Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1470
Peer-review started: February 23, 2022
First decision: April 5, 2022
Revised: April 19, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 27, 2022
Processing time: 153 Days and 14.9 Hours
The clinical efficacy and safety of vaccination against novel coronavirus disease 2019 (COVID-19) in patients with cirrhosis have not been evaluated yet.
To evaluate the clinical efficacy and safety of vaccination against COVID-19 in patients with cirrhosis.
This was a retrospective cohort study of patients with cirrhosis. The first cohort included patients vaccinated with Gam-COVID-Vac (Sputnik V); the second one consisted of unvaccinated controls.
The study included 89 vaccinated patients and 148 unvaccinated ones. There were 4 cases of COVID-19 in the vaccinated group and 24 cases in the unvaccinated group (P = 0.035). No severe cases of COVID-19 were revealed in the vaccinated group, while there were 12 ones in the unvaccinated group (P = 0.012) with 10 deaths detected (P = 0.012). The vaccine efficacy was 69.5% (95% confidence interval [CI]: 18.5%-94.4%) against symptomatic cases of COVID-19, 100% (95%CI: 25.1%-100.0%) against severe cases, and 100% (95%CI: 1.6%-100.0%) against death associated with COVID-19. The efficacy of full vaccination with revaccination against symptomatic cases of COVID-19 was 88.3% (95%CI: 48.0%-99.6%). The overall mortality rate was higher in the unvaccinated group than in the vaccinated group (17.1% vs 3.0%; P = 0.001). Higher Child-Turcotte-Pugh class cirrhosis (hazard ratio [HR] = 4.13, 95%CI: 1.82-9.35) and higher age (HR = 1.08, 95%CI: 1.04-1.15) were independent predictors of overall mortality, while vaccination had a protective effect (HR = 0.09, 95%CI: 0.01-0.76). There was no significant difference in liver-related mortality (P = 0.135) or the incidence of liver decompensation (P = 0.077), bleeding esophageal varices (P = 0.397), and vascular events (P = 0.651) between the two groups of patients.
Vaccination against COVID-19 in patients with cirrhosis is effective and safe.
Core Tip: The aim of the study was to evaluate the clinical efficacy and safety of vaccination against novel coronavirus disease 2019 (COVID-19) in patients with cirrhosis. No severe cases of COVID-19 were revealed in the vaccinated group. The vaccine efficacy was 69.5% (95% confidence interval [CI]: 18.5%-94.4%) against symptomatic cases of COVID-19, 100% (95%CI: 25.1%-100.0%) against severe cases, and 100% (95%CI: 1.6%-100.0%) against death associated with COVID-19. There was no significant difference in liver-related mortality, or the incidence of liver decompensation, bleeding esophageal varices, and vascular events between the two groups of patients. Vaccination against COVID-19 in patients with cirrhosis is effective and safe.
- Citation: Ivashkin V, Ismailova A, Dmitrieva K, Maslennikov R, Zharkova M, Aliev S, Bakhitov V, Marcinkevich V. Efficacy and safety of COVID-19 vaccination in patients with cirrhosis. World J Hepatol 2022; 14(7): 1470-1479
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1470.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1470
The new coronavirus infection 2019 (COVID-19) has become a challenge to the health services. At the time of this writing, more than a quarter of a billion people have been infected with COVID-19, and more than 5 million of them have died. Despite all the efforts of doctors, mortality from this infection remains high and its prevention through vaccination is urgently needed.
Although the main vaccines used in the world were shown to be highly effective in preventing COVID-19[1-6], the change of the dominant strain to the new variants led to a significant decrease in vaccination efficiency[7]. In addition, the immune response to vaccination decreases over time[8]. Therefore, the need for revaccination came up for discussion[8].
The main vaccines against COVID-19 lead to a moderate incidence of side effects, which are short-term and not dangerous in the vast majority[1-6]. However, there were some concerns that vaccination of patients with cirrhosis may lead to the decompensation of liver function or provoke bleeding esophageal varices. Immune paralysis observed in cirrhosis may lead to decreased efficacy of vaccination against different infections[9].
Recent articles have shown that a subset of cirrhotic patients has a poor antibody response to COVID-19 vaccination[10] and that several cirrhotic patients develop COVID-19 after full vaccination[11].
Cirrhosis is associated with an increased risk of mortality due to COVID-19 compared to non-cirrhotic patients[12,13]. Therefore, experts from the European Association for the Study of the Liver recommended COVID-19 vaccination of patients with cirrhosis without waiting for the results of studies on the efficacy and safety of the procedure in this cohort of patients[14].
Gam-COVID-Vac (Sputnik V) is a Russian vector two-component vaccine against COVID-19 that has shown its high efficiency in phase 3 clinical trials[1], as well as in an independent national-level comparative study in Hungary[14]. However, these data were obtained before the arrival of the COVID-19 delta surges.
The aim of this study was to evaluate the clinical efficacy and safety of COVID-19 vaccination and revaccination with Sputnik V in patients with cirrhosis.
This was a retrospective cohort study approved by the Ethics Committee of Sechenov University (Protocol 20-11) in accordance with the Helsinki Declaration.
The patients with cirrhosis, who were residents of Moscow, regularly monitored at the Clinic for Internal Diseases, Gastroenterology and Hepatology of Sechenov University or Consultative and diagnostic center № 2, did not undergo liver transplantation, and were alive as of June 1, 2021, were included in the study.
Patients, who caught COVID-19 before June 1, 2021 or who were vaccinated against COVID-19 with a vaccine other than Gam-COVID-Vac (Sputnik V), were excluded from the study. The diagnosis of cirrhosis was established based on biopsy data or a combination of clinical, laboratory, and instrumental data.
Patients in the vaccination group were injected with Sputnik V intramuscularly at a standard dose (0.5 mL) twice with an interval of 21-37 d between the doses. Patients in the subgroup of revaccination received the third (booster) dose (first component) of Sputnik V 6-8 mo after taking the first component of the vaccine.
Patients in the control (unvaccinated) group did not receive COVID-19 vaccination by the end of the observation period (November 30, 2021) and were not diagnosed with COVID-19 before the beginning of the observation period (June 1, 2021).
There were no special criteria for the selection of patients in the vaccination group. Vaccination was carried out at the will of the patients themselves.
All patients received standard of care treatment for cirrhosis according to its etiology and complications. There was no significant difference between groups in drugs used for the treatment of cirrhosis.
The primary outcome was the development of symptomatic COVID-19 during the observation period (from June 1, 2021 to November 30, 2021). We chose this period because the delta variant almost completely replaced other variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and became dominant in Russia in June 2021. The third (from June to August 2021) and fourth (from September to November 2021) surges of COVID-19 associated with the delta variant occurred in Moscow during this period. A symptomatic COVID-19 case was considered if a patient had a positive polymerase chain reaction (PCR) test of oropharyngeal or nasopharyngeal swab for SARS-CoV-2 and symptoms and/or signs of COVID-19 (fever, weakness, cough, shortness of breath, anosmia, ageusia, etc.). Both inpatients and outpatients were assessed in the study.
Patients were considered fully vaccinated 2 wk after receiving the second dose of the vaccine.
COVID-19 severity classification was carried out in accordance with the current guidelines of the World Health Organization.
Secondary outcomes included death due to COVID-19, death associated with complications of cirrhosis (liver-related death), death from all causes, and the incidence of liver decompensation, bleeding esophageal varices, and vascular events (myocardial infarction, stroke, transient ischemic attack, pulmonary embolism, and abdominal thrombosis [thrombosis of the portal or hepatic veins]).
Death due to COVID-19 was considered if a patient had a positive PCR test for SARS-CoV-2 and had a significant lung damage (areas of ground glass and/or consolidation occupying more than 25% of lung volumes according to chest computed tomography) or a cytokine storm (serum C-reactive protein level more than 60 mg/L), regardless of whether liver decompensation or vascular events developed or not.
When evaluating the efficacy of revaccination, the vaccinated patients were considered unvaccinated 6 mo after the administration of the first dose of Sputnik V. We chose this period because it has been shown that the serum level of anti-SARS-CoV-2-spike-RBD IgG was significantly reduced 6 mo after vaccination against COVID-19 with Sputnik V compared with the results in the first 3 mo after this vaccination[8]. Moreover, these antibodies were not detected in almost 70% of persons 6 mo after this vaccination, although they were detected in 94% of persons 3 mo after this vaccination[8].
Information about vaccination, COVID-19 cases and their severity, patient death and its cause, and development of complications of cirrhosis and vascular events was taken from the Unified Medical Information and Analytical System, which accumulates almost all medical information about the residents of Moscow.
The liver function was assessed before the beginning of the observation period using the Child-Turcotte-Pugh (CTP) classification based on the data of the last check-up of the patient.
Statistical analyses was performed with STATISTICA 10 software (StatSoft Inc., United States). The data are represented as median [interquartile range]. The difference in continuous variables was assessed by Mann-Whitney test. Fisher's exact test was used to assess the difference in categorical variables. Survival was assessed using the Kaplan-Meier estimator and Cox test. A Cox regression model was used to assess the influence of factors on patient survival and hazard ratio (HR). A P-value ≤0.05 was considered significant.
Vaccine efficacy was estimated by 100 × (1−IRR), where IRR (incidence rate ratio) is the calculated ratio of cases of COVID-19 per 1 person-year of the observation in the vaccinated group to the corresponding illness rate in the unvaccinated group; 95% confidence interval (95%CI) for vaccine efficacy was obtained by the Baptista-Pike method (on-line calculator https://rdrr.io/cran/ORCI/man/BPexact.CI.html was used)[15].
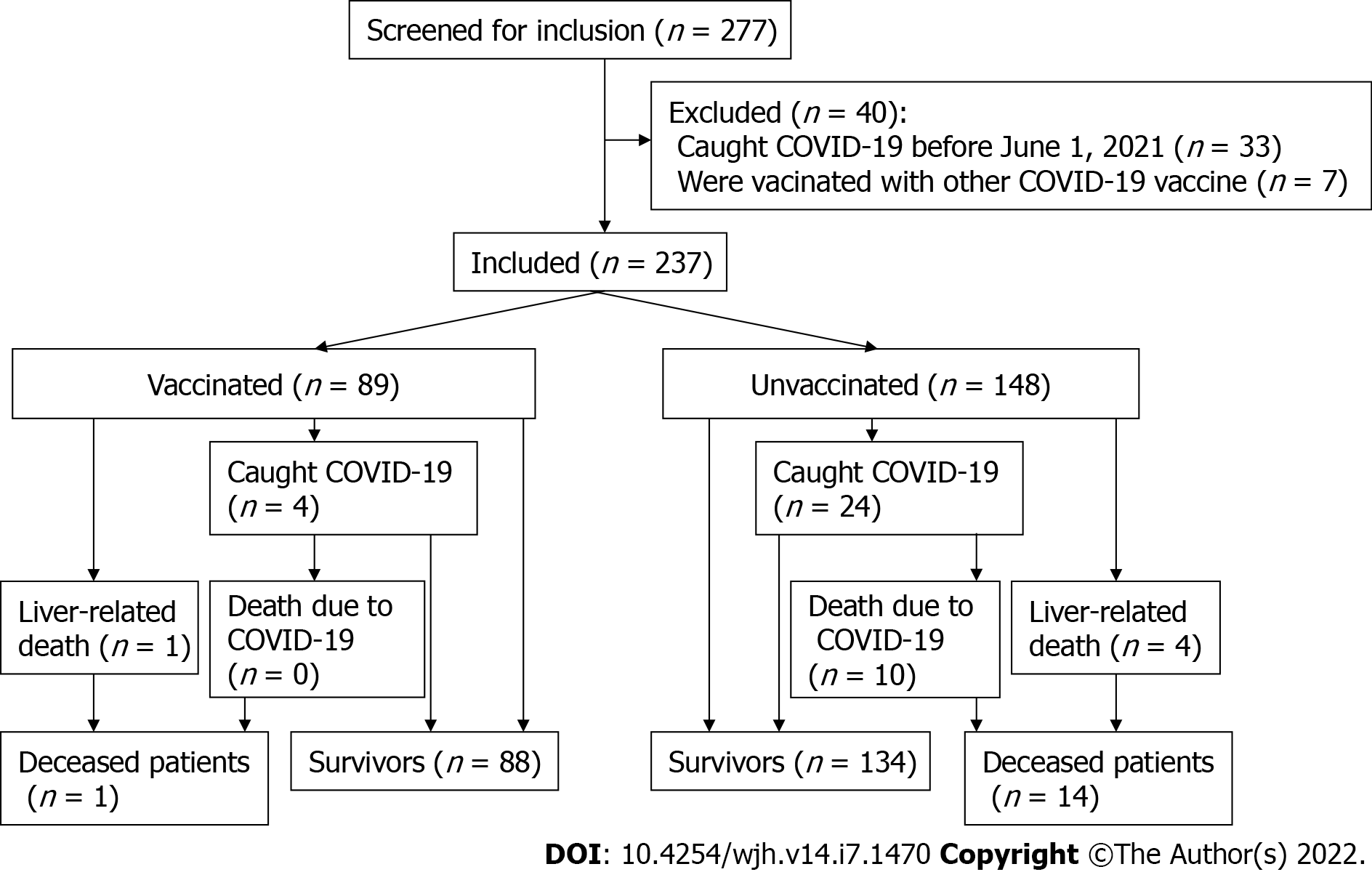
After excluding patients vaccinated with other vaccines (6 patients vaccinated with the EpiVacCorona peptide vaccine and 1 vaccinated with the CoviVac inactivated vaccine) and those who had had COVID-19 before the observation period (n = 33), a total of 237 patients with cirrhosis were enrolled in the study. Eighty-nine (37.6%) patients were vaccinated with Sputnik V, of whom 39 received the vaccine before the beginning of the observation period, and the rest did it during this period (Figure 1). If the patient was vaccinated during the observation period, then the period before the first dose injection was counted as an unvaccinated period, as well as all events that developed during it.
There was no significant difference between vaccinated and unvaccinated patients in age, gender distribution, severity and etiology of cirrhosis, or the presence of significant comorbidity (Table 1).
| Vaccinated (n = 89) | Unvaccinated (n = 148) | P value | |
| Age, yr | 59 [48-68] | 57 [47-64] | 0.161 |
| Male/Female | 50/39 | 66/82 | 0.055 |
| Child-Turcotte-Pugh class A | 52 (58.4%) | 72 (48.6%) | 0.092 |
| Child-Turcotte-Pugh classes B and C | 37 (41.6%) | 76 (51.4%) | |
| Etiology of cirrhosis | |||
| Hepatitis B virus | 3 (3.4%) | 9 (6.1%) | 0.275 |
| Hepatitis C virus | 16 (18.0%) | 29 (15.6%) | 0.449 |
| Alcohol | 41 (46.0%) | 56 (37.8%) | 0.094 |
| Metabolic associated liver disease | 5 (5.6%) | 7 (4.7%) | 0.492 |
| Autoimmune hepatitis | 1 (1.1%) | 9 (6.1%) | 0.059 |
| Primary biliary cholangitis | 9 (10.1%) | 8 (5.4%) | 0.136 |
| Primary sclerosing cholangitis | 1 (1.1%) | 2 (1.4%) | 0.684 |
| Wilson disease | 1 (1.1%) | 1 (0.7%) | 0.611 |
| Other | 0 | 6 (4.1%) | 0.057 |
| Mixed | 12 (13.5%) | 21 (14.2%) | 0.521 |
| Comorbidity | |||
| Diabetes mellitus | 18 (20.2%) | 21 (14.2%) | 0.151 |
| Ischemic heart disease | 7 (7.9%) | 6 (4.1%) | 0.170 |
| Cancer | 9 (10.1%) | 12 (8.1%) | 0.381 |
| Hepatocellular carcinoma | 1 (1.1%) | 4 (2.7%) | 0.379 |
| Asthma | 3 (3.4%) | 2 (1.4%) | 0.274 |
| Chronic obstructive pulmonary disease | 5 (5.6%) | 2 (1.4%) | 0.071 |
COVID-19 was detected significantly more often in unvaccinated individuals than in vaccinated ones. COVID-19 occurred in 4 vaccinated patients: 17 d, 3.0, 6.1, and 7.2 mo after injection of the first dose of the vaccine. Thus, the first case should be considered as incompletely vaccinated, and the third and fourth ones as unrevaccinated. Severe COVID-19 was detected in 50.0% of unvaccinated patients infected with the coronavirus and in none of vaccinated patients. None of vaccinated patients died of COVID-19. Ten deaths due to COVID-19 were registered in the unvaccinated group, which accounted for 41.7% of patients with COVID-19 in this group. However, there was no significant difference in the incidence of mild and moderate cases of COVID-19 between vaccinated and unvaccinated patients (Table 2).
| Vaccinated (n = 33.8 patient-yr) | Unvaccinated (n = 82.1 patient-yr) | P value | |
| COVID-19 cases | |||
| Total | 4 (11.8%)/3 (8.9%)1 | 24 (29.2%) | 0.035/0.0131 |
| Mild | 1 (3.0%) | 7 (8.5%) | 0.260 |
| Moderate | 3 (8.9%)/2 (5.9%)1 | 5 (6.1%) | 0.431/0.6661 |
| Severe | 0 | 12 (14.6%) | 0.012 |
| Death | |||
| Overall | 1 (3.0%) | 14 (17.1%) | 0.001 |
| Associated with COVID-19 | 0 | 10 (12.2%) | 0.012 |
| Liver-related | 1 (3.0%) | 4 (4.9%) | 0.135 |
| Non-COVID-19 complications (cases) | |||
| Liver decompensation | 4 (11.9%) | 21 (25.6%) | 0.077 |
| Bleeding esophageal varices | 2 (5.9%) | 8 (9.7%) | 0.394 |
| Myocardial infarction | 0 | 1 (1.2%) | 0.707 |
| Pulmonary embolism | 0 | 1 (1.2%) | 0.707 |
| Stroke | 0 | 0 | - |
| Transient ischemic attack | 1 (3.0%) | 0 | 0.293 |
| Abdominal thrombosis | 0 | 0 | - |
The efficacy of vaccination was 69.5% (95%CI: 18.5%-94.4%) against symptomatic cases of COVID-19, 100% (95%CI: 25.1%-100.0%) against COVID-19 severe cases, and 100% (95%CI: 1.6%-100.0%) against death due to COVID-19.
The overall mortality and mortality associated with COVID-19 were lower among the vaccinated patients than among the unvaccinated ones. There was no significant difference in liver-related mortality, as well as in the overall incidence of liver decompensation, bleeding esophageal varices, and vascular events (P = 0.651) between the two groups of patients (Table 2). Among patients with cirrhosis of CTP classes B and C, there were also no significant differences in the incidence of liver decompensation (44.0% vs 51.8% per person-year; P = 0.500) and bleeding esophageal varices (22.0% vs 16.1% per person-year; P = 0.504) between vaccinated and unvaccinated patients.
All cases of liver decompensation, bleeding esophageal varices, and transient ischemic attack in the vaccination group occurred later than 3 mo after vaccination and are extremely unlikely to be associated with it. The only patient in the vaccination group died more than 7 mo after vaccination from liver decompensation following bleeding esophageal varices.
Higher CTP class cirrhosis and higher age were significant predictors of overall mortality, while vaccination had a protective effect, according to the results of multiple Cox regression (Table 3).
| Predictor | P value | Hazard ratio |
| Age | 0.001 | 1.08 (95%CI: 1.04-1.15) |
| Vaccination | 0.027 | 0.09 (95%CI: 0.01-0.76) |
| Child-Turcotte-Pugh class | 0.001 | 4.13 (95%CI: 1.82-9.35) |
| Diabetes mellitus | 0.363 | |
| Ischemic heart disease | 0.595 | |
| Cancer | 0.751 | |
| Asthma | 0.342 | |
| Chronic obstructive pulmonary disease | 0.851 |
During the observation period, 39 patients had to be revaccinated, as they had more than 6 mo after the injection of the first vaccine dose. Nineteen (43.8%) of them were revaccinated. There were no cases of COVID-19, liver decompensation, bleeding esophageal varices, or vascular event after revaccination. If we consider unrevaccinated patients 6 mo after the injection of the first vaccine dose as unvaccinated (adjustment for the need for revaccination), the efficacy of full vaccination among patients with cirrhosis against symptomatic cases of COVID-19 was 88.3% (95%CI: 48.0-99.6%).
The incidence of COVID-19 in unrevaccinated patients was not significantly different from that in unvaccinated patients (39.0% vs 29.2% per person-year; P = 0.661).
There were no cases of COVID-19 or deaths among vaccinated CTP class A cirrhosis patients (20.8 person-years). Among unvaccinated CTP class A cirrhosis patients (40.4 person-years), COVID-19 developed in 8 (19.8% per person-year) ones, and in 3 (7.4% per person-year) of them it was severe and resulted in death. The efficacy of vaccination against symptomatic cases of COVID-19 was 100.0% (95%CI: 16.1-100.0%) among CTP class A cirrhosis patients.
Among the fully vaccinated patients with cirrhosis of CTP classes B and C adjusted for the need for revaccination (11.6 person-years), there was 1 (8.6% per person-year) case of COVID-19 that was moderate. Among these unvaccinated and unrevaccinated patients (42.1 person-years), there were 18 (42.8% per person-year) cases of COVID-19 (with 2 cases that developed later than 6 mo after the first dose of vaccine injection), including 9 (21.4% per person-year) severe ones, of which 7 (16.6% per person-year) resulted in death. The efficacy of full vaccination with revaccination against symptomatic cases of COVID-19 was 79.9% (95%CI: 11.4-99.5%) among CTP B and C cirrhosis patients.
Among patients with cirrhosis of CTP classes B and C, overall mortality was significantly lower in the vaccinated group than in the unvaccinated group (7.7% vs 26.4% per person-year; P = 0.010).
Patients with cirrhosis have a high risk of poor outcome of COVID-19. The high mortality rate (34.0%) among these patients was shown in the first study on this topic[15]. In our study, the mortality rate among unvaccinated patients with cirrhosis was 38.4%, which is significantly higher than the mortality rate among patients with COVID-19 in the general population of Moscow over the same period (about 4%). Thus, the prevention of the development of COVID-19 in this group of patients is an urgent task for health care system.
The presence of impaired immune function in patients with cirrhosis[9] has raised concerns that vaccination against COVID-19 may be of lower efficacy. In a recent study, it was shown that antibodies to SARS-CoV-2 were not found 4 wk after vaccination in 3.8% of patients with cirrhosis, and were too low in 19% of them[10]. Interestingly, the percentage of insufficient responders to the vaccine did not differ significantly between patients with cirrhosis and pre-cirrhotic stages of chronic liver disease[10]. There are publications describing COVID-19 in vaccinated cirrhotic patients. COVID-19 occurred in 6 patients with cirrhosis later than 2 wk after receiving the second dose of vaccine (criterion for full vaccination). Half of them required hospitalization, but none of them needed admission to the intensive care unit and none of them died[11]. In our study, COVID-19 developed only in 3 fully vaccinated patients and was also non-severe.
Our study is the first that describes the clinical efficacy of COVID-19 vaccination in cirrhosis. It was 69.5% against symptomatic cases of COVID-19 and 100% against severe cases and death due to COVID-19. However, the immune response to vaccination fades over time and antibodies to SARS-CoV-2 are retained in the blood only in one third of healthy persons 6 mo after the administration of the first dose of the Gam-COVID-Vac vaccine[8]. Therefore, it is not surprising that 2 out of 3 fully vaccinated patients who caught COVID-19 did it later than 6 mo after the injection of the first vaccine dose in our study. The incidence of COVID-19 in unrevaccinated patients was not significantly different from that of unvaccinated patients. Thus, revaccination of patients with cirrhosis within the sixth month after the injection of the first vaccine dose is highly recommended. None of the revaccinated patients caught COVID-19. The efficacy of full vaccination with revaccination against symptomatic COVID-19 was 88.3%.
High efficacy of vaccination was also observed in patients with cirrhosis of CTP classes B and C, which, taking into account the need for revaccination, was almost 80%.
Interestingly, the incidence of non-severe COVID-19 did not differ between the vaccinated and unvaccinated groups. Thus, vaccination protects against severe COVID-19 and death from this disease. The development of non-severe COVID-19 in vaccinated persons with cirrhosis is quite possible and should not be considered as an indicator of the ineffectiveness of vaccination.
Since COVID-19 is characterized by the development of thrombotic complications[16], there were concerns that vaccination against this infection could also contribute to their development, especially in persons with compromised hemostasis system which includes patients with cirrhosis[18]. Although a large study has shown that vaccination with certain types of vaccines is associated with an increased risk of developing thrombotic complications, this risk is negligible[19]. Therefore, one of the objectives of our study was to assess the risk of developing vascular thrombotic complications of vaccination in cirrhosis. In our study, the development of these complications was rare and their incidence did not differ significantly between vaccinated and unvaccinated patients.
The most discussed complications of COVID-19 vaccination are immune thrombotic thrombocytopenia[20] and myocarditis[21]. In our study, there were no cases of these complications, which, however, can be explained by the small number of included patients and the extremely rare reported incidence of these events[20-21].
We did not observe the onset of liver decompensation or bleeding esophageal varices associated with vaccination. The incidence of these events as well as mortality associated with complications of cirrhosis did not differ significantly between groups of vaccinated and unvaccinated patients.
Thus, we can state the excellent safety of Gam-COVID-Vac vaccination in patients with cirrhosis, including patients with CTP classes B and C cirrhosis.
Analyzing the overall mortality, we found that vaccination is an independent factor predicting the survival of patients with cirrhosis.
The need for revaccination should be emphasized. In our study, 2 out of 3 cases of COVID-19 in fully vaccinated patients were within 2 mo after 6 post-vaccination months, while there was only 1 this case within this six-month post-vaccination period.
The strength of our study is that it is the first to describe the efficacy and safety of vaccination against COVID-19 among patients with cirrhosis in the time of the delta variant dominance.
Although we tested only one vaccine in our study, we believe that the remaining major COVID-19 vaccines have a similar effect in patients with cirrhosis, as their efficacy was comparable in a recent national-level Hungarian study[14].
The limitation of our work is its retrospective nature. However, it is hardly possible to conduct randomized controlled trials on this topic in the pandemic. Another limitation is the fact that patients themselves decided whether they would be vaccinated or not, which can lead to selection bias. However, as shown in Table 1, the vaccinated and unvaccinated groups did not differ significantly in the main indicators.
Vaccination of patients with cirrhosis against COVID-19 with Gam-COVID-Vac is effective and safe. Revaccination should be carried out within the sixth month after the injection of the first dose of the vaccine.
Patients with cirrhosis have a high risk of poor prognosis when developing novel coronavirus disease 2019 (COVID-19).
The clinical efficacy and safety of vaccination against the COVID-19 in patients with cirrhosis have not been evaluated yet.
To evaluate clinical efficacy and safety of vaccination against COVID-19 in patients with cirrhosis.
This was a retrospective cohort study of patients with cirrhosis. The first cohort included patients vaccinated with Gam-COVID-Vac (Sputnik V); the second one consisted of unvaccinated controls.
There were 4 cases of COVID-19 in the vaccinated group and 24 cases in the unvaccinated group (P = 0.035). No severe cases of COVID-19 were revealed in the vaccinated group, while there were 12 ones in the unvaccinated group (P = 0.012) with 10 deaths detected (P = 0.012). The vaccine efficacy was 69.5% (95%CI: 18.5%-94.4%) against symptomatic cases of COVID-19, 100% (95%CI: 25.1%-100.0%) against severe cases, and 100% (95%CI: 1.6%-100.0%) against death associated with COVID-19. There was no significant difference in liver-related mortality, or the incidence of liver decompensation, bleeding esophageal varices, and vascular events between the two groups of patients.
Vaccination against COVID-19 in patients with cirrhosis is effective and safe.
The effectiveness of vaccinating patients with cirrhosis against COVID-19 with different vaccines should be compared.
The authors are grateful to Piskareva O and Kosabutskaya N, specialists of the Department of Medical Statistics of the Consultative and Diagnostic Center № 2.
| 1. | Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Semikhin AS, Simakova YV, Tokarskaya EA, Egorova DA, Shmarov MM, Nikitenko NA, Gushchin VA, Smolyarchuk EA, Zyryanov SK, Borisevich SV, Naroditsky BS, Gintsburg AL; Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1097] [Cited by in RCA: 1119] [Article Influence: 223.8] [Reference Citation Analysis (0)] |
| 2. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 11172] [Article Influence: 1862.0] [Reference Citation Analysis (1)] |
| 3. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7958] [Article Influence: 1591.6] [Reference Citation Analysis (1)] |
| 4. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3505] [Article Influence: 701.0] [Reference Citation Analysis (0)] |
| 5. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2024] [Cited by in RCA: 1880] [Article Influence: 376.0] [Reference Citation Analysis (0)] |
| 6. | Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, Pullukçu H, Batum Ö, Şimşek Yavuz S, Turhan Ö, Yıldırmak MT, Köksal İ, Taşova Y, Korten V, Yılmaz G, Çelen MK, Altın S, Çelik İ, Bayındır Y, Karaoğlan İ, Yılmaz A, Özkul A, Gür H, Unal S; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 740] [Cited by in RCA: 663] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 7. | Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375:331-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 8. | Chahla RE, Tomas-Grau RH, Cazorla SI, Ploper D, Vera Pingitore E, López MA, Aznar P, Alcorta ME, Vélez EMDM, Stagnetto A, Ávila CL, Maldonado-Galdeano C, Socias SB, Heinze D, Navarro SA, Llapur CJ, Costa D, Flores I, Edelstein A, Kowdle S, Perandones C, Lee B, Apfelbaum G, Mostoslavsky R, Mostoslavsky G, Perdigón G, Chehín RN. Long-term analysis of antibodies elicited by SPUTNIK V: A prospective cohort study in Tucumán, Argentina. Lancet Reg Health Am. 2022;6:100123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 293] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 10. | Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Moon AM, Webb GJ, García-Juárez I, Kulkarni AV, Adali G, Wong DK. SARS-CoV-2 Infections Among Patients With Liver Disease and Liver Transplantation Who Received COVID-19 Vaccination. Hepatol Commun. 2021;6:889-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Middleton P, Hsu C, Lythgoe MP. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 14. | Vokó Z, Kiss Z, Surján G, Surján O, Barcza Z, Pályi B. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin Microbiol Infect. 2021;28:398-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Baptista J, Pike M. Exact two-sided confidence limits for the odds ratio in a 2x2 table. J R Stat Soc. 1977;26:214-220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (2)] |
| 17. | Ali MAM, Spinler SA. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc Med. 2021;31:143-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, Harrison DA, Griffin SJ, Sheikh A, Coupland CAC. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 20. | Iba T, Levy JH, Warkentin TE. Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia. Crit Care Med. 2022;50:e80-e86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Salah HM, Mehta JL. COVID-19 Vaccine and Myocarditis. Am J Cardiol. 2021;157:146-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lal A, United States; Ramesh PV, India; Sivanand N, India S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL