Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1235
Peer-review started: January 11, 2022
First decision: February 15, 2022
Revised: March 1, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: June 27, 2022
Processing time: 163 Days and 1.6 Hours
The rise in prevalence of non-alcoholic fatty liver disease (NAFLD) mirrors the obesity epidemic. NAFLD is insidious but may gradually progress from simple steatosis to steatohepatitis, fibrosis and cirrhosis and/or hepatocellular carcinoma. Intervention strategies to ameliorate developmental programming of NAFLD may be more efficacious during critical windows of developmental plasticity.
To review the early developmental factors associated with NAFLD.
Databases MEDLINE via PubMed, and EMBASE and Reference Citation Analysis were searched and relevant publications up to April 30, 2021 were assessed. Original research studies that included risk factors associated with early development of NAFLD in human subjects were included. These factors include: Maternal factors, intrauterine and prenatal factors, post-natal factors, genetic and ethnic predisposition, childhood and adolescence environmental factors. Studies were excluded if they were review articles or animal studies, case reports or conference abstracts, or if NAFLD was not clearly defined and assessed radiologically.
Of 1530 citations identified by electronic search, 420 duplicates were removed. Of the 1110 citations screened from title and abstract, 80 articles were included in the final analysis. Genetic polymorphisms such as patatin-like phospholipase domain-containing protein 3 (PNPLA3) and membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) were associated with increased risk of NAFLD. Familial factors such as maternal obesogenic environment and parental history of hepatic steatosis was associated with offspring NAFLD. Longer duration of exclusive breastfeeding in infancy was associated with a lower risk of developing NAFLD later in life while metabolic dysfunction and/or obesity in adolescence was associated with increased risk of NAFLD. Studies relating to socioeconomic factors and its association with NAFLD reported confounding results.
Maternal metabolic dysfunction during pregnancy, being exclusively breastfed for a longer time postnatally, diet and physical activity in childhood and adolescence are potential areas of intervention to decrease risk of NAFLD.
Core Tip: Prevalence of non-alcoholic fatty liver disease (NAFLD) in adolescents has more than doubled in the last two decades, with its downstream complications placing an increasing burden on healthcare systems globally. The aim of this study is to review the early developmental factors associated with NAFLD and potentially identify areas where intervention can be made to halt the progress to steatohepatitis, fibrosis and cirrhosis and/or hepatocellular carcinoma which may develop later in life.
- Citation: Quek SXZ, Tan EXX, Ren YP, Muthiah M, Loo EXL, Tham EH, Siah KTH. Factors early in life associated with hepatic steatosis. World J Hepatol 2022; 14(6): 1235-1247
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1235.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1235
The global prevalence of non-alcoholic fatty liver disease (NAFLD) is approaching 30%, in keeping with the growing obesity epidemic[1]. With rising obesity rates worldwide, the prevalence of NAFLD is set to increase markedly in the near future. The pathogenesis of NAFLD has been considered to be a “multi-hit” disease, where epigenetic, genetic, and environmental factors may interplay to cause progressive disease. The initial hit in this multifactorial process may be early in life – during pre-conception, in-utero, infancy and early childhood[2]. For example, the predictive adaptive response hypothesis has been proposed to explain the phenomenon where poor conditions during childhood increase the risk of metabolic diseases later in life[3,4]. Being aware of factors associated with early development of NAFLD allows physicians to alter the natural course of disease progression, be it through lifestyle or pharmacological interventions. This is made even more important as the mainstay of treatment of NAFLD at present is weight loss, which is often difficult to achieve and sustain[5]. Interventional strategies to ameliorate the developmental programming of NAFLD may thus potentially be more efficacious during the critical windows of developmental plasticity, negating the need for strategies to reverse NAFLD later in life. This systematic review aims to review the early developmental factors associated with NAFLD. These factors include maternal and paternal factors, intrauterine factors, postnatal factors such as breastfeeding, lifestyle factors in adolescence including sleep, physical activity, nutrition and presently still non-modifiable factors such as genetic polymorphisms.
The study was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards (Supplementary Table 1). A comprehensive search of databases and conference proceedings to identify all relevant studies up to April 30, 2021, was performed. The following electronic databases were searched: Medline via PubMed, Embase, and Reference Citation Analysis. We use both text words and medical subject heading terms. The literature search strategy was adapted to suit each database. Our study was only restricted to full text articles in English language. For example, on PubMed, we used the combination of the following medical subject heading terms: “non-alcoholic fatty liver disease” and “Risk” and “maternal-fetal relations” or "maternal nutritional physiological phenomena" or "maternal exposure" or "maternal-fetal exchange" or "maternal behavior" or "obesity, maternal" or "maternal age" or "maternal inheritance" or "maternal health" or "educational status" or "pregnancy complications, infectious" or "gestational weight gain" or "perinatal care" or "prenatal nutritional physiological phenomena" or "prenatal care" or "prenatal education" or "prenatal exposure delayed effects" or "prenatal diagnosis" or "embryonic and fetal development" or "growth and development" or "fetal weight" or "pregnancy" or "fetal therapies" or "parents" or "parent-child relations" or "paternal exposure" or "paternal behavior" or "paternal age" or "nutrition assessment" or "child nutrition disorders" or "nutrition disorders" or "infant nutrition disorders" or "diet, food, and nutrition" or "nutritional physiological phenomena" or "fetal nutrition disorders" or "child nutrition sciences" or "nutritional status" or "nutritional sciences" or "adolescent nutritional physiological phenomena" or "exercise".
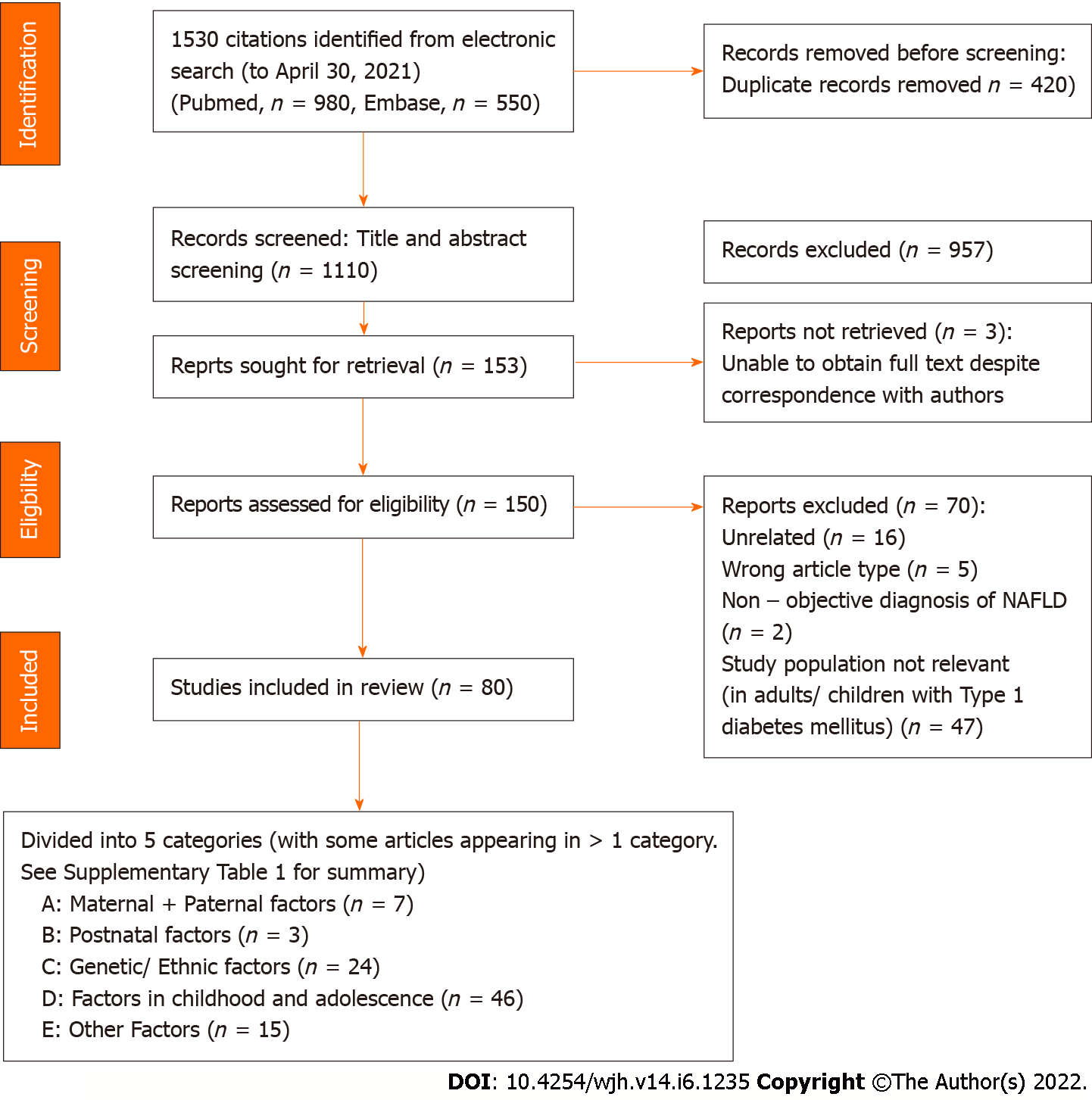
The methods for data collection and analysis were based on the Cochrane Handbook of Systematic Reviews for Interventions. Corresponding authors were contacted through electronic mail for clarification when required. Literature search and data review was performed using a case report form independently by pairs of investigators (SQ, ET, RYP) and each article was independently inspected to verify that they met the pre-specified inclusion criteria. Duplicates were excluded. Discordance during the data extraction process was resolved by consensus between the two reviewers and or consultation with a third and senior investigator (ET, KS). The study selection process is summarized in Figure 1.
Original research studies that included risk factors associated with early development of NAFLD in human subjects were included in this study. The risk factors of particular interest included: maternal factors, intrauterine and prenatal factors, post-natal factors, genetic and ethnic predisposition, childhood and adolescence environmental factors – such as (but not limited to) nutrition and physical activity. Studies were excluded if they were review articles or animal studies, case reports or conference abstracts, or if NAFLD was not clearly defined in the study. Definition of hepatic steatosis in this review was based on hepatic ultrasound, computed tomography or magnetic resonance imaging.
Of 1530 citations identified by electronic search, 420 duplicates were removed. Of the 1110 citations screened from title and abstract, 957 articles were excluded. Full text review of the 150 potentially relevant citations was performed to assess eligibility for inclusion into study and 70 reports were excluded. 80 articles were included in the final analysis (Figure 1).
Seven articles included assessment of maternal, paternal and intrauterine factors on the risk of development of NAFLD. Maternal diabetes and pre-natal obesity was associated with increased odds of offspring NAFLD[6] (Supplementary Table 1). This was also reported in Patel et al[7]’s study of 1215 patients, where maternal diabetes was found to be associated with offspring NAFLD, with an adjusted odds ratio (OR) of 6.74 (95%CI 2.47-18.40). Moreover, a higher pre-pregnancy body mass index (BMI) was associated with greater odds of fatty liver in offspring, even when adjusted for confounding factors, with adjusted OR 2.72 (95%CI 1.20-6.15, P < 0.001). Similarly, in a study from Australia[6], which studied NAFLD in adolescents at the age of 17 years, prevalence of NAFLD was associated with maternal obesity and maternal weight gain ≥ 6.0 kg by 18th week of gestation. These studies suggest that the in-utero environment can be associated with development of NAFLD later in life. On the contrary, a smaller study by Rajindrajith et al[8] reported in 499 adolescents that maternal or paternal history of metabolic syndrome was not associated with NAFLD.
Parental diagnosis of fatty liver was also associated with NAFLD in offspring. Long et al[9] reported that a larger proportion of patients with parental history of hepatic steatosis was diagnosed with hepatic steatosis (21.3% vs 12.6%, P = 0.004). After adjustment for confounding factors such as insulin resistance, BMI, age and gender, the odds of hepatic steatosis in an individual remained increased in individuals with at least one parent diagnosed with hepatic steatosis. Similar findings were reported in a study from India[10], which included 99 subjects. The authors reported that presence of NAFLD in one and two parents increased the odds of an offspring being diagnosed with NAFLD by 3.9 and 6.7 times respectively. Long and colleagues found that parental history of hepatic steatosis was only a significant risk factor for NAFLD among subjects without cardiometabolic risk factors: (16.1% vs 5.2%, P < 0.001) in those with no cardiometabolic coexisting disease compared to (30.3% vs 28.5%, P = 0.78) in patients with cardiometabolic risks[9].
Malnutrition pre- and postnatally was reported in two studies to be associated with prevalence of NAFLD in offsprings[6,11,12]. A study from China studied 8752 patients and classified them into various categories in relation to the Great Chinese famine in 1959-196112. They analysed the prevalence of various metabolic diseases in relation to famine exposure. In that study, authors found that the prevalence of NAFLD was higher in participants born around the time of the Great Chinese famine; prenatally (born 1960-1961) and postnatally (born 1957-1958) exposed subjects, compared to those who were non-exposed (born 1963-1964): Prevalence 23.0%, 22.9%, 17.3% respectively (P = 0.02). Consequently, the adjusted OR of offspring NAFLD in prenatally and postnatally exposed compared to non-exposed women for NAFLD were 2.42 (95%CI 1.14-5.16) and 1.61 (95%CI 1.29-2.01) respectively (P < 0.05).
Other socioeconomic factors at time of birth may be associated with NAFLD development however there is heterogeneity in available literature. For example, Ayonrinde et al[11] reported that lower socioeconomic status at birth was significantly associated with increased risk of NAFLD in male adolescents residing in Australia. On the other hand, a study from Sri Lanka by Rajindrajith et al[8] that studied 499 adolescents found that parental highest education and family income were not associated with NAFLD in adolescents.
Five studies examined postnatal factors associated with NAFLD (Supplementary Table 2). Duration of breastfeeding was negatively associated with NAFLD. Rajindrajith et al[8] reported more subjects who had NAFLD were breastfed less than four months, compared to non-NAFLD subjects (33.3% vs 17.1%, P = 0.02). A similar finding was reported from an Australian cohort, where exclusive breastfeeding in excess of six months was associated with decreased odds of NAFLD development at adolescence (OR 0.64, 95%CI 0.43-0.94, P = 0.02)[11] which persisted after adjustment for adolescent dietary patterns. Moreover, breastfeeding without supplementary milk for ≥ 6 mo compared with < 6 mo was associated with decreased prevalence of severe steatosis (3.5% vs 7.7%, P = 0.005). Additionally, some studies[13,14] have also reported that a more rapid increase in weight in early life is associated with NAFLD. However, these studies have not been included in this systematic review, as the diagnosis of hepatic steatosis was not determined by imaging a priori.
45 studies reported factors in childhood and adolescence that are associated with NAFLD (Supplementary Table 3). We have further divided this section into obesity as defined by BMI, other anthropometric factors, metabolic dysfunction, dietary, physical activity and sleep, and menarche in females.
Obesity (BMI) and changes in weight: A BMI > 25 kg/m2 in Sri Lankan adolescents was shown by Rajindrajith et al[8] in a study to be significantly associated with NAFLD. Adolescents with NAFLD also had a higher amount of total body fat (P < 0.001) and subcutaneous fat (P < 0.001) than those without NAFLD. Studies from China also supported these findings[15,16]. For example, Yan et al[16], in a cohort study of 1350 subjects, reported that overweight or obese children were more likely to develop NAFLD in adulthood: males and females OR 2.49 (95%CI 1.51-4.11) and OR 3.34 (95%CI 1.77-6.29) respectively, P < 0.001. However, in a cohort of 242 adolescents undergoing bariatric surgery of which 59% had NAFLD, Shulman et al[17] showed that the presence of more severe stages of NAFLD on liver biopsy was not associated with BMI in this obese cohort.
It has been widely reported in adult NAFLD literature that weight gain was strongly associated with NAFLD. Interestingly, Virtue et al[18] found that a gain in BMI from 7 to 13 years of age conferred an increased risk of NAFLD and suggested that this increased risk may be irrespective of initial or attained BMI. In this large study population of 244464 children, adjusting for BMI z-score at age 7 years, the hazard ratio of adult NAFLD was 1.15 (95%CI 1.05-1.26) and 1.12 (95%CI 1.02-1.23) per 1-unit gain in BMI z-score in males and females, respectively. Importantly, the increased odds of development of NAFLD seen in obese children may potentially be ameliorated by obtaining a normal BMI by adulthood[16].
Other anthropometric/body fat measurements: Obese adolescents with NAFLD may have a different composition compared to those without NAFLD – with greater fat body mass and visceral fat compared to those without NAFLD[19]. Besides waist circumference, other measurements such as subcutaneous adipose tissue (SCAT), intra-abdominal adipose tissue (IAAT), and anthropometric measurements including suprailiac skinfold thickness and neck circumference were shown to be associated with NAFLD. Silveira et al[20] studied 182 obese sedentary children and adolescents and showed that a higher IAAT but not SCAT was positively associated with NAFLD. In another study of adolescent girls, suprailiac skinfold thickness was found to be independently associated with NAFLD (OR 1.14, 95%CI 1.08-1.20, P < 0.001)[21]. Peña-Vélez et al[22] reported neck circumference was larger in NAFLD pediatric patients compared to those without NAFLD (P < 0.001) and this was found to be an independent risk factor in multivariate analysis (OR 1.172, 95%CI 1.008-1.362, P = 0.038).
Metabolic dysfunction: Metabolic syndrome was shown to be associated with NAFLD, highlighted in four studies. In a study by Rajindrajith et al[8] consisting of 499 adolescents, 8.2% with NAFLD living in an urban Sri Lankan community, more children with NAFLD were to found to have metabolic derangements as compared to those without (85.8% vs 26.3% in controls, P < 0.0001). In this study, children with NAFLD had a significantly higher waist circumference, homeostatic model assessment for insulin resistance (HOMA-IR) result and hypertriglyceridemia. These results were mirrored by other studies from Poland, Egypt and Italy[23-25]. In the study by Prokopowicz et al[23], which studied 108 obese hospitalized children in Poland, fasting insulin concentration was the strongest independent factor associated with NAFLD. Alkassabany et al[24] reported that the odds for NAFLD increased in school children aged 6 to 18 years with an increased number of components of metabolic syndrome. When there were three or more components of metabolic syndrome, the OR of NAFLD was 158.3 (95%CI 87.4-202.9, P < 0.05)[24] as compared to school children without any components of the metabolic syndrome.
Most studies that investigated associations of insulin resistance and NAFLD reported a positive association with a few exceptions[19,21,26-28]. Pre-diabetes and diabetes was associated with a two-fold increased risk in non-alcoholic steatophepatitis (NASH)[25]. In a group of 520 obese children in China, it was also reported that high levels of fasting c-peptide was independently associated with NAFLD[29]. In another study, insulin like growth factor 1 (IGF-1) standard deviation scores (IGF-1 SDS) was significantly lower in adolescents with NAFLD as compared to the control group (OR 0.727, 95%CI 0.559-0.946, P = 0.017)[27]. In contrast, Jimenez-Rivera et al[28] reported in their study of 97 obese children that while median triglyceride (TG) level was higher in those with NAFLD (1.5 ± 0.9 vs 1.1 ± 0.5 mmol/L, P = 0.01), other factors such as HOMA-IR, hyperlipidemia and BMI were not associated with NAFLD. NAFLD was more prevalent in girls with polycystic ovarian syndrome (PCOS) compared to girls without (37.5% vs 15.1%, P = 0.003), and PCOS was found to be independently predictive of NAFLD (OR 2.99, 95%CI 1.01-8.82, P = 0.048)[22].
Dietary: NAFLD is predominantly a condition associated with net caloric excess. Thus it is unsurprising that total calorie intake was found to be significantly higher in overweight children with NAFLD compared to similarly overweight children without NAFLD by (approximately 250 kcal per day)[30]. Dissecting down to the type of macronutrient composition and its association with NAFLD, the total intake of carbohydrates trended higher in overweight children with NAFLD than those without (approximately 120 kcal per day). In particular, intakes of fructose[31] and glucose were higher in overweight children with NAFLD than those without, whereas intake of fat protein and fiber was similar. Similarly, Félix et al[32] also showed that high amounts of refined carbohydrates in diet was independently associated with the presence of NAFLD (OR 2.17, 95%CI 1.05-6.82, P = 0.038). Mosca et al[31] studied 271 biopsy-proven NAFLD obese children and found that fructose consumption in this group of patients was independently associated with NASH (OR 1.612, 95%CI 1.25-1.86, P = 0.001) after adjustment for confounders[31]. In a study from India performed on 242 undergraduate students, soft drink consumption was associated with NAFLD. The prevalence of NAFLD was 75% in the group who consumed ≥ 2 soft drinks per day, 16% in the group that consumed 1 soft drink per day and 4% in the group that consumed < 1 soft drink per day (P = 0.001). Importantly, Siddiqi and colleagues found no differences in baseline metabolic risks in those who consumed diet soft drinks which are often marketed as a healthier alternative, vs regular soft drinks in that study[33].
A Western diet, which is generally characterized by high intakes of take-away foods, red and processed meats, full-fat dairy products, fried potatoes, refined sugars and soft drinks, has been shown to be associated with a higher risk of NAFLD[34]. Liu et al[35] compared different types of diets (Chinese vs Western vs High Energy) of 1639 participants in Shandong Province, China. After adjustment for confounders, those with traditional Chinese dietary pattern had the lowest risk (OR 0.726, 95%CI 0.383-0.960, P < 0.05), while the Western dietary pattern was associated with an increased risk of NAFLD (OR 1.197, 95%CI 1.013-1.736, P < 0.01). On the contrary, in a study of 1170 adolescents in Australia, neither a Western dietary nor healthy dietary pattern at age 14 was associated with NAFLD at 17 years of age. Only in a subgroup of obese adolescents was a Western dietary pattern at 14 years significantly associated with NAFLD at age 17 years (OR 1.45, 95%CI 1.05-2.00, P = 0.03). Nevertheless, this became insignificant when it was further adjusted for duration of breastfeeding and maternal obesity[11].
The Mediterranean diet is beneficial in the treatment of NAFLD and has been included in guidance for adult NAFLD[36]. However, this has not been widely studied in pediatric literature. Della Corte et al[37] studied 243 obese children and performed liver biopsy on 100 out of 166 children who had hepatic steatosis on ultrasound. In that study, it was found that low adherence to Mediterranean diet was significantly higher in patients with NASH compared with patients without NASH or hepatic steatosis. Similar findings were reported in two other studies[38,39]. Moreover, poor adherence to Mediterranean diet was also correlated with liver damage, liver inflammation and fibrosis[37].
Docosahexaenoic (DHA) supplementation was studied in a double blind, parallel group placebo-controlled randomized trial by Pacifico et al[40]. The final analysis at 6 mo showed that liver fat was reduced by 53.4% (95%CI 33.4-73.4) in the group that received DHA supplementation, as compared with 22.6% (95%CI 6.2-39.0) in the placebo group (P = 0.04). Other metabolic factors such as fasting insulin and TG were also significantly reduced in the group treated with DHA supplementation.
Physical activity and sleep: A sedentary lifestyle was shown to be associated with an increased risk of NAFLD. Nier et al[30] compared normal weight healthy children with overweight children with NAFLD in Southern Germany aged 5 to 9 years. Normal weight children on average spent 140 min per day compared to overweight children with NAFLD who spent approximately 215 min per day on sedentary activities (i.e. handcrafting, drawing, reading, watching television or playing video games). While the previous study may be confounded by participants’ BMI, Félix et al[32] also reported that a sedentary lifestyle was a significant independent predictive factor of NAFLD in a study population that only included obese children (OR 3.35, 95%CI 1.97-11.76, P = 0.006). In another study by Trovato and colleagues, on multiple regression sleep shortage and wearing over-sized clothing was shown to be associated with NAFLD[39].
Menarche: Ryu et al[41] conducted a cross sectional study involving 76415 women, 9601 of whom were diagnosed with NAFLD and an inverse association between age of menarche and development of NAFLD was seen. The prevalence ratios for NAFLD for early menarche and menarche at 12, compared with menarche at 13 years were: 1.26 (95%CI 1.14-1.39) and 1.04 (95%CI 0.97-1.12) respectively, whereas prevalence ratio of menarche at 14, 15, and 16 to 18 years compared with menarche at 13 years were 0.94 (95%CI 0.89-1.00), 0.91 (95%CI 0.86-0.97), and 0.83 (95%CI 0.78-0.89), respectively (P < 0.001). Even adjusting for other variables, the association between time of menarche and NAFLD persisted. Similarly, Mueller et al[42] reported in their study of 1214 women that one-year earlier menarche was associated with higher prevalence of NAFLD at young adulthood even after adjustment for body weight (RR 1.15, 95%CI 1.07-1.24, P < 0.05).
Gene polymorphisms: Several gene polymorphisms were found to be associated with NAFLD and have been extensively reported in adult NAFLD (Supplementary Table 4). Patatin-like phospholipase domain-containing protein 3 (PNPLA3) is the most widely reported. Other polymorphisms reported in the included studies in this review include: Transmembrane 6 Superfamily Member 2 (TM6SF2), heme-oxygenase-1 (HO-1), and membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7).
PNPLA3 polymorphisms and its association with NAFLD in children and adolescents were reported in several studies[25,43]. A study of 230 Italian children with obesity reported that homozygous PNPLA3 carriers (GG genotype) showed the highest risk of NAFLD (OR 14.9, 95%CI: 4.3-51.5, P < 0.001). In a study comprising 1010 adolescents, Grandone et al[43] found that the E167K allele of TM6SF2 gene was associated with hepatic steatosis (P < 0.0001). Interestingly in subjects homozygous for the PNPLA3 148M allele, carrying this rare variant of TM6SF2 showed increased odds of 12.2 (CI 3.8-39.6, P ≤ 0.001) to have raised alanine transaminase (ALT) as compared to the remaining patients[43]. This is further substantiated by Zusi and colleagues[44], who found in their cohort of 514 obese children and adolescents that genetic variants in TM6SF2 rs58542926, Glucokinase regulatory protein (GCKR) rs1260326, PNPLA3 rs738409, and Elongation of Very Long chain fatty acids-2 (ELOVL2) rs2236212 were significantly associated with a higher risk of NAFLD. These genetic variants in TM6SF2, GCKR and PNPLA3 were also found to be independently associated with NAFLD.
Multiple studies also reported associations with the MBOAT7 gene. A study by Di Sessa et al[45] found that carriers of the MBOAT7 T allele showed both significantly higher ALT and Pediatric NAFLD Fibrosis Index (PNFI) values compared to non-carriers. The MBOAT7 rs641738 variant was also found to exert an additive effect with PNPLA3 and TM6SF2 variants on NAFLD risk in obese children. Other variants in the MBOAT7 gene were found not to be associated NAFLD, such as with the rs641738 single nucleotide polymorphism (SNP) as reported by Lin et al[46]. Additionally, Di Costanzo et al[25] reported no association identified between hepatic fat content and MBOAT7 genotypes. In addition, the HO-1 gene promoter polymorphism was found to play an important role in the development of NAFLD. Chang et al[47] reported that patients 6 to 17 years with L alleles to the HO-1 gene were at higher risk of developing pediatric NAFLD (OR 18.84, 95%CI 1.45-245.22, P = 0.025). SNPs analysed and found not to be associated with risk of NAFLD include rs62064119, rs2297508, rs11868035 and rs13306741 in the sterol regulatory element binding protein 1c (SREBP1c) gene as reported by Peng et al[48].
Ethnic factors associated with NAFLD in adolescence and adulthood: Few ethnic factors were also found to be associated with NAFLD. In a study conducted by Younossi et al[49] in the US, 11613 participants aged 20 or older were enrolled and assessed according to four major racial or ethnic groups: non-Hispanic whites, non-Hispanic blacks, Hispanics, and ‘‘Other,’’ (Aleut, Eskimo, American Indian, Asian, or Pacific Islander). Multivariate analysis showed that NASH was independently associated with being Hispanic, having a younger age, and having components of metabolic syndrome such as hypertension (P < 0.05). On the other hand, smaller studies such as Lee et al[50] from Canada found in a study of 57 children analysing age, ethnicity, total body fat, fat-free mass, visceral fat, abdominal subcutaneous fat, and cardiorespiratory fitness, ‘visceral fat’ was the only factor to be independently associated with increased odds of having hepatic steatosis (OR 1.12, 95%CI 1.04-1.21, P = 0.003).
NAFLD is associated with gut microbiota dysbiosis, which has been associated with increased intestinal permeability (Supplementary Table 5). Circulating zonulin levels, a known mediator of intestinal permeability and modulating intracellular tight junctions is increased in children with NAFLD. Circulating zonulin is also significantly correlated with histological severity of hepatic steatosis[51]. Lipopolysaccharide-binding protein (LBP), possible surrogates of intestinal barrier function, were found to be significantly higher in overweight children with NAFLD than in those without[30]. In another study from Romania, Belei and colleagues investigated small intestinal bacterial overgrowth (SIBO) by glucose hydrogen breath test in 445 children[52]. NAFLD was detected in 28 of 47 (59.5%) of the SIBO positive obese group, compared to only 8 of 78 (10.2%) of the SIBO negative obese group (P < 0.001) and 0/120 (0%) non-obese group (P < 0.001).
Other markers that have been studied in relation to NAFLD include serum 25-Hydroxyvitamin D levels uric acid levels. Two studies reported the association of serum 25-hydroxyvitamin D and NAFLD (Supplementary Table 5). The first study conducted in West Australia involved 994 adolescents, where vitamin D concentrations were measured at ages 14 and 17 years and liver ultrasonography was done at 17 years to diagnose NAFLD. In the cohort, 16% (n = 156) had NAFLD, of which 51% and 17% had insufficient or deficient vitamin D status respectively[53]. Lower serum vitamin D concentrations at 17 years were significantly associated with NAFLD (independent of BMI and insulin resistance OR 0.74, 95%CI 0.56-0.97, P = 0.029). The second study conducted in Turkey[54] reported 87 obese adolescents (n = 42 with NAFLD) also showed an association of serum vitamin D and NAFLD. The group of adolescents with NAFLD had significantly lower measurements of serum vitamin D than the non-NAFLD group (29.5 ± 18.4 vs 41.0 ± 17.9 ng/mL, P < 0.001).
Four studies described the association of serum uric acid levels and NAFLD. Two studies reported uric acid to be independently associated with histologically more advanced NAFLD after adjustment for measured confounders[27,31]. On the contrary, in a cross-sectional study including 129 obese children and adolescents, authors found no significant association between high levels of uric acid and NAFLD. Instead, in that study, uric acid levels was only associated with the presence of metabolic syndrome and age range. However, the severity of NAFLD was not further characterised in this study[55].
Prevalence of NAFLD in adolescents has more than doubled in the last two decades[56]. Without early intervention, young patients with NAFLD can also develop steatohepatitis and finally decompensated cirrhosis and/or hepatocellular carcinoma later in life. Here, we have reported several studies which suggest that a maternal obesogenic environment has been associated with NAFLD in offspring. Lifestyle factors in early childhood and adolescence such as a sedentary lifestyle, sleep deficit and/or a high calorie, high carbohydrate diet are also factors associated with hepatic steatosis; while a longer period of exclusive breastfeeding, a Mediterranean diet and DHA supplementation appears to be protective. In the studies looking at gene polymorphisms, we also see that even among those who are obese and have metabolic risk factors, there are gene polymorphisms (PNPLA3, TM6SF2, HO-1 and MBOAT7) that increase the risk of NAFLD.
NAFLD may be the first silent manifestation of metabolic syndrome and predictive of other metabolic diseases, which contribute to adverse health outcomes. Metabolic syndrome and NAFLD is often linked to nutrient excess and obesity, though not all that are obese are metabolically unhealthy and vice versa. The adipose tissue expandability hypothesis by Virtue and Puig suggests that capacity to store lipids by expanding adipose tissue is variable in different individuals. When capacity is reached, adipose tissue then gets stored in ectopic tissues like the muscle and liver. Increase in visceral adipose tissue appears to be associated with metabolic disorders[17]. This concept suggests that instead of obesity in general, fat distribution, adipose tissue functionality and presence of insulin resistance are the likely key drivers of metabolic syndrome and NAFLD[18], which often exist as a continuum.
At present, there are no current FDA approved pharmacological agents to treat NAFLD. A 7%-10% weight loss is the first line treatment for adult NAFLD, which is often challenging, and difficult to maintain. Developmental plasticity is the ability where a given genotype may produce different phenotypes in response to different environments[57,58]. An exposure to a suboptimal condition during critical period of developmental programming can result in a diseased state. It is thought that the programmable windows of human obesity, which is tightly associated with NAFLD, may exist during periods of greatest weight velocity[59]. Thus, it is imperative to also target these modifiable factors associated with NAFLD early in life.
A maternal obesogenic environment has been associated with NAFLD in offspring via various potential mechanisms, associated with maternal insulin resistance, including inflammation, hormones and fetal hypoxia[2,60,61]. In animal model studies, it was shown that a maternal obesogenic diet was associated with fetal fatty liver in absence of fetal or maternal adiposity[62]. This is suggestive that maternal circulating hormones, lipids or cytokines could result in hepatic steatosis in-utero[63-65]. Other studies show that exposure to maternal obesogenic diet in early life was associated with an increased expression of hepatic transcription factor SREBP1c and its co-activators in offspring[66,67]. Furthermore, animal studies support that exposure to an obesogenic diet in early life may have long lasting consequences during these periods of developmental plasticity. Studies in mice and macaques have shown that in offsprings exposed to these diets, weaning to standard control diet does not completely reverse NAFLD, even in the absence of obesity in offspring[68,69].
While maternal obesity has been widely reported to be associated with NAFLD, poor nutrition in the form of undernourishment in-utero has also been associated with NAFLD in offspring[70-73]. This hypothesis was explored by Zheng et al[12] as summarized in the results section earlier. However, the mechanisms which potentiate metabolic dysfunction and/or NAFLD in the offspring, remains unclear. In murine studies, exposure to undernourishment in the in-utero environment increased activation of de novo lipogenesis is observed in parallel with the occurrence of NAFLD[74]. This was also associated with increased carbohydrate responsive element binding protein and SREBP1c expression at both transcriptional and protein levels.
Ex-utero, breastfeeding may confer some protective effects against NAFLD later in life. Breast milk contains high levels of oligosaccharides, which are complex sugars with substantial prebiotic effects for desired gut microbial growth. Additionally, it is also a rich source of long chain polyunsaturated fatty acids that has been reported to suppress de novo lipogenesis via inhibition of SREBP-1c[75,76]. However, these hypothesized mechanisms have yet to be clearly proven in the setting of NAFLD as protection against NAFLD.
While we present a comprehensive review of early developmental factors of NAFLD, which is not widely reported, our study is not without limitations. Firstly, there are only seven studies looking at maternal, paternal and postnatal factors associated with NAFLD. Secondly, most of the studies included in this systematic review are cross-sectional or cohort studies, and thus an inherent limitation of such studies is its inability to prove causation. For example, in most studies that reported in-utero factors, genetic polymorphisms, a major confounding factor, was not concurrently studied. Furthermore, post-natal factors such as lifestyle factors in childhood and adolescence have been associated with NAFLD. In particular, a high calorie, high refined carbohydrate and/or Western diet have been associated with NAFLD and adherence to Mediterranean diet was protective. Hepatic steatosis develops when the rate of fatty acid uptake and synthesis is greater than the rate that the liver can oxidise and export fatty acid[77]. It is difficult to establish if such dietary choices directly affect hepatic lipid metabolism independent of obesity, as obese individuals have more free fatty acid being released from adipose tissues with increase delivery and uptake into the liver. Nevertheless, this is an inherent limitation in any observational cross sectional study. Fourthly, only patients with imaging findings of hepatic steatosis were included, and we were unable to choose studies that only included patients who had secondary causes for hepatic steatosis and other liver diseases excluded. However, we expect that the proportion of patients who have viral hepatitis would be low[78]. Lastly, NAFLD is an umbrella term consisting of simple steatosis, steatohepatitis and/or fibrosis. Those with NASH and/or fibrosis are at highest risk of cardiovascular mortality and liver related morbidity. In this review, only 5 studies further graded severity of NAFLD by histology and reported factors associated with NAFLD severity grade, thus limiting the reporting of factors associated with severe NAFLD.
In spite of these limitations, our review serves as a useful overview and identifies areas in which further interventional studies can be considered. Measures to ameliorate the developmental programming of NAFLD should be introduced in early life, during times of developmental plasticity to elicit the most effective benefits. Some potential areas for further studies would be maternal control of metabolic dysregulation, maternal dietary intake during gestation and breastfeeding for example. Furthermore, studies in lean individuals with NAFLD are needed and would help to identify risk factors without the confounder of BMI.
In summary, our systematic review summarizes the current available literature on early developmental factors associated with hepatic steatosis. Maternal in utero environment, breastfeeding and nutritional, physical and genetic factors are associated with NAFLD. This time period in early life is potentially a time of developmental plasticity and may be a window of opportunity for early intervention to alter the natural course of this increasingly common and potentially debilitating disease.
Prevalence of non-alcoholic fatty liver disease (NAFLD) in adolescents has more than doubled in the last two decades, with its downstream complications placing an increasing burden on healthcare systems globally.
At present, there is a paucity of treatment options NAFLD. In line with the developmental origins of heath and disease (DOHaD) concept, we hope to identify factors in early life where possible intervention can be instituted to prevent the development of NAFLD later in life.
To review the early developmental factors associated with NAFLD and potentially identify areas where intervention can be made to halt the progress to steatohepatitis, fibrosis and cirrhosis and/or hepatocellular carcinoma which may develop later in life.
Original research studies that included risk factors associated with early development of NAFLD in human subjects were identified from databases MEDLINE via PubMed, and EMBASE and relevant publications up to April 30, 2021 were assessed.
Genetic polymorphisms, familial factors such as maternal obesogenic environment and parental history of hepatic steatosis was associated with offspring NAFLD. Longer duration of exclusive breastfeeding in infancy was associated with a lower risk of developing NAFLD later in life while metabolic dysfunction and/or obesity in adolescence was associated with increased risk of NAFLD.
Our systematic review summarizes the current available literature on early developmental factors associated with hepatic steatosis. Maternal in utero environment, breastfeeding and nutritional, physical and genetic factors are associated with NAFLD.
Maternal metabolic dysfunction during pregnancy, being exclusively breastfed for a longer time postnatally, diet and physical activity in childhood and adolescence are potential areas where research and interventions can be explored to prevent the development of NAFLD. Studied in lean individuals with NAFLD are needed and would help to identify risk factors without the confounder of BMI.
| 1. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1946] [Article Influence: 389.2] [Reference Citation Analysis (33)] |
| 2. | Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol. 2017;14:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 431] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 4. | Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc Biol Sci. 2005;272:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Dale KS, Mann JI, McAuley KA, Williams SM, Farmer VL. Sustainability of lifestyle changes following an intensive lifestyle intervention in insulin resistant adults: Follow-up at 2-years. Asia Pac J Clin Nutr. 2009;18:114-120. [PubMed] |
| 6. | Ayonrinde OT, Adams LA, Mori TA, Beilin LJ, de Klerk N, Pennell CE, White S, Olynyk JK. Sex differences between parental pregnancy characteristics and nonalcoholic fatty liver disease in adolescents. Hepatology. 2018;67:108-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Patel S, Lawlor DA, Callaway M, Macdonald-Wallis C, Sattar N, Fraser A. Association of maternal diabetes/glycosuria and pre-pregnancy body mass index with offspring indicators of non-alcoholic fatty liver disease. BMC Pediatr. 2016;16:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Rajindrajith S, Pathmeswaran A, Jayasinghe C, Kottahachchi D, Kasturiratne A, de Silva ST, Niriella MA, Dassanayake AS, de Silva AP, de Silva HJ. Non-alcoholic fatty liver disease and its associations among adolescents in an urban, Sri Lankan community. BMC Gastroenterol. 2017;17:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Long MT, Gurary EB, Massaro JM, Ma J, Hoffmann U, Chung RT, Benjamin EJ, Loomba R. Parental non-alcoholic fatty liver disease increases risk of non-alcoholic fatty liver disease in offspring. Liver Int. 2019;39:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Sood V, Khanna R, Rawat D, Sharma S, Alam S, Sarin SK. Study of Family Clustering and PNPLA3 Gene Polymorphism in Pediatric Non Alcoholic Fatty Liver Disease. Indian Pediatr. 2018;55:561-567. [PubMed] |
| 11. | Ayonrinde OT, Oddy WH, Adams LA, Mori TA, Beilin LJ, de Klerk N, Olynyk JK. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol. 2017;67:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Zheng X, Ren W, Gong L, Long J, Luo R, Wang Y. The Great Chinese Famine Exposure in Early Life and the Risk of Nonalcoholic Fatty Liver Disease in Adult Women. Ann Hepatol. 2017;16:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Breij LM, Kerkhof GF, Hokken-Koelega AC. Risk for Nonalcoholic Fatty Liver Disease in Young Adults Born Preterm. Horm Res Paediatr. 2015;84:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Breij LM, Kerkhof GF, Hokken-Koelega AC. Accelerated infant weight gain and risk for nonalcoholic fatty liver disease in early adulthood. J Clin Endocrinol Metab. 2014;99:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Rong Y, Chun-Yan N, Hong-Xin Z, Lu Y, Wen W, Yu T. Association of Adolescent Obesity with Nonalcoholic Fatty Liver Disease and Related Risk Factors in Xi 'an, China. Ann Hepatol. 2018;17:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Yan Y, Hou D, Zhao X, Liu J, Cheng H, Wang Y, Mi J. Childhood Adiposity and Nonalcoholic Fatty Liver Disease in Adulthood. Pediatrics. 2017;139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 782] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 18. | Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 2008;6:e237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Sanches PL, de Piano A, Campos RM, Carnier J, de Mello MT, Elias N, Fonseca FA, Masquio DC, da Silva PL, Corgosinho FC, Tock L, Oyama LM, Tufik S, Dâmaso AR. Association of nonalcoholic fatty liver disease with cardiovascular risk factors in obese adolescents: the role of interdisciplinary therapy. J Clin Lipidol. 2014;8:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Silveira LS, Monteiro PA, Antunes Bde M, Seraphim PM, Fernandes RA, Christofaro DG, Freitas Júnior IF. Intra-abdominal fat is related to metabolic syndrome and non-alcoholic fat liver disease in obese youth. BMC Pediatr. 2013;13:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Ayonrinde OT, Adams LA, Doherty DA, Mori TA, Beilin LJ, Oddy WH, Hickey M, Sloboda DM, Olynyk JK, Hart R. Adverse metabolic phenotype of adolescent girls with non-alcoholic fatty liver disease plus polycystic ovary syndrome compared with other girls and boys. J Gastroenterol Hepatol. 2016;31:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Peña-Vélez R, Garibay-Nieto N, Cal-Y-Mayor-Villalobos M, Laresgoiti-Servitje E, Pedraza-Escudero K, García-Blanco MDC, Heredia-Nieto OA, Villanueva-Ortega E. Association between neck circumference and non-alcoholic fatty liver disease in Mexican children and adolescents with obesity. J Pediatr Endocrinol Metab. 2020;33:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Prokopowicz Z, Malecka-Tendera E, Matusik P. Predictive Value of Adiposity Level, Metabolic Syndrome, and Insulin Resistance for the Risk of Nonalcoholic Fatty Liver Disease Diagnosis in Obese Children. Can J Gastroenterol Hepatol. 2018;2018:9465784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Alkassabany YM, Farghaly AG, El-Ghitany EM. Prevalence, risk factors, and predictors of nonalcoholic fatty liver disease among schoolchildren: a hospital-based study in Alexandria, Egypt. Arab J Gastroenterol. 2014;15:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Di Costanzo A, Pacifico L, Chiesa C, Perla FM, Ceci F, Angeloni A, D'Erasmo L, Di Martino M, Arca M. Genetic and metabolic predictors of hepatic fat content in a cohort of Italian children with obesity. Pediatr Res. 2019;85:671-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Nobili V, Mantovani A, Cianfarani S, Alisi A, Mosca A, Sartorelli MR, Maffeis C, Loomba R, Byrne CD, Targher G. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. J Hepatol. 2019;71:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Liang S, Cheng X, Hu Y, Song R, Li G. Insulin-like growth factor 1 and metabolic parameters are associated with nonalcoholic fatty liver disease in obese children and adolescents. Acta Paediatr. 2017;106:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Jimenez-Rivera C, Hadjiyannakis S, Davila J, Hurteau J, Aglipay M, Barrowman N, Adamo KB. Prevalence and risk factors for non-alcoholic fatty liver in children and youth with obesity. BMC Pediatr. 2017;17:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Han X, Xu P, Zhou J, Liu Y, Xu H. Fasting C-peptide is a significant indicator of nonalcoholic fatty liver disease in obese children. Diabetes Res Clin Pract. 2020;160:108027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Nier A, Brandt A, Conzelmann IB, Özel Y, Bergheim I. Non-Alcoholic Fatty Liver Disease in Overweight Children: Role of Fructose Intake and Dietary Pattern. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, Alisi A, Byrne CD. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 32. | Félix DR, Costenaro F, Gottschall CB, Coral GP. Non-alcoholic fatty liver disease (Nafld) in obese children- effect of refined carbohydrates in diet. BMC Pediatr. 2016;16:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Siddiqi Z, Karoli R, Fatima J, Khanduri S, Varshneya S, Ahmad SS. Soft Drinks Consumption and the Risk of Nonalcoholic Fatty Liver Disease. J Assoc Physicians India. 2017;65:28-32. [PubMed] |
| 34. | Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'Sullivan TA, Ayonrinde OT, Olynyk JK, Black LJ, Beilin LJ, Mori TA, Hands BP, Adams LA. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. 2013;108:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (3)] |
| 35. | Liu X, Peng Y, Chen S, Sun Q. An observational study on the association between major dietary patterns and non-alcoholic fatty liver disease in Chinese adolescents. Medicine (Baltimore). 2018;97:e0576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3288] [Article Influence: 328.8] [Reference Citation Analysis (7)] |
| 37. | Della Corte C, Mosca A, Vania A, Alterio A, Iasevoli S, Nobili V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutrition. 2017;39-40:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Cakir M, Akbulut UE, Okten A. Association between Adherence to the Mediterranean Diet and Presence of Nonalcoholic Fatty Liver Disease in Children. Child Obes. 2016;12:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 39. | Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 40. | Pacifico L, Bonci E, Di Martino M, Versacci P, Andreoli G, Silvestri LM, Chiesa C. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2015;25:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Ryu S, Chang Y, Choi Y, Kwon MJ, Kim CW, Yun KE, Jung HS, Kim BK, Kim YJ, Ahn J, Cho YK, Kim KH, Chung EC, Shin H, Cho J. Age at menarche and non-alcoholic fatty liver disease. J Hepatol. 2015;62:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Mueller NT, Pereira MA, Demerath EW, Dreyfus JG, MacLehose RF, Carr JJ, Terry JG, Jacobs DR Jr. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: The CARDIA study. Obesity (Silver Spring). 2015;23:468-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Grandone A, Cozzolino D, Marzuillo P, Cirillo G, Di Sessa A, Ruggiero L, Di Palma MR, Perrone L, Miraglia Del Giudice E. TM6SF2 Glu167Lys polymorphism is associated with low levels of LDL-cholesterol and increased liver injury in obese children. Pediatr Obes. 2016;11:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Zusi C, Mantovani A, Olivieri F, Morandi A, Corradi M, Miraglia Del Giudice E, Dauriz M, Valenti L, Byrne CD, Targher G, Maffeis C. Contribution of a genetic risk score to clinical prediction of hepatic steatosis in obese children and adolescents. Dig Liver Dis. 2019;51:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Di Sessa A, Umano GR, Cirillo G, Del Prete A, Iacomino R, Marzuillo P, Del Giudice EM. The Membrane-bound O-Acyltransferase7 rs641738 Variant in Pediatric Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2018;67:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Lin YC, Chang PF, Chang MH, Ni YH. Genetic determinants of hepatic steatosis and serum cytokeratin-18 fragment levels in Taiwanese children. Liver Int. 2018;38:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Chang PF, Lin YC, Liu K, Yeh SJ, Ni YH. Heme oxygenase-1 gene promoter polymorphism and the risk of pediatric nonalcoholic fatty liver disease. Int J Obes (Lond). 2015;39:1236-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Peng XE, Chen FL, Liu W, Hu Z, Lin X. Lack of association between SREBF-1c gene polymorphisms and risk of non-alcoholic fatty liver disease in a Chinese Han population. Sci Rep. 2016;6:32110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore). 2012;91:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 50. | Lee S, Kuk JL. Visceral fat is associated with the racial differences in liver fat between black and white adolescent boys with obesity. Pediatr Diabetes. 2017;18:660-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Pacifico L, Bonci E, Marandola L, Romaggioli S, Bascetta S, Chiesa C. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:17107-17114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. The relationship between non-alcoholic fatty liver disease and small intestinal bacterial overgrowth among overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2017;30:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Black LJ, Jacoby P, She Ping-Delfos WC, Mori TA, Beilin LJ, Olynyk JK, Ayonrinde OT, Huang RC, Holt PG, Hart PH, Oddy WH, Adams LA. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol. 2014;29:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Pirgon O, Cekmez F, Bilgin H, Eren E, Dundar B. Low 25-hydroxyvitamin D level is associated with insulin sensitivity in obese adolescents with non-alcoholic fatty liver disease. Obes Res Clin Pract. 2013;7:e275-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Cardoso AS, Gonzaga NC, Medeiros CC, Carvalho DF. Association of uric acid levels with components of metabolic syndrome and non-alcoholic fatty liver disease in overweight or obese children and adolescents. J Pediatr (Rio J). 2013;89:412-418. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162:496-500.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 57. | Hochberg Z. Developmental plasticity in child growth and maturation. Front Endocrinol (Lausanne). 2011;2:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Gyllenhammer LE, Entringer S, Buss C, Wadhwa PD. Developmental programming of mitochondrial biology: a conceptual framework and review. Proc Biol Sci. 2020;287:20192713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 59. | Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003;46:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 60. | Taricco E, Radaelli T, Rossi G, Nobile de Santis MS, Bulfamante GP, Avagliano L, Cetin I. Effects of gestational diabetes on fetal oxygen and glucose levels in vivo. BJOG. 2009;116:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 61. | Li M, Reynolds CM, Segovia SA, Gray C, Vickers MH. Developmental Programming of Nonalcoholic Fatty Liver Disease: The Effect of Early Life Nutrition on Susceptibility and Disease Severity in Later Life. Biomed Res Int. 2015;2015:437107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 63. | Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 2006;27:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Cao L, Mao C, Li S, Zhang Y, Lv J, Jiang S, Xu Z. Hepatic insulin signaling changes: possible mechanism in prenatal hypoxia-increased susceptibility of fatty liver in adulthood. Endocrinology. 2012;153:4955-4965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 66. | Kjaergaard M, Nilsson C, Rosendal A, Nielsen MO, Raun K. Maternal chocolate and sucrose soft drink intake induces hepatic steatosis in rat offspring associated with altered lipid gene expression profile. Acta Physiol (Oxf). 2014;210:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Pruis MG, Lendvai A, Bloks VW, Zwier MV, Baller JF, de Bruin A, Groen AK, Plösch T. Maternal western diet primes non-alcoholic fatty liver disease in adult mouse offspring. Acta Physiol (Oxf). 2014;210:215-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, Grove KL, Friedman JE. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes. 2014;63:2702-2713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Kruse M, Seki Y, Vuguin PM, Du XQ, Fiallo A, Glenn AS, Singer S, Breuhahn K, Katz EB, Charron MJ. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology. 2013;154:3565-3576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Abenavoli L. Influence of Famine in Women with Non-Alcoholic Fatty Liver Disease. Ann Hepatol. 2017;16:826-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Hepatic structural alteration in adult programmed offspring (severe maternal protein restriction) is aggravated by post-weaning high-fat diet. Br J Nutr. 2007;98:1159-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab. 2007;292:E1702-E1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 73. | George LA, Zhang L, Tuersunjiang N, Ma Y, Long NM, Uthlaut AB, Smith DT, Nathanielsz PW, Ford SP. Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am J Physiol Regul Integr Comp Physiol. 2012;302:R795-R804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Howie GJ, Sloboda DM, Vickers MH. Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br J Nutr. 2012;108:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Takeuchi Y, Yahagi N, Izumida Y, Nishi M, Kubota M, Teraoka Y, Yamamoto T, Matsuzaka T, Nakagawa Y, Sekiya M, Iizuka Y, Ohashi K, Osuga J, Gotoda T, Ishibashi S, Itaka K, Kataoka K, Nagai R, Yamada N, Kadowaki T, Shimano H. Polyunsaturated fatty acids selectively suppress sterol regulatory element-binding protein-1 through proteolytic processing and autoloop regulatory circuit. J Biol Chem. 2010;285:11681-11691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 76. | González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, Rodés J, Clària J. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1587] [Article Influence: 99.2] [Reference Citation Analysis (1)] |
| 78. | Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6:589-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (25)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji G, China; Wang T, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH