis B virus (HBV) or hepatitis C virus (HCV) infection related cirrhosis. AFP accuracy to detect early-stage HCC remains sub-optimal even when combined with US, increasing the accuracy by only 6%-8%[4,6]. AFP levels may also vary in patients with HBV and HCV-related cirrhosis following flares of viral replication or disease progression with further fluctuations being observed in patients with cirrhosis whenever the underlying liver disease exacerbates[7]. Moreover, early-stage HCCs express AFP in only 10%-20% of cases[8].
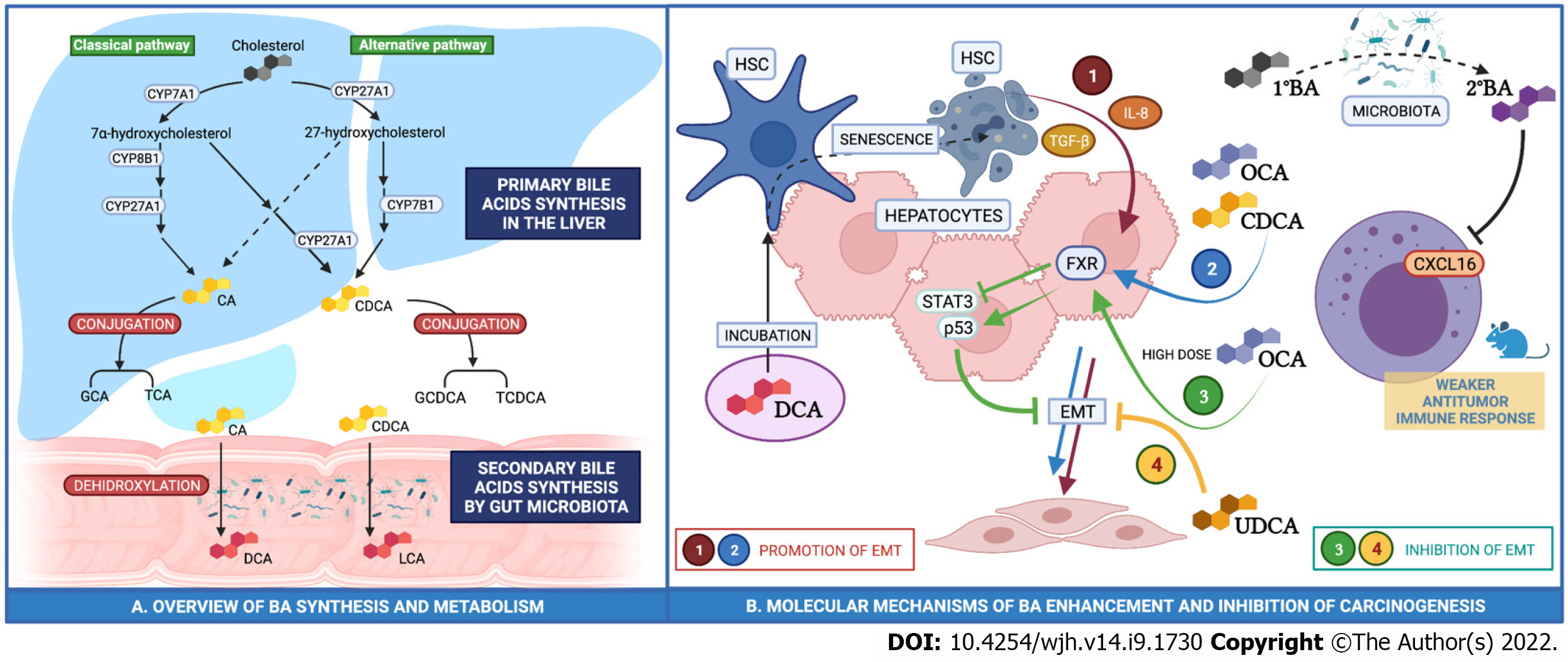
Bile acids (BA) include a variety of lipid compounds that are synthesized in hepatocytes, secreted into the biliary tree and stored in the gall bladder. The primary bile acids (cholic (CA) and chenodeoxycholic acid (CDCA)) are dehydroxylated to secondary bile acids by gut microbiota, reabsorbed in the intestine and conjugated in the liver (Figure 1). The amount of BA recirculating within the enterohepatic circulation is constant (approximately 3 g). As little as 0.5 mg of BAs spill over into the systemic circulation and are subsequently excreted into urine. Bile acid composition in serum and urine is thought to be proportional to concentration in the gallbladder.
