Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1621
Peer-review started: March 16, 2022
First decision: April 28, 2022
Revised: May 11, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 27, 2022
Processing time: 162 Days and 18.1 Hours
Renal failure is an independent prognostic factor for survival in patients with cirrhosis. Equations to calculate serum creatinine significantly overestimate the glomerular filtration rate (GFR). Plasma clearance of direct biomarkers has been used to improve the accuracy of evaluations of GFR in this population, but no study has simultaneously measured plasma and urinary clearance, which is the gold standard.
To study calculated plasma and urinary concentrations of iohexol, based on the kinetics of samples collected over 24 h from cirrhotic patients with three different grades of ascites.
One dose of iohexol (5 mL) was injected intravenously and plasma concentrations were measured 11 times over 24 h in nine cirrhotic patients. The urinary concentration of iohexol was also measured, in urine collected at 4, 8, 12 and 24 h.
The plasma and urinary curves of iohexol were similar; however, incomplete urinary excretion was detected at 24 h. Within the estimated GFR limits of our population (> 30 and < 120 mL/min/1.73 m²), the median measured GFR (mGFR) was 63.7 mL/min/1.73 m² (range: 41.3–111.3 mL/min/1.73 m²), which was an accurate reflection of the actual GFR. Creatinine-based formulas for estimating GFR showed significant bias and imprecision, while the Brochner–Mortensen (BM) equation accurately estimated the mGFR (r = 0.93).
Plasma clearance of iohexol seems useful for determining GFR regardless of the ascites grade. We will secondly devise a pharmacokinetics model requiring fewer samples andvalidate the BM equation.
Core tip: Accurately evaluating glomerular filtration rate (GFR) in cirrhotic patients is critical to optimize their management and identify patients who should be prioritized for liver transplantation, and informs discussion of double liver–kidney transplantation. Until now, no formula or direct method for measuring GFR was available. This prospective pilot study is the first to systematically describe the plasma and urinary concentrations of iohexol, based on the kinetics of samples collected over 24 h from cirrhotic patients with three different ascites grades. The next step will be to construct a Bayesian estimator from a limited number of samples.
- Citation: Carrier P, Destere A, Giguet B, Debette-Gratien M, Essig M, Monchaud C, Woillard JB, Loustaud-Ratti V. Iohexol plasma and urinary concentrations in cirrhotic patients: A pilot study. World J Hepatol 2022; 14(8): 1621-1632
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1621.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1621
Impaired renal function is an independent prognostic factor in patients with cirrhosis, particularly decompensated patients. In addition, chronic renal impairment after liver transplantation (prevalence = 15%) is an independent predictor of mortality[1]. Serum creatinine has been incorporated into the Model for End-stage Liver Disease (MELD) as a prognostic factor. The MELD predicts mortality at 3 mo.
Guidelines recommend double liver–kidney transplantation in cases where the measured glomerular filtration rate (mGFR) is < 30 mL/min/1.73 m²[2]. Accurate evaluation of the GFR is essential to optimize the management of cirrhotic patients and to identify those who should be prioritized for liver transplantation, and can also inform the discussion of double liver–kidney transplantation[3,4].
Serum creatinine and creatinine clearance, calculated using equations such as the Cockcroft and Gault, Modification of Diet in Renal Disease-4 (MDRD-4), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), and MDRD-6 equations, tend to overestimate the GFR of cirrhotic patients by approximately 23 ± 23 mL/min/1.73 m2[5,6], particularly in cases of advanced liver disease. The cystatin-C-based and combined cystatin C and creatinine equations show promise but have not been validated in cirrhotic patients. To date, no equation has shown efficiency for direct measurement of GFR based on inulin, iohexol, or 51Cr-EDTA, which are considered the gold standards in some liver transplantation centers. Iohexol has the advantages of being nontoxic (at the dose used to estimate the GFR), filtered by the glomerulus, and not reabsorbed or secreted by the tubules; it is also inexpensive compared to inulin and Cr-EDTA. However, only a few studies have assessed iohexol in compensated and noncirrhotic populations[7-9]. Moreover, those studies had several limitations, such as use of plasma samples only, or insufficient samples or a small collection time window. Finally, no study has measured iohexol urinary clearance, which remains the gold standard.
Our main aim was to determine the urinary and plasma concentrations of iohexol in a pilot population of nine cirrhotic patients with different grades of ascites, based on full pharmacokinetics (PK) profiles obtained over 24 h. The secondary objectives were to assess whether 24 h was sufficient to recover the entire administered dose from the urine, and to compare the plasma clearance of iohexol among different GFR evaluation methods [CKD-EPI, MDRD-4 and 6, the Royal Free Hospital (RFH) formula, and the Brochner–Mortensen (BM) formula]. Finally, we evaluated the influence of covariates on plasma iohexol clearance measurements, particularly ascites.
Eligible patients were > 18 years old and had advanced liver disease with different grades of ascites (three without ascites, three with grade I, and three with grade II or III) and a potential indication for liver transplantation. The inclusion and exclusion criteria are detailed in Supplemental material 1. This study was conducted in full compliance with the European and French guidelines of Good Clinical Practice, the most up-to-date Declaration of Helsinki (Seoul 2008), and the International Conference on Harmonization, Harmonized Tripartite Guideline for Good Clinical Practice in the European Community. This study was approved by the Independent Ethics Committee of Limoges and relevant authorities. All patients provided written informed consent to participate in this study and have their blood samples analyzed. This study was registered at EudraCT (2018-002778-35), and on Clinic
Eligible patients were screened during routine medical consultations in the Hepato-Gastroenterology Department of Limoges University Hospital (V 1 visit). Patients were given time to decide on whether they wanted to participate in the study and, where applicable, consent was obtained. Whether patients met the inclusion and exclusion criteria was checked during a second visit (V0 visit). The inclusion of each patient was finalized during the V1 visit at the Clinical Investigation Center of Limoges. Clinical and biological data were collected before iohexol was injected.
Patients received a single 5 mL bolus of iohexol (Omnipaque®, 5 mL; GE Healthcare, Chicago, IL, USA). Over the next 24 h, 11 blood samples were collected for measurement of iohexol plasma concentrations at 15, 30, 60 and 90 min, and 2, 3, 4, 6, 8, 12 and 24 h. In addition, urine was collected at 4, 8, 12 and 24 h, and voiding volumes were measured. Liquid consumption was quantified and 300 mL water was provided at 3 and 6 h. Liquids were provided by the attending physician according to the clinical condition of the patients. Diuretics were systematically withdrawn during the urine collections.
Iohexol was measured in serum and urine samples, using a sensitive and specific method based on liquid chromatography coupled with tandem mass spectrometry, in the Pharmacology Unit of the University Hospital of Limoges. The internal standard was ioversol and the limit of quantification was 1 mg/L.
A noncompartmental analysis was performed using PKanalix (Lixoft, Antony, France) to determine the plasma clearance of iohexol. Urinary clearance was measured using the formula U × V/P, where U is the urinary concentration, V is the urinary output, and P is the plasma concentration of the marker. The mean concentration of iohexol from three urine samples (collected at 0–4, 4–8 and 8–12 h) was used in the formula; the plasma concentration was measured in the middle of each urine collection (2, 6 and 10 h). As no sample was available at 10 h, it was calculated using the first-order process from samples taken at 8 and 12 h: C10 = C8 × e –ktwith k being calculated using the same formula based on t8 and t12.
The reference iohexol clearance was analyzed using linear correlation and a Bland–Altman plot. The relationships between covariates and the reference clearance were studied using linear regression and a scatter plot (for continuous covariates) or the Mann–Whitney test and boxplots (for categorical covariates). The covariates of interest were the ascites grade, age, weight (at inclusion and 24 h), albumin, natriuresis, diuretics (type and dose) and other drugs that could affect the GFR, and biological markers of liver failure or portal hypertension (bilirubin, albumin, international normalized ratio, platelet count, Child–Pugh class, and MELD score).
Nine male patients were included in our study. Three other patients were screened and signed the informed consent form at the V0 visit, but subsequently withdrew their consent. The characteristics of the nine patients are shown in Table 1.
| Characteristics | Median (min-max) |
| Age (yr) | 60 (47-70) |
| Sex ratio (M:F) | 1 |
| Etiology of cirrhosis | Alcohol 78% - mixed1 22% |
| MELD | 17 (8-33) |
| Child Pugh | 7 (5-12) |
| Serum creatinine (µmol/L) | 87 (53-142) |
| CKD-EPI (mL/min/1.73 m²) | 83 (46-120) |
| MDRD 4 (mL/min/1.73 m²) | 78 (44-144) |
| MDRD 6 (mL/min/1.73 m²) | 86 (46-134) |
| RFH (mL/min/1.73 m²) | 55 (32-105) |
| BM formula (mL/min/1.73 m²) | 70 (40-139) |
| mCl plas (mL/min) | 64 (41-111) |
| mCl urin (mL/min) | 59 (42-100) |
| Serum albumin (g/L) | 34.2 (26-37.7) |
| SBP (mmHg) | 120 (101-140) |
| DBP (mmHg) | 70 (60-89) |
| Weight (kg) | 96 (70-143) |
| BMI (kg/m²) | 32.8 (22.3-48.3) |
| Natremia (mmol/L) | 132 (118-139) |
| Diuretics | 56% |
| Bilirubin (µmol/L) | 29.9 (10.2-159) |
| ALKP (UI/L) | 136 (73-323) |
The results of noncompartmental analysis of iohexol blood concentrations and the urinary clearance results are presented in Table 2.
| Patient no. | Cl plas (mL/min) | Cl urin (mL/min) | K plas (min-1) | Cmax (mg/L) | Vd (mL) | AUC 0-inf (mg/h/L) | AUC 0-24 h (mg/h/L) | % |
| 1 | 63.71 | 52.89 | 0.003211 | 284 | 19839.7 | 846.26 | 830.171 | -1.9 |
| 2 | 55.92 | 46.33 | 0.002345 | 196 | 23852.5 | 964.113 | 924.802 | -4.1 |
| 3 | 58.70 | 48.17 | 0.002031 | 148 | 28897.5 | 918.45 | 860.117 | -6.4 |
| 4 | 76.25 | 79.24 | 0.002644 | 154 | 28830.0 | 707.119 | 686.323 | -2.9 |
| 5 | 111.26 | 93.53 | 0.003635 | 227 | 30607.4 | 484.599 | 480.014 | -0.9 |
| 6 | 79.01 | 74.79 | 0.004057 | 202 | 19475.6 | 682.396 | 680.013 | -0.3 |
| 7 | 41.27 | 42.26 | 0.001994 | 173 | 20689.5 | 1306.38 | 1226.09 | -6.1 |
| 8 | 79.88 | 99.89 | 0.003817 | 247 | 20924.1 | 674.929 | 671.568 | -0.5 |
| 9 | 63.39 | 58.17 | 0.001947 | 140 | 32556.8 | 850.604 | 779.724 | -8.3 |
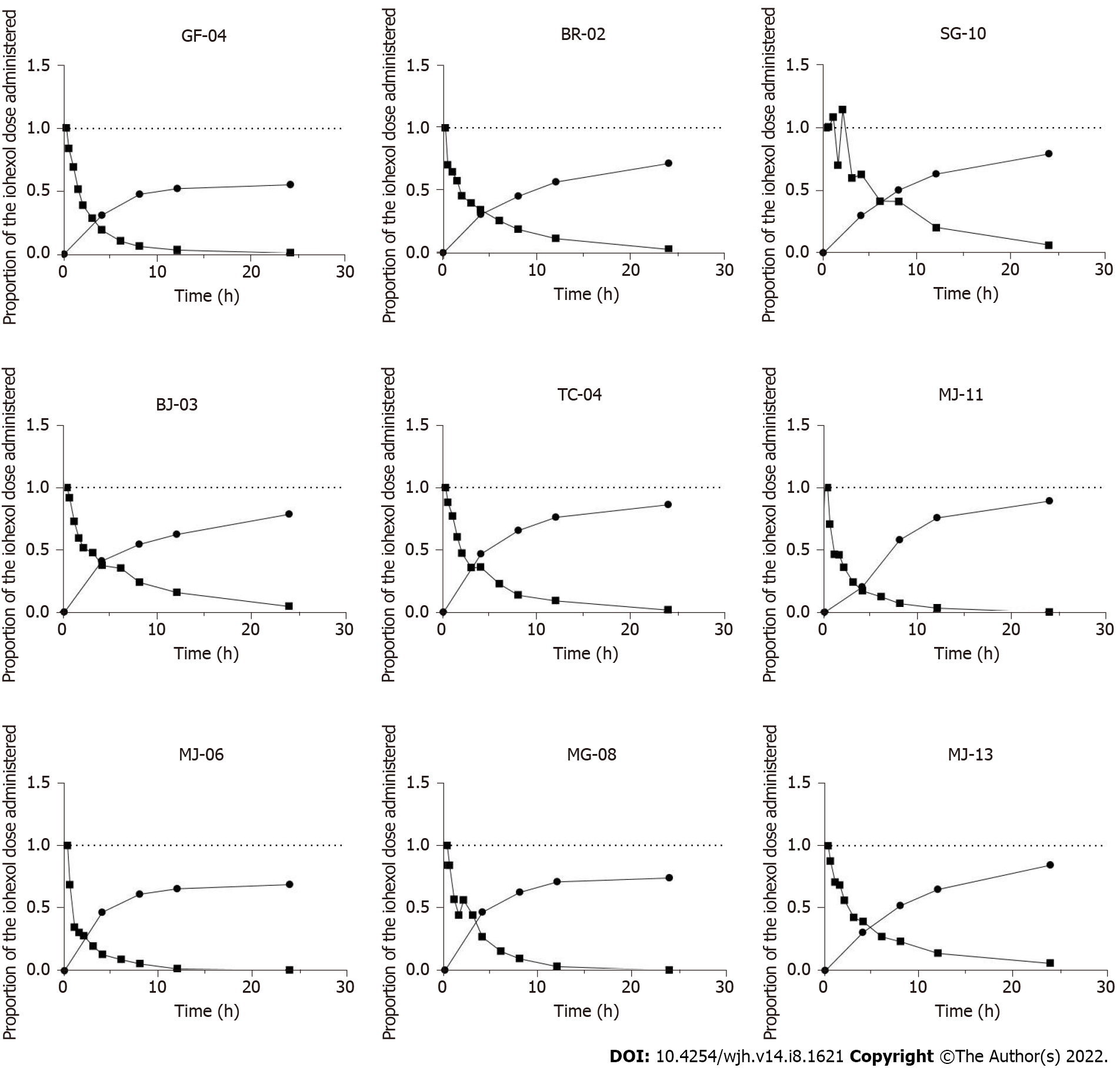
Overall, the plasma concentration decay was similar in all patients, with two distinct phases: a rapid distribution phase (phase 1; first 2 h) and a slower phase (phase 2; elimination phase) (Figure 1). Iohexol was not detected in the plasma after 24 h, which allowed us to extrapolate the area under the curve (AUC) for 0–24 h to AUC 0-∞.
Cumulative urinary curves were similar, and showed the opposite pattern to the plasma curves. However, the dose administered was not fully recovered from the urine after 24 h; the volume collected varied from 60% to 90% of the dose injected (Figure 1).
Notably, iohexol was measured in the ascites of Patient #1 at 24 h, who had grade 2–3 ascites and needed paracentesis: A low concentration (14 mg/L) was observed, suggesting negligible accumulation of iohexol in the ascites.
The median plasma and urinary clearance iohexol concentrations over 24 h were 64 mL/min (range: 41–111 mL/min) and 59 mL/min (range: mL/min) respectively. Relative to the body surface area of each patient, the median plasma clearance was 61 mL/min/1.73 m2.
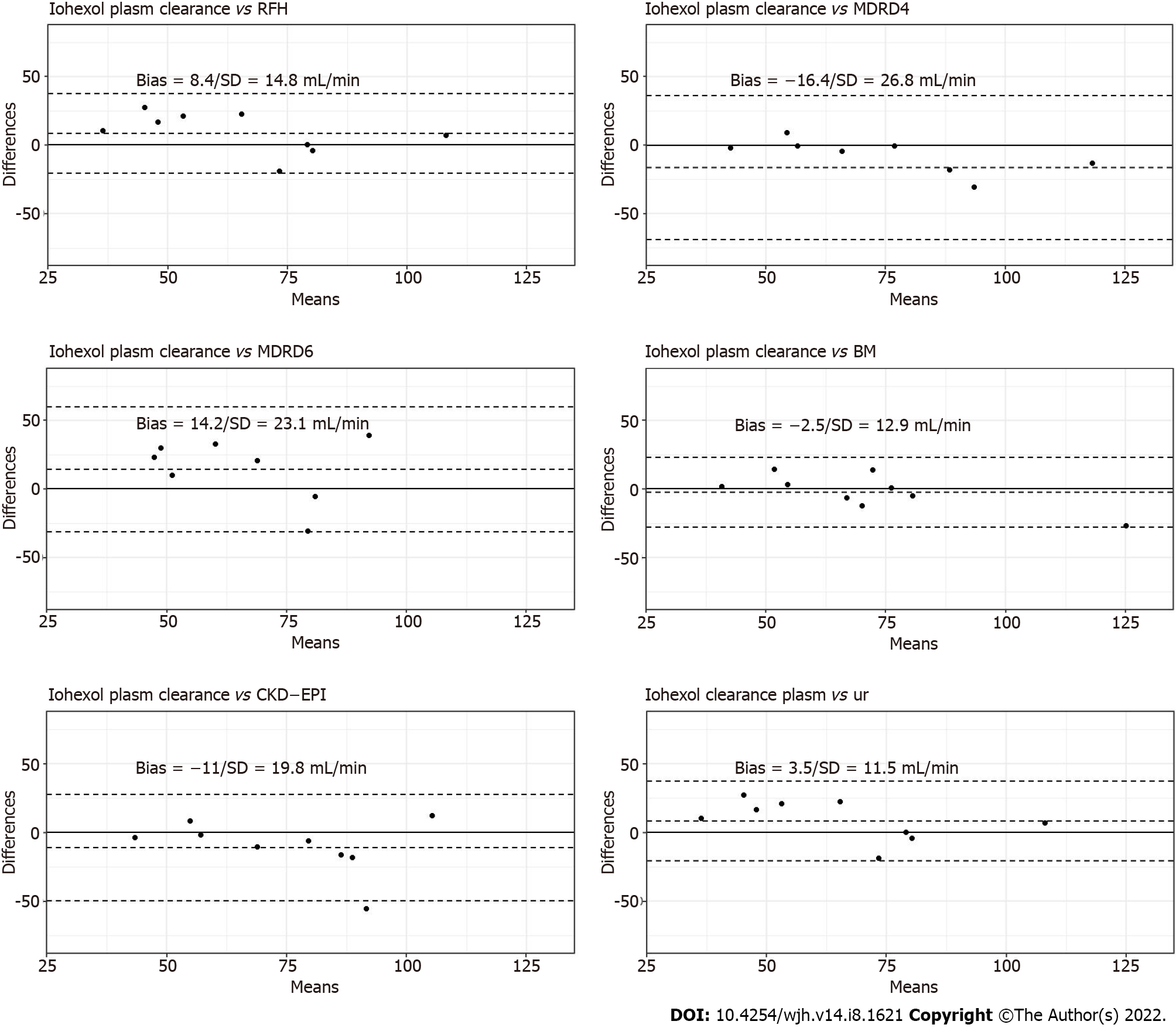
The Bland–Altman plots of the urinary clearance by plasma clearance of iohexol are shown in Figure 2. The correlation between urinary clearance and iohexol plasma clearance was strong (r = 0.84). The mean ± standard deviation (SD) difference between plasma clearance and urinary clearance was 3.46 ± 11.5 mL/min.
The Bland–Altman plots of the plasma clearance of iohexol according to the creatinine-based equations, RFH equation and BM equation are presented in Figure 2. The BM formula produced the lowest mean ± SD difference from the measured plasma clearance of iohexol (−2.48/12.90 mL/min/1.73 m2).
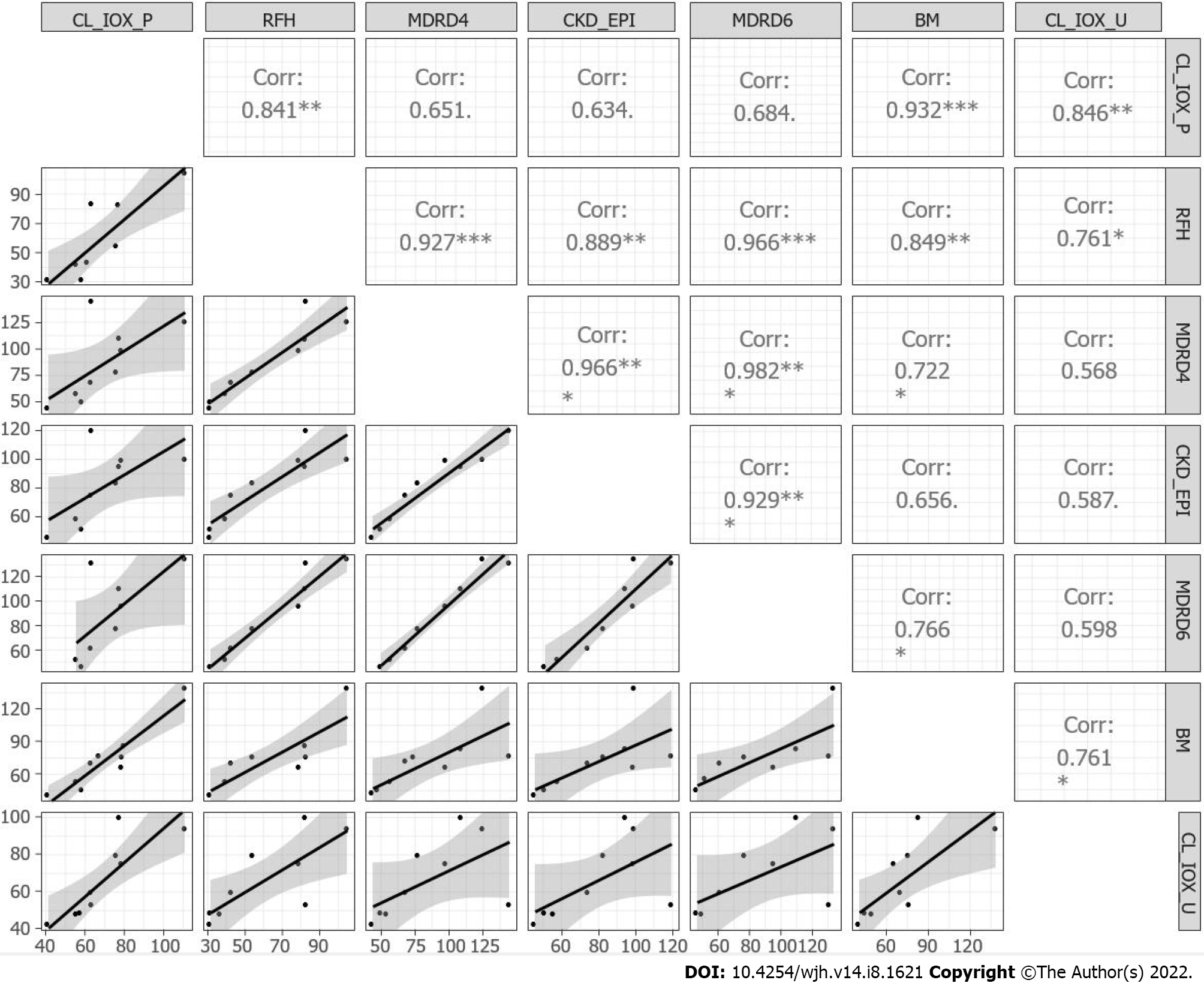
The regression matrix and scatterplot for the plasma clearance and urinary clearance of iohexol, for the creatinine-based equations, RFH equation and BM equation, are presented in Figure 3. A weak correlation was detected between plasma and urinary iohexol clearance for the creatinine-based equations, while a strong correlation was observed for the BM formula (r = 0.93).
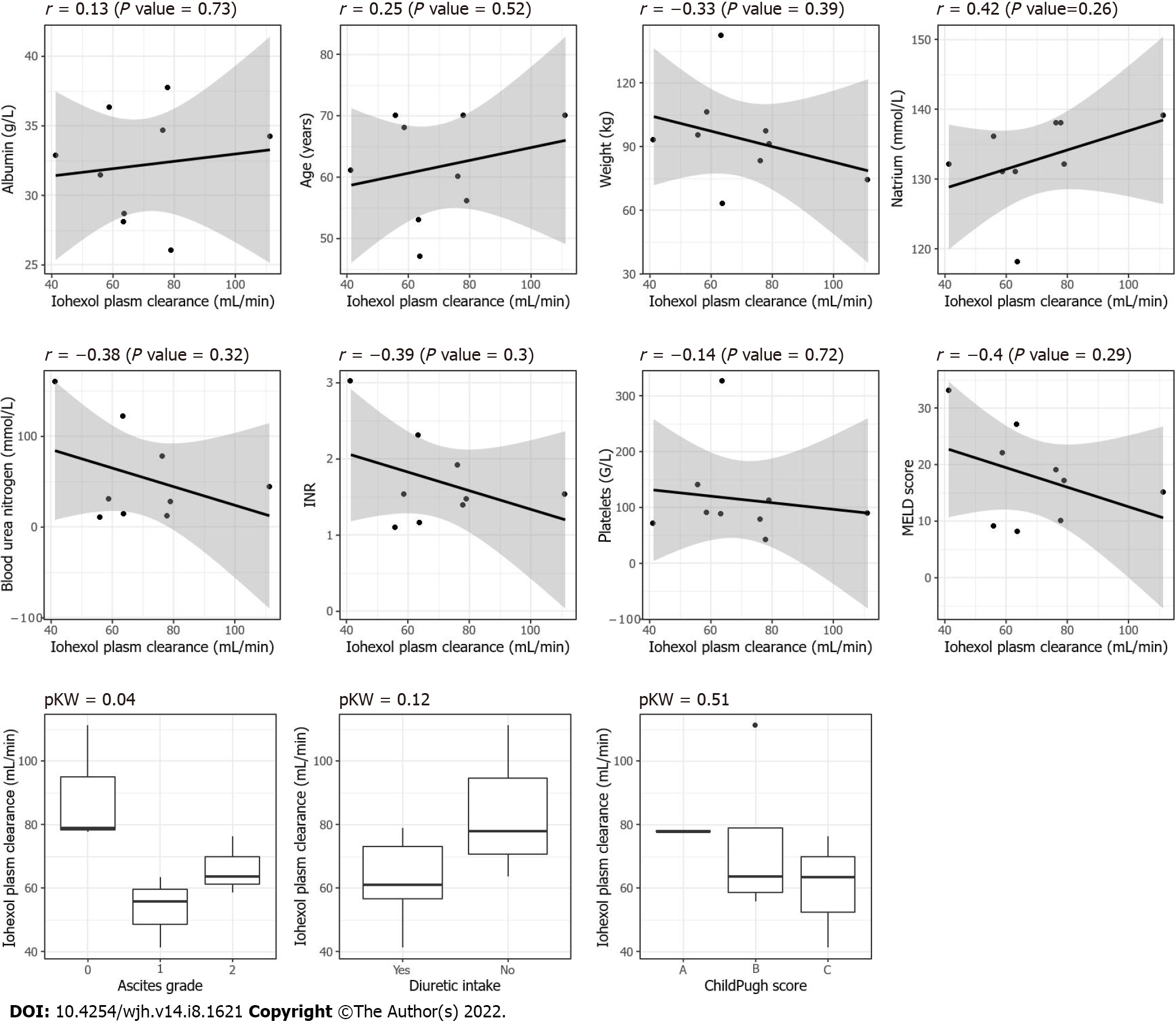
None of the clinically or biologically relevant covariates significantly affected the measured plasma clearance of iohexol (Figure 4).
The distribution volume of iohexol estimated based on noncompartmental analysis of the nine patients was compared according to the presence versus absence of ascites (grade I vs II/III vs absence of ascites) (Supplementary Figure 1). A trend was observed graphically, but there was no significant difference among the three groups (Kruskal–Wallis, P = 0.8359).
This pilot study was the first to confirm the relevance of the plasma clearance of iohexol in a cirrhotic population, by comparison with urinary clearance (the gold standard). In addition, it also confirmed the low correlations between the estimates of creatinine-based equations and the measured GFR, and showed a good performance of the BM equation in cirrhotic patients.
Iohexol is a nonionic contrast agent that is replacing inulin as the marker of choice for determining GFR. Iohexol is characterized by low extrarenal excretion and weak protein binding, and is neither secreted nor reabsorbed by the kidney. Moreover, it is nontoxic and inexpensive. The safety of iohexol has been extensively studied and confirmed in numerous studies. The doses of injected iohexol (5 or 10 mL) are more than 20 times lower than those used for computed tomography (CT); the exclusion of patients with known contrast medium reactions explains the reportedly high safety[10].
We assessed both the plasma (11 samples/patient) and urinary clearance (four separate measurements) of iohexol, based on full PK profiles of a population of cirrhotic patients with different ascites grades. Few data are available regarding the utility of this biomarker for this population[8], and no study has validated the plasma clearance of iohexol as a direct measure of GFR by comparison with urinary clearance, which remains the gold standard. Other methodological limitations of previous studies included blood sample collection over only 5 h, plasma not being sampled before 1 h, and lack of consideration of the third compartment (ascites) in which iohexol may accumulate over time (except in one study, in which ascites samples were not available after 4 h)[8].
Urinary clearance is the most accurate method to determine the filtering capacity of the kidneys, particularly in patients presenting with ascites or voluminous edema. Ideally, urinary samples should be collected every hour, with a plasma sample obtained in the middle part of the measurement period. However, as this is difficult in routine clinical practice, we collected urine at 4-h intervals and extrapolated the plasma concentration at 10 h. This may have led to imprecision in the iohexol urinary clearance estimates, and could partially explain the difference between the plasma clearance and urinary clearance. In the future, we will use a urometer in all patients given the difficulties of collecting urine.
Surprisingly, the total dose of iohexol administered was not fully recovered in the urine at 24 h, regardless of ascites grade. Between 60% and 90% of the initial dose was eliminated from the urine, although only three patients achieved the 90% clearance rate and none had an estimated GFR (eGFR) < 30 mL/min/1.73 m2 at baseline.
Three hypotheses are proposed based on the iohexol urinary elimination curves. The first is that iohexol is not a good marker to measure GFR. However, many studies have shown that the plasma clearance of iohexol provides similar results to inulin and 51Cr-EDTA measurements[11,12], except in patients with cirrhosis, and it is used as a reference for measuring GFR. Additionally, measurements of plasma clearance of these biomarkers are considered as accurate as urinary clearance measurements[10,13].
The second hypothesis is that the collection of samples over more than 48 h, i.e., until complete elimination of the iohexol in the urine, provides more accurate data, although this is almost impossible in routine clinical practice. Most studies focusing on the urinary clearance of other markers, such as inulin and Cr-EDTA, have demonstrated that systematic urine collection is difficult and rarely complete, such that interpretations of the results are prone to error. We had difficulty obtaining the entire urine output of Patient #1, and collected only approximately 60% of the initially injected dose of iohexol from this patient’s urine at 24 h.
The third hypothesis is that iohexol is mainly stored in ascites or edema. Slack et al[8] analyzed iohexol concentrations in the ascites and plasma of three patients. Iohexol equilibrated between the blood and ascites compartments after 4 h, but blood and ascites samples were not available beyond 4 h, limiting the interpretability of their results. Those authors also compared iohexol and Cr-EDTA plasma clearance, and showed a small difference (1.3 mL/min/1.73 m2). Our study was not designed to analyze iohexol in ascites, but we evaluated the ascites concentration of iohexol in one patient with grade 2–3 ascites who had benefited from paracentesis, immediately after collecting the plasma and urine samples at 24 h. Iohexol was present, albeit at a low concentration (14 mg/L), indicating that ascites was not the main storage location for iohexol. A question raised by the present study is where is the iohexol stored in decompensated cirrhosis patients?
Iohexol plasma clearance was significantly different However, the distribution volume was not significantly affected by ascites (Supplementary Figure 1) within the GFR range estimated in our patients (30–120 mL/min/1.73 m2). This supports the hypothesis that the difference in plasma iohexol clearance observed between patients with and without ascites is attributable to a true difference in renal function, as opposed to iohexol clearance measurement error.
A strength of this study was that we collected a large number of plasma samples, allowing highly accurate plasma iohexol concentration curves to be constructed. The decrease in plasma iohexol concentrations during phase 2 (elimination phase) followed first-order elimination, and the amount of iohexol detected in blood at 24 h was at or below the detection limit (1 ng/mL). Thus, the half-life of iohexol did not vary significantly among ascites grades, or according to the presence or absence of ascites. This result suggests that the characteristics of cirrhotic patients, such as ascites, have a minor effect on iohexol clearance. The correlation coefficient between the complete plasma clearance of iohexol and estimated urinary clearance was strong (R² = 0.846). However, we were unable to draw conclusions regarding GFR values < 30 or > 120 mL/min/1.73 m², as these were not measured in this study. High-precision evaluation of the GFR in this range is useful, as patients with an eGFR < 30 mL/min/1.73 m² are considered as candidates for immediate double transplantation[14].
We conclude that measurements of plasma clearance of iohexol are probably as accurate (and less cumbersome and more feasible) as urinary clearance measurements to estimate the GFR in cirrhotic patients, with consideration of the limitations mentioned above. Therefore, we used plasma clearance as a reference for GFR assessment based on the comparisons conducted in this study.
The creatinine-based equations were not useful, with the possible exception of the RHF formula; the correlation coefficients between the complete plasma clearance of iohexol and GFR estimated by the serum creatinine-based formulas were small. The coefficients ranged from 0.634 (for the CKD-EPI equation) to 0.684 (for the MDRD-6 equation), which agrees with literature data indicating that the MDRD-6 equation is probably the most accurate[15]. The MDRD-6 was proposed as the reference (by US consensus guidelines) to identify candidates for simultaneous liver and kidney transplantation[14]. The RFH formula, which was introduced more recently[16], was the most accurate creatinine-based equation for evaluating the GFR in our population (R² = 0.841) but showed large variability (SD = 8.44/14.79 mL/min/1.73 m². Moreover, the RFH formula has not been widely validated.
An unexpected result in our cirrhosis patients was the strong correlation between the measured plasma clearance and GFR estimated by the BM equation[17]; the difference between the estimated and measured GFR was lowest for this equation, for which dispersion on the Bland–Altman plot was also the smallest. The BM equation is not affected by the initial rapid phase of plasma clearance of iohexol (< 1 h; distribution phase). The BH equation requires fewer plasma samples (n = 4 in this study), which are obtained during phase 2 (elimination), and provides accurate estimates of the GFR. However, it has not been validated in terms of the third compartment (ascites).
The main limitation of this study was the small number of patients included. However, this was a descriptive pilot study aiming to elucidate the behavior of iohexol in the plasma and urine through full PK profiles (i.e., with early and late samples obtained over a period of at least 24 h), as this has not been explored before in a cirrhotic population. The small number of patients may also explain why variables known to affect GFR were not significant (e.g., diuretic intake).
Patients with different ascites grades were recruited prospectively and consecutively, but unfortunately, as all participants were male, there was a recruitment bias with respect to gender. Although cirrhosis mainly affects men, women are equally affected by overestimates of the GFR by serum creatinine-based formulas, particularly during the pretransplant period. Women have relatively low serum creatinine levels and are therefore likely to be disadvantaged by graft allocation systems based on the MELD score. As an illustration, after the MELD score was adopted to allocate liver transplants, the proportion of male transplant recipients increased, and the waiting list mortality rate for women was higher than for men. Women scored higher when creatinine was replaced with the mGFR in the MELD scoring system. Therefore, this is essential to ensure equal access to liver transplantation between genders[15,18,19].
Patient #7 had a maximum concentration of iohexol higher than that measured at the end of perfusion. This may have been due to the iodine injection that this patient received for a CT scan 15 d previously. Thus, patients with severe cirrhosis are likely to benefit from radiological examinations involving an iodine injection that may interfere with iohexol plasma clearance.
Finally, no control group of healthy patients or patients without cirrhosis was included in this study. However, two previous studies that compared the performance of iohexol and inulin in terms of estimating plasma and renal clearance in healthy subjects obtained comparable results. If we assume that 100% of inulin is recovered in the urine in a healthy population, and that renal clearance is the same using iohexol, we can further assume that 100% of iohexol will be recovered[20,21].
Obtaining a full PK profile remains difficult in clinical practice, as 11 plasma samples are needed over 24 h. This can only be achieved in a small proportion of patients with highly complex profiles, particularly before liver transplantation. The next steps will be to investigate the performance of the BM equation in a larger cohort of cirrhotic patients, and to construct a PK model for cirrhotic patients for estimating iohexol plasma clearance based on a limited number of samples.
Accurate evaluation of GFR in cirrhotic patients is critical, but no formula or direct measurement method has been available until now. Even though urinary clearance is considered the gold standard, we showed that it requires urine to be collected over more than 24 h, which is not feasible in practice. Our study suggests that the plasma clearance of iohexol is more valuable for determining the GFR in cirrhotic patients than urinary clearance is, and that specific patient characteristics, such as ascites, have a minor effect on mGFR. The next step will be to construct a PK model in a larger cirrhotic cohort that requires fewer samples, to simplify the mGFR estimate. In addition, the validity of the BM equation must be confirmed.
To date, no method for measuring the glomerular filtration rate (GFR) based on either creatinine or an exogenous marker, which is both reliable and applicable in clinical practice in cirrhotic patients with different degrees of decompensation, is available.
We urgently need accurate methods to measure GFR in cirrhotic patients; renal failure being a key prognostic factor in decompensated cirrhosis, particularly in the pre and post-transplant period.
Describing the complete pharmacokinetic (PK) study of iohexol in blood and urine as an appropriate and inexpensive marker is essential to subsequently construct a PK model from a limited number of samples.
This pilot study included nine patients with different ascites grades, who received a single 5-mL bolus of iohexol, with the collection of 11 blood samples and all the urine volume (in four samples) over a period of 24 h.
Iohexol was almost no longer detected in plasma at 24 h that allowed us to extrapolate the area under the curve (AUC) 0–24 h to AUC 0–∞. The dose recovery in urine varied from 60% to 90% of the dose injected. The correlation between urine clearance and iohexol plasma clearance was strong. As expected, a low correlation with the estimated GFR (eGFR) calculated by creatinine-based equations was observed contrary to the Brochner–Mortensen (BM) equation, which exhibited a high correlation.
This study confirmed the relevance of the plasma clearance of iohexol in the cirrhotic population. It also suggests a high accuracy of the BM equation and confirms the low correlation with eGFR estimated by creatinine-based equations.
A future study based on a larger cohort of cirrhotic patients with different ascites grades will be performed to devise a PK model allowing the estimation of iohexol plasma clearance from a limited number of samples and to investigate the performance of the BM equation.
We thank Sarah Demay, Karen Poole, Céline Rigaud and Ludovic Micallef for their precious help.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemical research methods
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Italy; Ke X, China S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | Durand F, Francoz C, Asrani SK, Khemichian S, Pham TA, Sung RS, Genyk YS, Nadim MK. Acute Kidney Injury After Liver Transplantation. Transplantation. 2018;102:1636-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK). Am J Transplant. 2008;8:2243-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Carrier P, Debette-Gratien M, Loustaud-Ratti V. Serum creatinine in cirrhotic patients: a cornerstone. AME Med J 2018; 3; Accessed 17 September 2021. Available from: https://amj.amegroups.com/article/view/4703. |
| 4. | Garcia-Pagan JC, Francoz C, Montagnese S, Senzolo M, Mookerjee RP. Management of the major complications of cirrhosis: Beyond guidelines. J Hepatol. 2021;75 Suppl 1:S135-S146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Carrier P, Debette-Gratien M, Essig M, Loustaud-Ratti V. Beyond serum creatinine: which tools to evaluate renal function in cirrhotic patients? Hepatol Res. 2018;48:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Francoz C, Prié D, Abdelrazek W, Moreau R, Mandot A, Belghiti J, Valla D, Durand F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver Transpl. 2010;16:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Francoz C, Nadim MK, Baron A, Prié D, Antoine C, Belghiti J, Valla D, Moreau R, Durand F. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology. 2014;59:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Slack A, Tredger M, Brown N, Corcoran B, Moore K. Application of an isocratic methanol-based HPLC method for the determination of iohexol concentrations and glomerular filtration rate in patients with cirrhosis. Ann Clin Biochem. 2014;51:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | González-Alayón C, Porrini E, Luis-Lima S, Negrín-Mena N, Moreno M, Morales-Arráez D, González-Rinne F, Díaz-Martín L, Gaspari F, González-Delgado A, Ferrer-Moure C, Ortiz-Arduán A, Hernandez-Guerra M. Estimated glomerular filtration rate by formulas in patients with cirrhosis: An unreliable procedure. Liver Int. 2022;42:884-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Delanaye P, Melsom T, Ebert N, Bäck SE, Mariat C, Cavalier E, Björk J, Christensson A, Nyman U, Porrini E, Remuzzi G, Ruggenenti P, Schaeffner E, Soveri I, Sterner G, Eriksen BO, Gaspari F. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J. 2016;9:700-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol. 2010;52:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Benz-de Bretagne I, Le Guellec C, Halimi JM, Gatault P, Barbet C, Alnajjar A, Büchler M, Lebranchu Y, Andres CR, Vourcʼh P, Blasco H. New sampling strategy using a Bayesian approach to assess iohexol clearance in kidney transplant recipients. Ther Drug Monit. 2012;34:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Kalafateli M, Wickham F, Burniston M, Cholongitas E, Theocharidou E, Garcovich M, O'Beirne J, Westbrook R, Leandro G, Burroughs AK, Tsochatzis EA. Development and validation of a mathematical equation to estimate glomerular filtration rate in cirrhosis: The royal free hospital cirrhosis glomerular filtration rate. Hepatology. 2017;65:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Bröchner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 718] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Yoo JJ, Kim SG, Kim YS, Lee B, Lee MH, Jeong SW, Jang JY, Lee SH, Kim HS, Kim YD, Cheon GJ. Estimation of renal function in patients with liver cirrhosis: Impact of muscle mass and sex. J Hepatol. 2019;70:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 20. | Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almén T. Determining 'true' glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2008;42:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Brown SC, O'Reilly PH. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol. 1991;146:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 4.8] [Reference Citation Analysis (0)] |