The biochemical modifications that BAs undergo during this cycle reflect their functions as dietary fat emulsifiers. Importantly however, it is now widely recognized that BAs have a diverse array of functions to regulate cellular metabolic, inflammatory and proliferative phenotypes, mediated through a series of discrete BA receptors.
Farnesoid X receptor (FXR) is a nuclear receptor expressed in hepatocytes and enterocytes and is most potently activated by CDCA. FXR activation within the liver downregulates lipogenesis and enhances lipolysis preventing liver fat accumulation whilst in the intestine, it promotes inflammation and insulin resistance. Takeda G protein-coupled receptor 5 (TGR5) is a bile acid-specific G protein-coupled receptor that activates various intracellular signaling pathways. Pregnane X receptor (PXR) and constitutive androstane receptor (CAR) are BA nuclear receptors involved in the regulation of drug metabolism and BA conjugation[9].
In this review, we will discuss the evidence supporting the role of BAs and their receptors in the pathogenesis of HCC focusing largely on human models. In addition, we discuss the potential utility of BA profiling as a risk stratification and early diagnostic tool in the management of patients at risk of developing HCC.
FXR-null mice accumulate BAs and develop spontaneous HCC in approximately 90% of cases[10]. In addition, liver-specific FXR-knockout mice also develop spontaneous HCC in 20% of cases whilst intestine-specific FXR-knockout only develop HCC in 5% of cases. The rate of HCC onset increases with diethylnitrosamine (DEN) treatment, a well-recognised driver of the development of HCC, as well as treatment with cholic acid[11]. Conversely, in a humanized rodent model of NASH-associated HCC induced by DEN and high fat choline-deficient diet, activation of FXR via administration of obeticholic acid (OCA), a synthetic bile acid FXR potent agonist, upregulated p53 and downregulated STAT3, a regulatory pattern that limits apoptosis and cancer promotion[12].
The anti-tumor immune response is also modulated by BA. Increased conversion of primary to secondary bile acids by an altered gut-microbiota has been associated with CXCL16 downregulation in Natural Killer T-cells (NK) and this has been proposed to exert a weaker antitumor immune response[13].
In addition to FXR, other BA receptors have been implicated in the pathogenesis of HCC, although they have been studied in less detail. TGR5 activation may be involved in the anti-tumor immune response. In a tumor-bearing murine model, administration of ursodeoxycholic acid restrained T-reg Cell activation working through the TGR5-AMPK-PKA (AMP-kinase, protein kinase A) axis, resulting in carboxyl terminus of Hsc70-interacting protein mediated ubiquitination and subsequent degradation of TGF-beta[14].
To date there are currently no studies that have specifically examined the role of CAR and PXR in the pathogenesis of HCC and this is clearly an area where further research is required. PXR is expressed in the intestine and has been suggested to have a role in the pathogenesis of colon cancer[15]. However, the specific pathways maybe different between colon and liver and therefore dedicated studies in HCC models are warranted.
Evidence from rodent studies would therefore suggest that BA accumulation alongside suppression of FXR expression may act synergistically in promoting carcinogenesis. However, it is important to note that there are fundamental differences in BA synthesis and metabolism between rodents and humans (exemplified by the exclusive generation of muricholic acids and their metabolites in rodents) and this may limit the interpretation of rodent derived data.
The data from human in vitro models are complex but do suggest a potential role for BA in HCC pathogenesis. In Huh-7 and Hep3B cell models, physiological doses of OCA and CDCA promote epithelial-mesenchymal transition (EMT) through the expression of TGF-Beta[16]. EMT is a process that entails changes in the shape of cells with loss of polarity, cell-cell adhesion, and gain of spindle shape, migratory and invasive potential and may be critical for the malignant transformation of hepatocytes. Furthermore, in human hepatoma HepG2 cells, CDCA promotes cellular proliferation and reduces sensitivity to the chemotherapeutic agent 5-fluorouracil[17]. These data would suggest that BA may promote HCC development, however, there may also be a dose-dependency of effect. When administered in higher doses, OCA suppresses cell growth and induces cell death[16].
Hepatic stellate cells (HSC) are a subgroup of hepatic cells that account for 10% of the total cell mass of the liver and are fundamental in driving hepatic fibrosis. They maintain a quiescent phenotype in normal liver and transdifferentiate to myofibroblasts after a liver injury. There is evidence to suggest that DCA may indirectly promote hepatocellular carcinogenesis through initiation of HSC senescence. Incubation of HSC with DCA drives inflammation through the release of TGF-beta and IL-8 and both of these cytokines are able to modify the expression of adhesion molecules promoting EMT and subsequent HCC risk[18].
Ursodeoxycholic acid, a secondary BA produced by the gut microbiota causes a dose- and time-dependent increase in HepG2 cell apoptosis by activating the mitochondrial cell death pathway[19]. In addition, taurocholic acid (TCA) has an antiproliferative effect on HepG2 increasing the expression of adhesion molecules and promoting apoptosis, that may inhibit or even reverse EMT[20].
Taken together, all these data would suggest that BAs may play a role in the regulation of cellular phenotype that may predispose to the development of HCC (Figure 1), but the mechanisms are complex and currently poorly understood.
Based on the preclinical evidence, it has been proposed that specific patterns of serum and urine BAs concentrations may predict HCC risk or facilitate the early detection of HCC. There are many potential benefits of such approach. Firstly, this may reduce the number of percutaneous liver biopsies needed. Secondly, it may enhance the rate of early diagnosis with the potential to improve clinical outcome.
Based upon in vitro and preclinical observations, BA profiling has been used as a tool to predict the subsequent development of HCC. Wang et al[21] reported the results of a retrospective study and showed that elevated total serum bile acids (TBA) levels were an independent risk factor for the future development of HCC. After adjusting for liver fibrosis using a non-invasive risk stratification tool (AST to platelet ratio index, APRI) and the presence of ascites, total BA levels were found to be elevated in those patients who later developed HCC on a background of HBV-related cirrhosis.
More recently, a further study has reported an increased of risk of developing HCC in cirrhotic patients with increased total BA. In addition, after adjustment for potential confounders, the taurochenodeoxycholic acid/glycochenodeoxycholic acid (TCDCA/GCDCA) and the taurodeoxycholic acid/ glycodeoxycholic acid (TDCA/GDCA) ratios were both associated with a higher risk of developing HCC four years later. In contrast, a decrease in the TCA/CDCA ratio was associated with a reduction in the risk of HCC[22].
In a multi-centre, prospective observational cohort study, Stepien et al[23] defined the metabolic perturbations that precede the diagnosis of HCC. Among fourteen metabolites identified in the study, elevated serum levels of glycocholic acid (GCA) and GCDCA were associated with an increased risk of developing HCC. However, it is important to note that in this study, comparison was made against healthy controls matched to patients with HCC at the time of case occurrence. A comparison to patients with established cirrhosis was not made and therefore this limits the interpretation of their findings. However, these results are consistent with those of previously reported studies suggesting that elevated TBA, and specifically primary bile acid levels, are associated with an increased risk of developing HCC.
The mechanisms that underpin these observations remain poorly understood. Detailed studies examining the role of BA conjugation in the development of HCC have not been performed. However, diets high in saturated and milk-derived fats specifically increase serum levels of taurine-conjugated bile acids in rodents[24]. Increased taurine-conjugated bile acids levels may therefore be a surrogate marker providing a reflection of dietary composition that could drive HCC risk through the progression to advanced NAFLD.
Current screening strategies for the early detection of HCC in patients with advanced liver disease are heavily reliant on US imaging. However, several studies have tried to use measurements of serum (and in some cases urine) BA profiles as an early detection strategy.
Han et al[25] measured selected BAs (CDCA, GCA and GCDCA) in 3 distinct patient groups: One group with HCC, one group with cirrhosis but not HCC and a third group of healthy controls. In both the HCC and cirrhosis groups, levels of GCDCA and GCA were found to be higher than in healthy controls. CDCA levels were reduced in patients with HCC compared to both patients with cirrhosis and healthy control subjects, suggesting a potential protective effect against tumorigenesis[25]. In the same study, samples of HCC tumor tissue were also analyzed. Levels of all three bile acids were reduced and it is possible that this may provide an environment which allows HCC to develop by increasing inflammation and disrupting the efficacy of the immune response (Figure 1).
In a retrospective, case-control study that incorporated an analysis of the serum metabolome to identify potential markers for HCC, total BA concentrations were higher in patients with HCC [with and without diabetes mellitus type 2 (T2DM)] compared to patients with T2DM but without HCC and to healthy controls[26].
Several other studies have also confirmed higher levels of specific bile acids (most frequently GCA, but also taurodeoxycholic acid, taurocholate, glycocholate, CDCA and cholic acid) in the serum or plasma of patients with HCC when compared to healthy controls[27-33]. However, there is some variability as to the underlying etiology of chronic liver disease predisposing to HCC in these cohorts and direct comparison of BA profiles amongst these etiologies has not been made. Some of the published data are not entirely consistent and highlight the critical importance of matching, clinically relevant comparator cohorts. GCA and GDCA were found to be significantly decreased in a cohort of patients with HCC when compared to those with cirrhosis, whilst they were increased with respect to healthy volunteers[34]. Another study using samples mainly from patients with HCV and HBV related cirrhosis and HCC has shown that GCA, GCDCA, TCA, and TDCA were decreased in patients with HCC compared to those with cirrhosis[35]. Taken together, these data would seem to suggest that the changes in GCA and GDCA are the most consistent in the majority, although not all, of the published studies and could offer biomarker potential. However, on their own in isolation and without additional biomarkers (or the use of machine learning strategies) they are unlikely to offer sufficient specificity or sensitivity as an early detection test. A summary of findings from all the published studies is presented in Table 1.
| Ref. | Type of study | Clinical cohort (No.) | Control cohort | Underlying chronic liver disease | No. of subjects (Male) | Samples | Outcome vs control | Outcome vs cirrhosis | |
| 1 | Wang et al[17], 2016 | Retrospective | Cirrhosis without HCC (1082) | NA | HBV | 2262 (1710) | Serum | Increased TBA | Increased TBA-> risk factor for HCC |
| 2 | Thomas et al[22], 2021 | Case-Control | HCC (100) | Healthy Match (age, gender, dialect group) | MAFLD/ Cryptogenetic | 200 (150) | Serum | Increased TBA and CPBA | Increased TBA and CPBA -> risk factor for HCC |
| 3 | Stepien et al[23], 2020 | Case-Control | Cirrhosis without HCC (129) | Healthy Match (age, sex, centre etc.) | Any | 258 (176) | Serum | Increased TBA and CPBA -> risk factor vs healthy control | Increased TBA and CPBA |
| 4 | Han et al[25], 2019 | Cross-Sectional | HCC (30) | Healthy (30) Cirrhosis (30) | HBV | 90 (58) | Serum | Serum GCDCA reduced in HCC | Serum GCDCA reduced. GCDCA, CDCA, GCA in HCC tissue are reduced. |
| 5 | Sun et al[26], 2020 | Cross-Sectional | HCC (16) HCC-T2DM (10) | Healthy (27) T2DM (27) | NAFLD/ Cryptogenetic | 80 (50) | Serum | Increased TBA in HCC +/- T2DM vs T2DM and healthy | |
| 6 | Hsu et al[27], 2017 | Case Control | HCC (121) | HBV positive non-cirrhotic | HBV | 242 (242) | Serum | Increased TDCA, CA, TC, GC | |
| 7 | Li et al[28], 2017 | Case Control | HCC (14) | Healthy | NA | 28 | Plasma/ Urine | Urine and Plasma GCA 3-24 times increased in HCC | |
| 8 | Luo et al[29], 2018 | Cross-Sectional | HCC (516) | Cirrhosis Healthy | NA | 1448 (1076) | Serum | GCA and Phe-Trp validated for HCC prevention and detection | GCA (increased) and Phe-Trp validated for HCC prevention and detection |
| 9 | Ikegami et al[30], 2016 | Case Control | HCC (11) | Healthy | NASH | 79 | Serum | Increased PBA in NASH-HCC vs NASH | |
| 10 | Ressom et al[35], 2012 | Prospective | HCC (78) | Cirrhosis | HCV | 262 (165) | Serum | Metabolites of PBA are downregulated in HCC (GCDCA, GCA) | |
| 11 | Xiao et al[34], 2012 | Cross-Sectional | HCC (40) | Cirrhosis | HCV | 89 (64) | Serum | GCA, GDCA increased | GCA, GDCA reduced |
| 12 | Banales et al[31], 2019 | Cross-sectional | PSC (20), CCA (20), HCC (20) | Healthy | NA | 80 (55) | Serum | GCA elevated in HCC vs control | |
| 13 | Patterson et al[38], 2011 | Case Control | HCC (30) | Healthy (6), Cirrhosis (7), AML (22) | NA | 53 (35) | Plasma | Fetal BAs increased in HCC vs AML | |
| 14 | El-Mir et al[39], 2001 | Cross-sectional | HCC (27) | Cirrhosis (49), Viral Hepatitis (11), Liver Metastasis (19), Healthy (26) | NA | 132 (91) | Urine | Increased Delta(4)- and/or allo-bile acids in urine | |
| 15 | Changbumrung et al[30], 1990 | Cross-sectional | CCA (25), HCC (75) | Healthy (21) | NA | 121 (121) | Serum | Glyco-BA:Tauro-BA increased in CCA and HCC vs control | |
| 16 | Stepien et al[33], 2021 | Case Control | HCC (233) | Healthy (233) | Any | 466 (306) | Plasma | Increased total BAs and TC in HCC vs control |
Familial intrahepatic cholestasis is a group of rare genetic disorders involving bile acid transport and synthetic defects characterized by BA accumulation in liver parenchyma. Although available data are limited, there is some evidence to suggest, a risk of early onset HCC in these groups of patients[36]. Specific bile acids are found predominantly in fetal life or in rare genetic disorders of bile acid synthesis. 7α-hydroxy-3-oxochol-4-en-24-oic acid and 3-oxochol-4,6-dien-24-oic acid are not normally secreted in adults, but have been identified in plasma and urine of patients with cirrhosis and HCC[37, 38]. An additional study has also suggested that Delta(4)- and/or allo-bile acids levels in urine are increased in patients with HCC[39]. Other chronic liver conditions are also associated with changes in BA levels that can predispose to malignancy. In cases of primary biliary cholangitis and primary sclerosing cholangitis, BA accumulation enhances necrosis and apoptosis of hepatocytes through mitochondrial damage, membrane disruption and ROS production. The chronic damage with oxidative stress and pro-inflammatory microenvironment can promote carcinogenesis[9].
Rather than focusing on individual BA levels, machine learning algorithms applied to BA profiles have been used successfully to differentiate benign from malignant hepatobiliary strictures[40]. Combining BAs and machine learning has not been applied to the diagnosis of HCC diagnosis but may offer a strategy to improve diagnostic and prognostic performance.
There are important inconsistencies and limitations in the published data that make direct comparisons challenging and limit the interpretation of study results. Most studies used liquid chromatography mass spectrometry to quantity bile acids and their metabolites. However, the methodology is not standardized and therefore results from different laboratories may not be completely reproducible or comparable. Race, age, gender, diet, medication, circadian rhythm are factors that can influence BAs basal concentration. Therefore, BA pool composition rather than absolute BA concentrations should be considered to overcome or adjust for individual differences. Furthermore, since the composition of BA profiles are not consistent, it is difficult to find consensus over the definition of a standard pool[41]. Study designs are also heterogeneous. Some of the reports are case-control studies designed to detect differences in the risk of development of HCC, whilst others are cross-sectional studies or retrospective studies aiming to identify differences in the concentration of specific biomarkers between patients with HCC and subjects at high risk or healthy control participants. Since most cases HCC develops on a background of cirrhosis (of differing aetiologies), it is essential that a biomarker is accurate enough to discriminate patients with disease-specific cirrhosis from those with HCC. Head-to-head comparisons of BAs measurements against the standard screening approaches of US+/-AFP have not been performed. However, these studies would be crucial next step in order to assess the potential clinical advantage of using BA profiles in replacement or addition to US+/-AFP as screening strategy.
In conclusion, there is a growing body of evidence detailing a role for BAs and their signaling in the pathogenesis of HCC. It is likely that this translates to clinical alterations in BA profiles that have been measured in serum and urine from patients with HCC and those with cirrhosis. What remains uncertain is how these observations may translate to the development of meaningful biomarkers that might help guide clinical management or predict clinical outcome. Adopting a standardized approach to the measurement of BAs, combined with innovative approaches to the analysis and interpretation, perhaps including the use of artificial intelligence and machine learning, may facilitate their meaningful clinical use to enhance patient care.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56629] [Article Influence: 7078.6] [Reference Citation Analysis (134)] |
| 2. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1393] [Article Influence: 174.1] [Reference Citation Analysis (1)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6397] [Article Influence: 799.6] [Reference Citation Analysis (9)] |
| 4. | McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 5. | Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204-209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (4)] |
| 7. | Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434-441. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. 2021;18:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 302] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 10. | Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (7)] |
| 11. | Kong B, Zhu Y, Li G, Williams JA, Buckley K, Tawfik O, Luyendyk JP, Guo GL. Mice with hepatocyte-specific FXR deficiency are resistant to spontaneous but susceptible to cholic acid-induced hepatocarcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2016;310:G295-G302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Attia YM, Tawfiq RA, Gibriel AA, Ali AA, Kassem DH, Hammam OA. Activation of FXR modulates SOCS3/Jak2/STAT3 signaling axis in a NASH-dependent hepatocellular carcinoma animal model. Biochem Pharmacol. 2021;186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1137] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 14. | Shen Y, Lu C, Song Z, Qiao C, Wang J, Chen J, Zhang C, Zeng X, Ma Z, Chen T, Li X, Lin A, Guo J, Cai Z. Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β degradation. Nat Commun. 2022;13:3419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, Zhu L, Kaubisch A, Wang L, Pullman J, Whitney K, Kuro-o M, Roig AI, Shay JW, Mohammadi M, Mani S. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121:3220-3232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Kainuma M, Takada I, Makishima M, Sano K. Farnesoid X Receptor Activation Enhances Transforming Growth Factor β-Induced Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Wang C, Yang M, Zhao J, Li X, Xiao X, Zhang Y, Jin X, Liao M. Bile salt (glycochenodeoxycholate acid) induces cell survival and chemoresistance in hepatocellular carcinoma. J Cell Physiol. 2019;234:10899-10906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Nguyen PT, Kanno K, Pham QT, Kikuchi Y, Kakimoto M, Kobayashi T, Otani Y, Kishikawa N, Miyauchi M, Arihiro K, Ito M, Tazuma S. Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:3255-3268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Wu D, Zhou J, Yin Z, Liu P, Zhao Y, Liu J. Ursodeoxycholic acid induced apoptosis of human hepatoma cells HepG2 and SMMC-7721 bymitochondrial-mediated pathway. Zhonghua Yixue Zazhi. 2014;94:3522-3526. [DOI] [Full Text] |
| 20. | Zhang X, Nan D, Zha C, He G, Zhang W, Duan Z. Long-term intervention of taurocholic acid over-expressing in cholestatic liver disease inhibits the growth of hepatoma cells. Cell Mol Biol. 2020;66:65-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Wang H, Shang X, Wan X, Xiang X, Mao Q, Deng G, Wu Y. Increased hepatocellular carcinoma risk in chronic hepatitis B patients with persistently elevated serum total bile acid: a retrospective cohort study. Sci Rep. 2016;6:38180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Thomas CE, Luu HN, Wang R, Xie G, Adams-Haduch J, Jin A, Koh WP, Jia W, Behari J, Yuan JM. Association between Pre-Diagnostic Serum Bile Acids and Hepatocellular Carcinoma: The Singapore Chinese Health Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Stepien M, Keski-Rahkonen P, Kiss A, Robinot N, Duarte-Salles T, Murphy N, Perlemuter G, Viallon V, Tjønneland A, Rostgaard-Hansen AL, Dahm CC, Overvad K, Boutron-Ruault MC, Mancini FR, Mahamat-Saleh Y, Aleksandrova K, Kaaks R, Kühn T, Trichopoulou A, Karakatsani A, Panico S, Tumino R, Palli D, Tagliabue G, Naccarati A, Vermeulen RCH, Bueno-de-Mesquita HB, Weiderpass E, Skeie G, Ramón Quirós J, Ardanaz E, Mokoroa O, Sala N, Sánchez MJ, Huerta JM, Winkvist A, Harlid S, Ohlsson B, Sjöberg K, Schmidt JA, Wareham N, Khaw KT, Ferrari P, Rothwell JA, Gunter M, Riboli E, Scalbert A, Jenab M. Metabolic perturbations prior to hepatocellular carcinoma diagnosis: Findings from a prospective observational cohort study. Int J Cancer. 2021;148:609-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104-108. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1472] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 25. | Han J, Qin WX, Li ZL, Xu AJ, Xing H, Wu H. Tissue and serum metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Clinica Chimica Acta. 2019;488:68-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. |
Sun Y, Zhu M, Zhao H, Ni X, Chang R, Su J, et al Serum Fibroblast Growth Factor 19 and Total Bile Acid Concentrations Are Potential Biomarkers of Hepatocellular Carcinoma in Patients with Type 2 Diabetes Mellitus.
|
| 27. | Hsu JK, Lin CL, Liu CJ, Huang CJ, Yu MW. Identification of serum metabolite profiles associated with the risk of developing hepatitis B-related hepatocellular carcinoma using metabolomics. J Hepatol. 2017;66:S247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Li H, Zhao H, Li Q, Meng D, Li Z. Analysis of Glycocholic Acid in Human Plasma and Urine from Hepatocellular Carcinoma Patients. Chromatographia. 2017;80:209-215. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 30. | Ikegami T, Honda A, Miyazaki T, Yara S, Matsuzaki Y. Characteristic features of serum bile acids profile in patients with nonalcoholic steatohepatitis with hepatocellular carcinoma. Mol Cancer Res. 2016;14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Banales JM, Iñarrairaegui M, Arbelaiz A, Milkiewicz P, Muntané J, Muñoz-Bellvis L, La Casta A, Gonzalez LM, Arretxe E, Alonso C, Martínez-Arranz I, Lapitz A, Santos-Laso A, Avila MA, Martínez-Chantar ML, Bujanda L, Marin JJG, Sangro B, Macias RIR. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology. 2019;70:547-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 32. | Changbumrung S, Tungtrongchitr R, Migasena P, Chamroenngan S. Serum unconjugated primary and secondary bile acids in patients with cholangiocarcinoma and hepatocellular carcinoma. J Med Assoc Thai. 1990;73:81-90. [PubMed] |
| 33. | Stepien M, Lopez-Nogueroles M, Lahoz A, Kühn T, Perlemuter G, Voican C, Ciocan D, Boutron-Ruault MC, Jansen E, Viallon V, Leitzmann M, Tjønneland A, Severi G, Mancini FR, Dong C, Kaaks R, Fortner RT, Bergmann MM, Boeing H, Trichopoulou A, Karakatsani A, Peppa E, Palli D, Krogh V, Tumino R, Sacerdote C, Panico S, Bueno-de-Mesquita HB, Skeie G, Merino S, Ros RZ, Sánchez MJ, Amiano P, Huerta JM, Barricarte A, Sjöberg K, Ohlsson B, Nyström H, Werner M, Perez-Cornago A, Schmidt JA, Freisling H, Scalbert A, Weiderpass E, Christakoudi S, Gunter MJ, Jenab M. Prediagnostic alterations in circulating bile acid profiles in the development of hepatocellular carcinoma. Int J Cancer. 2022;150:1255-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Xiao JF, Varghese RS, Zhou B, Nezami Ranjbar MR, Zhao Y, Tsai TH, Di Poto C, Wang J, Goerlitz D, Luo Y, Cheema AK, Sarhan N, Soliman H, Tadesse MG, Ziada DH, Ressom HW. LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort. J Proteome Res. 2012;11:5914-5923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, Cheema AK, Tadesse MG, Goldman R, Shetty K. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43:20-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 37. | Mocan T, Kang DW, Molloy BJ, Jeon H, Spârchez ZA, Beyoğlu D, Idle JR. Plasma fetal bile acids 7α-hydroxy-3-oxochol-4-en-24-oic acid and 3-oxachola-4,6-dien-24-oic acid indicate severity of liver cirrhosis. Sci Rep. 2021;11:8298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Patterson AD, Maurhofer O, Beyoglu D, Lanz C, Krausz KW, Pabst T, Gonzalez FJ, Dufour JF, Idle JR. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011;71:6590-6600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 39. | El-Mir MY, Badia MD, Luengo N, Monte MJ, Marin JJ. Increased levels of typically fetal bile acid species in patients with hepatocellular carcinoma. Clin Sci (Lond). 2001;100:499-508. [PubMed] |
| 40. | Negrini D, Zecchin P, Ruzzenente A, Bagante F, De Nitto S, Gelati M, Salvagno GL, Danese E, Lippi G. Machine Learning Model Comparison in the Screening of Cholangiocarcinoma Using Plasma Bile Acids Profiles. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Chen T, Zhou K, Sun T, Sang C, Jia W, Xie G. Altered bile acid glycine : taurine ratio in the progression of chronic liver disease. J Gastroenterol Hepatol. 2022;37:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen S, China; Pan Y, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL