Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.592
Peer-review started: May 18, 2021
First decision: June 22, 2021
Revised: July 4, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 27, 2022
Processing time: 309 Days and 22.4 Hours
Acute kidney injury (AKI) in cirrhosis is important complication with poor outcomes. And infections are common cause for acute decompensation. Infections in cirrhosis lead to acute deterioration of hemodynamics leading to precipitation of AKI.
To study predictors of mortality in patients with infection-associated AKI in cirrhosis.
This was a prospective, observational study conducted at tertiary care centre from January 2018 till April 2019. Total 119 participants with cirrhosis of liver presenting with AKI were included into the study. AKI was defined as per international club of Ascites-AKI criteria 2015. Patients were grouped into infection AKI and non-infection AKI. Non-infection AKI included patients with diuretic induced AKI and pre-renal AKI. Logistic regression analysis was used to determine predictors of mortality at 28-d.
Out of 119 patients, alcohol (n = 104) was most common etiology of cirrhosis. The infection AKI included 67 (56%) patients and non-infection AKI (n = 52) included pre-renal AKI in 36 (30%) and diuretic-induced AKI in 16 (14%) patients. Infection AKI had significantly higher bilirubin, higher international normalized ratio (INR), low serum sodium, higher total leukocyte count (TLC) and higher prevalence of hepatic encephalopathy (HE) as compared to non-infection AKI. Infection AKI had higher progression of AKI (19/67 vs 2/52; P = 0.01) and 28-d mortality (38/67 vs 4/5; P ≤ 0.01) as compared to non-infection AKI. At 28-d, non-survivors (n = 42) had significantly higher bilirubin, higher INR, low serum sodium, higher TLC and higher prevalence of HE as compared to survivors (n = 77). On subgroup analysis of Infection AKI group, on multivariate analysis, serum bilirubin as well as presence of HE were independent predictors of 28-d mortality. There was no significant difference of mortality at 90-d between two groups.
Infection AKI in cirrhosis has a dismal prognosis with higher 28-d mortality as compared to non-infection AKI. Serum bilirubin and presence of HE predict 28-d mortality in infection AKI.
Core Tip: The infections in cirrhosis are the most common cause for acute decompensation and organ failure. Acute kidney injury (AKI) in cirrhosis is itself an indicator for worsening hemodynamics. In the present study, we compared infection associated AKI and non-infection AKI. We found higher 28-d mortality in infection AKI than non-infection AKI. In addition to altered hemodynamics, pathogen associated molecular patterns and damage-associated molecular patterns produced as a result of sepsis contribute to multiorgan failure, especially renal dysfunction. Moreover, higher bilirubin and presence of hepatic encephalopathy predicted 28-d mortality in patients with infection AKI. This provides an insight that the combination of infection and AKI in cirrhosis portends a dismal prognosis and therefore, on admission, early identification of infection and aggressive management may improve outcome in these patients.
- Citation: Gupta T, Ranga N, Goyal SK. Predictors of mortality at 28-days in infection associated acute kidney injury in cirrhosis. World J Hepatol 2022; 14(3): 592-601
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/592.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.592
The onset of jaundice, ascites, hepatic encephalopathy (HE), or gastrointestinal (GI) bleed marks the decompensation of a well-compensated chronic liver disease. The occurrence of ascites is an important benchmark in history of cirrhosis as it tilts the balance[1]. It signifies the presence of clinically significant portal hypertension, liver cell dysfunction, hypoalbuminemia, alteration in hemodynamics due to imbalance of vasoconstrictors and vasodilators in the splanchnic and systemic circulation. The splanchnic pooling of blood and systemic vasodilation leads to reduced effective arterial blood volume over a period of time resulting in refractory ascites. Ascites predisposes a patient with cirrhosis to increased incidence of spontaneous bacterial peritonitis (SBP) and acute kidney injury (AKI). In 25-year inception cohort study of patients with cirrhosis by D’Amico et al[2], it was shown that as stages of cirrhosis progress from 1 to 6, there is decreased 5-year survival. On competing risk analysis, they showed 0.50-0.97 risk of death within 1-year of onset of infections, renal failure or acute-on-chronic liver failure (ACLF) in decompensated cirrhosis[3,4].
The unique structural organization and dual blood supply of the liver plays important role in its immune function. Cirrhosis is associated with immune dysfunction. There is associated impaired Kupffer cell function, sinusoidal capillarization with continuous basement membrane formation leading to impaired exchange of cargo between sinusoidal blood and hepatocytes[5]. The gut dysbiosis in cirrhosis leads to increased portal blood endotoxemia, increased lipopolysaccharide levels which due to portosystemic shunting bypasses the liver and reaches directly in systemic circulation. This increases the risk of acquiring bacterial infections in cirrhosis. In addition, there is reduced neutrophil count due to splenic sequestration, associated neutrophil dysfunction with reduced chemotaxis, reduced monocyte and macrophage function with and impaired phagocytosis, impaired natural killer cell function. The CD4 and CD8 T cell function is also reduced[6-8]. Liver dysfunction leads to reduced complement proteins and hypoalbuminemia. All these factors predispose patients with cirrhosis towards acquiring infection. Sepsis, on the other hand is a precursor to multiorgan dysfunction. Therefore, we aimed to compare infection associated AKI with non-infection AKI in patients with cirrhosis of liver. We also determined the predictors of mortality in patients with infection AKI.
This was a prospective observational study which included consecutive patients with liver cirrhosis with AKI admitted in Department of Medicine at Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, India from Jan 2018 to June 2019. Liver cirrhosis was defined as per clinical, biochemical, and radiological parameters on ultrasound (nodular liver, portal vein diameter > 13 mm, splenomegaly, presence of collateral) and liver biopsy if needed. After written and informed consent, patients aged 18-70 years with cirrhosis of the liver presenting with AKI were included into the study. Patients aged < 18 years or > 70 years, pregnancy, chronic respiratory disease, chronic kidney disease, and hepatocellular carcinoma were excluded from the study. A detailed history and clinical examination were performed in all patients. AKI was defined as an increase in serum creatinine ≥ 0.3 mg/dL within 48 h; or a percentage increase in serum creatinine ≥ 50% from baseline which is known, or presumed, to have occurred within the prior 7 d. Baseline serum creatinine was defined as serum creatinine obtained in the previous 3 mo. In patients with more than one value within the previous 3 mo, the value closest to the admission time to the hospital was taken as the baseline value.
AKI staging was done as per international club of Ascites criteria; Stage 1 was defined as an increase in serum creatinine ≥ 0.3 mg/dL or ≥ 1.5-2 ULN from baseline, Stage 2 as an increase in serum creatinine > 2-3 ULN from baseline and Stage 3 as increase in serum creatinine > 3 ULN from baseline or serum creatinine ≥ 4.0 mg/dL with an acute increase ≥ 0.3mg/dL or initiation of renal replacement therapy. Further “Progression” of AKI was defined as progression to a higher stage and/or need of renal replacement therapy and “Regression” was defined as regression of AKI to a lower stage. The response to treatment was defined as “Full response” when serum creatinine value decreased to within 0.3 mg/dL of the baseline value, “Partial response” if reduction of serum creatinine ≥ 0.3mg/dL above baseline value and “No response” if there was no response in creatinine values. All patients were followed till 3 months to evaluate for 28-d and 90-d mortality. On admission, all patients were evaluated for the presence of infection by performing ascitic fluid analysis, blood culture, urine examination, urine culture, sputum gram stain and culture, chest X-Ray, and any other body fluid examination as indicated.
Pneumonia: Any new lung infiltrate with either symptom (cough, sputum, pleuritic pain, dyspnoea) or rales/crepitation on auscultation with components of systemic inflammatory response, i.e., temperature > 38 °C or < 36 °C or TLC > 10000/mm3 or < 4000/mm3 or respiratory rate > 20/min or PaCO2 < 32 mmHg or pulse > 90/min.
SBP: Either ascitic fluid PMNs (polymorphonuclear) > 250 cells/mm3 with/without a positive ascitic fluid culture.
Spontaneous bacterial empyema: Either pleural fluid PMNs > 250 cells/mm3 with positive culture or > 500 cells/mm3 irrespective of culture positivity.
Bacteraemia: Blood culture positivity without any source of infection.
Urinary tract infection (UTI): Urine microscopy showing WBC > 10/high power field with/without positive culture.
All patients with AKI were grouped into infection AKI, diuretic induced AKI and pre-renal AKI. Diuretic induced AKI was defined as patients who were on diuretics (furosemide and spironolactone) for the control of ascites with negative work up for infection or pre-renal causes. Pre-renal AKI was defined in patients with cirrhosis presenting with upper GI bleed, fluid losses due to diarrhea or vomiting etc. and with negative work up for infections and no history of diuretics. Diuretic induced AKI and pre-renal AKI were grouped as non-infection AKI.
All continuous variables were taken as mean ± SD (range) or median [IQR; Q1, Q3] and categorical variables as frequency and percentages. For comparison of continuous variables, Mann–Whitney U test/Student t-test and for categorical variables, χ2 and Fisher exact tests were used. 28-and 90-d mortality was assessed using survival analysis. P < 0.05 was taken as significant. SPSS v21.0 (IBM, USA) was used for analysis.
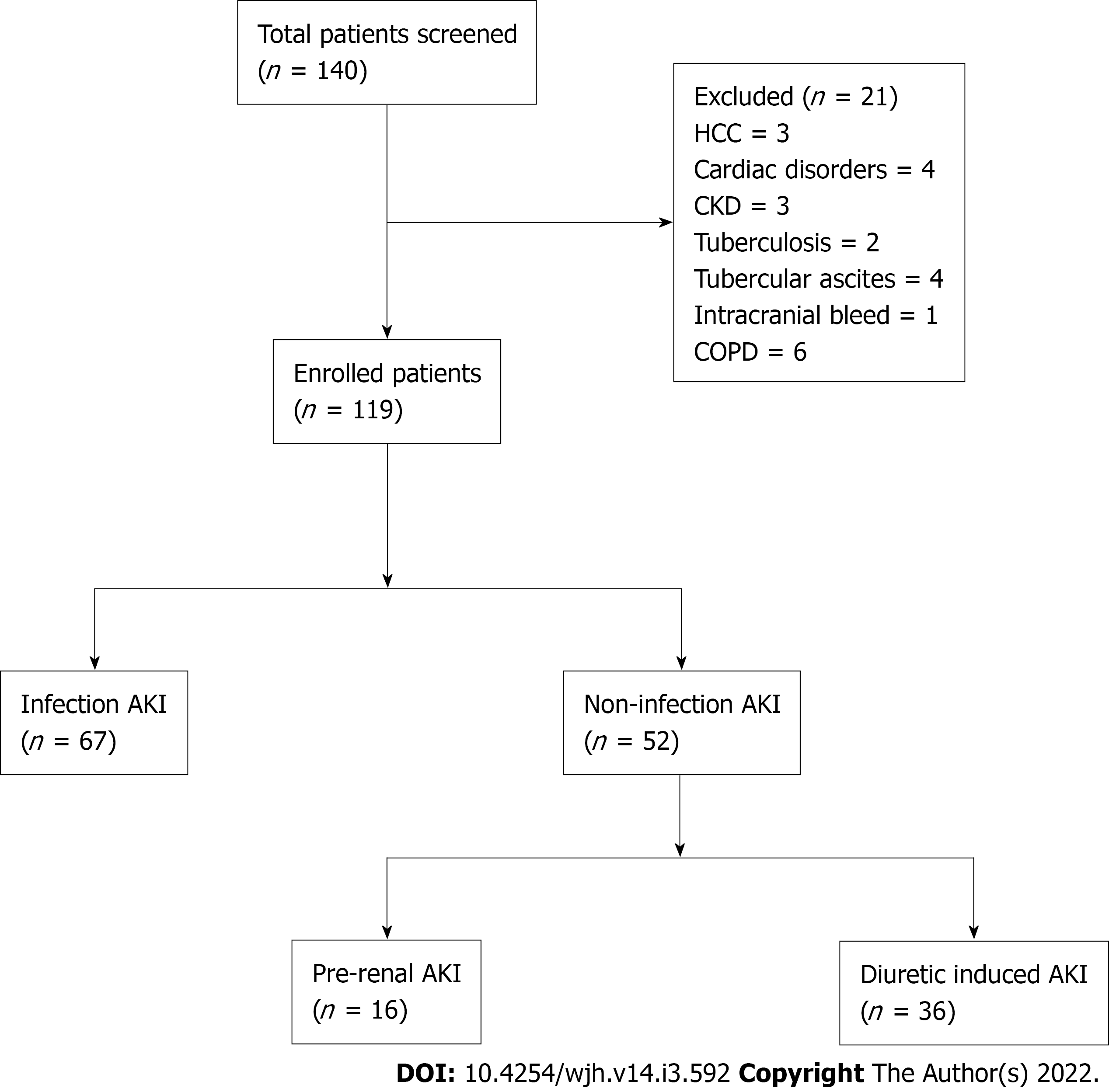
Out of 140 patients of cirrhosis with AKI were admitted during the study period, 21 patients did not fulfil inclusion criteria (Figure 1). Finally, 119 patients of cirrhosis with AKI were included into the study.
The most common etiology of cirrhosis was alcohol (n = 98), chronic hepatitis B and C (n = 5 each), non-alcoholic steatohepatitis related cirrhosis (n = 4), both alcohol and chronic hepatitis C (n = 4), both alcohol and chronic hepatitis B (n = 2), and autoimmune cirrhosis (n = 1).
Among 119 patients, infection with AKI was present in 67 (56%). Non-infection AKI included 36 (30%) patients with pre-renal and 16 (14%) patients with diuretic induced AKI (Figure 1). Out of 67 patients of infection AKI, SBP was present in 30 (45%), pneumonia in 9 (13%), cellulitis in 7 (10%), UTI in 2 (3%), splenic abscess in 1 (1.5%) and source of infection unidentified in 18 (27%) patients.
At baseline, Infection AKI group had higher creatinine (2.6 mg/dL vs 2.2 mg/dL, P = 0.016) as compared to non-infection AKI. Further, infection AKI group had higher mean serum bilirubin, higher INR, lower serum albumin, lower serum sodium, higher haemoglobin, higher TLC, and higher prevalence of HE than non-infection AKI group respectively (Table 1).
| Variables | Infection AKI (n = 67) | Non-infection AKI (n = 52) | P value |
| Age (yr, mean ± SD) | 42 ± 23 | 41 ± 21 | 0.23 |
| Males, n (%) | 58 (86%) | 47 (90%) | 0.31 |
| Hb (gm/dL) | 8.5 (3.6-14.7) | 8.1 (3-14) | 0.037 |
| TLC (× 103/mm3) | 17 (2-40) | 7.8 (2.5-18) | 0.001 |
| Platelet count (× 109/L) | 110 (60-200) | 130 (80-220) | 0.335 |
| Bilirubin (mg/dL) | 11.3 (0.8-46.6) | 4.4 (0.8-27.9) | 0.003 |
| INR | 2.1 (0.9-3.9) | 1.9 (0.9-3.6) | 0.045 |
| Albumin (gm/dL) | 2.3 (1.6-3.9) | 2.5 (1-3.7) | 0.04 |
| Creatinine (mg/dL) | 2.6 (1.4-6) | 2.2 (1.2-5.4) | 0.016 |
| Sodium (mEq/L) | 132.5 (116-164) | 135.9 (120-151) | 0.04 |
| HE, n | 47 | 22 | 0.03 |
| CTP | 12 (6-15) | 11 (6-14) | 0.73 |
| MELD | 27 (11-38) | 24 (10-35) | 0.95 |
Infection AKI had higher progression of AKI (19/67 vs 2/52; P = 0.01) and higher 28-d mortality (38/67 vs 4/52; P < 0.001) than non-infection AKI group respectively. In non-infection AKI group, four non-survivors belonged to prerenal AKI. At 90-d, there was no significant difference of mortality among infection AKI and non-infection AKI group (49/67 vs 13/52; P = 0.2) respectively (Table 2).
| Mortality | Infection AKI (n = 67) | Non-infection AKI (n = 52) | P Value | |
| Diuretic-induced (n = 16) | Pre-renal (n = 36) | |||
| 28-d (n = 42) | 38 | 0 | 4 | < 0.0001 |
| 90-d (n = 20) | 11 | 3 | 6 | 0.206 |
Overall, out of 119 patients, at 28-d, there were 77 survivors and 42 non-survivors. On univariate analysis, survivors had lower serum bilirubin, lower INR, lower TLC, and lower prevalence of HE compared to non-survivors. The multivariate analysis revealed higher bilirubin and presence of HE to predict 28-d mortality (Table 3).
| Variables | Survivors (n = 77) | Non-survivors (n = 42) | Univariate | Multivariate |
| Age (yr, mean ± SD) | 41 ± 21 | 40 ± 22 | 0.73 | - |
| Males, n (%) | 69 (86.9%) | 36 (85.7%) | 0.41 | - |
| Hb (gm/dL) | 8.3 (4-14) | 8.4 (3.4-14) | 0.838 | - |
| TLC (× 103/mm3) | 11 (2.5-37) | 17.4 (2-39) | 0.001 | - |
| Platelet count (× 109/L) | 114.6 (100-200) | 130 (60-220) | 0.520 | - |
| Bilirubin (mg/dL) | 4.2 (0.5-30) | 15.7 (0.2-46) | 0.001 | < 0.001 |
| INR | 1.8 (1-3.7) | 2.1 (1.2-3.8) | 0.006 | - |
| Albumin (gm/dL) | 2.4 (1-3.7) | 2.4 (1.8-3.9) | 0.689 | - |
| Sodium (mEq/L) | 135 (116-164) | 131 (120-146) | 0.336 | - |
| HE, n | 32 | 37 | < 0.001 | < 0.01 |
In subgroup analysis of Infection AKI group, non-survivors (n = 38) had higher TLC, higher bilirubin, higher INR and higher prevalence of HE as compared to survivors (n = 29). On multivariate analysis, serum bilirubin and presence of HE were independent predictors of 28-d mortality (Table 4).
| Variables | Survivors (n = 29) | Non-survivors (n = 38) | Univariate | Multivariate |
| Age (yr, mean ± SD) | 40 ± 21 | 40 ± 22 | 0.81 | - |
| Males, n (%) | 23 (79%) | 35 (92%) | 0.9 | - |
| Hb (gm/dL) | 8.1 (4-14) | 7.5 (3.4-14) | 0.06 | - |
| TLC (× 103/mm3) | 10 (2.5-36) | 18.3 (2-39) | 0.001 | - |
| Platelet count (× 109/L) | 112 (65-203) | 125 (60-220) | 0.520 | - |
| Bilirubin (mg/dL) | 4.6 (1.2-30) | 16.3 (1.5-46) | 0.004 | 0.01 |
| INR | 1.9 (1.3-3.7) | 2.1 (1.1-3.8) | 0.005 | - |
| Albumin (gm/dL) | 2.3 (1-3.6) | 2.4 (1.5-3.9) | 0.73 | - |
| Sodium (mEq/L) | 135 (116-154) | 132 (119-148) | 0.45 | - |
| HE, n | 10 | 37 | < 0.001 | < 0.01 |
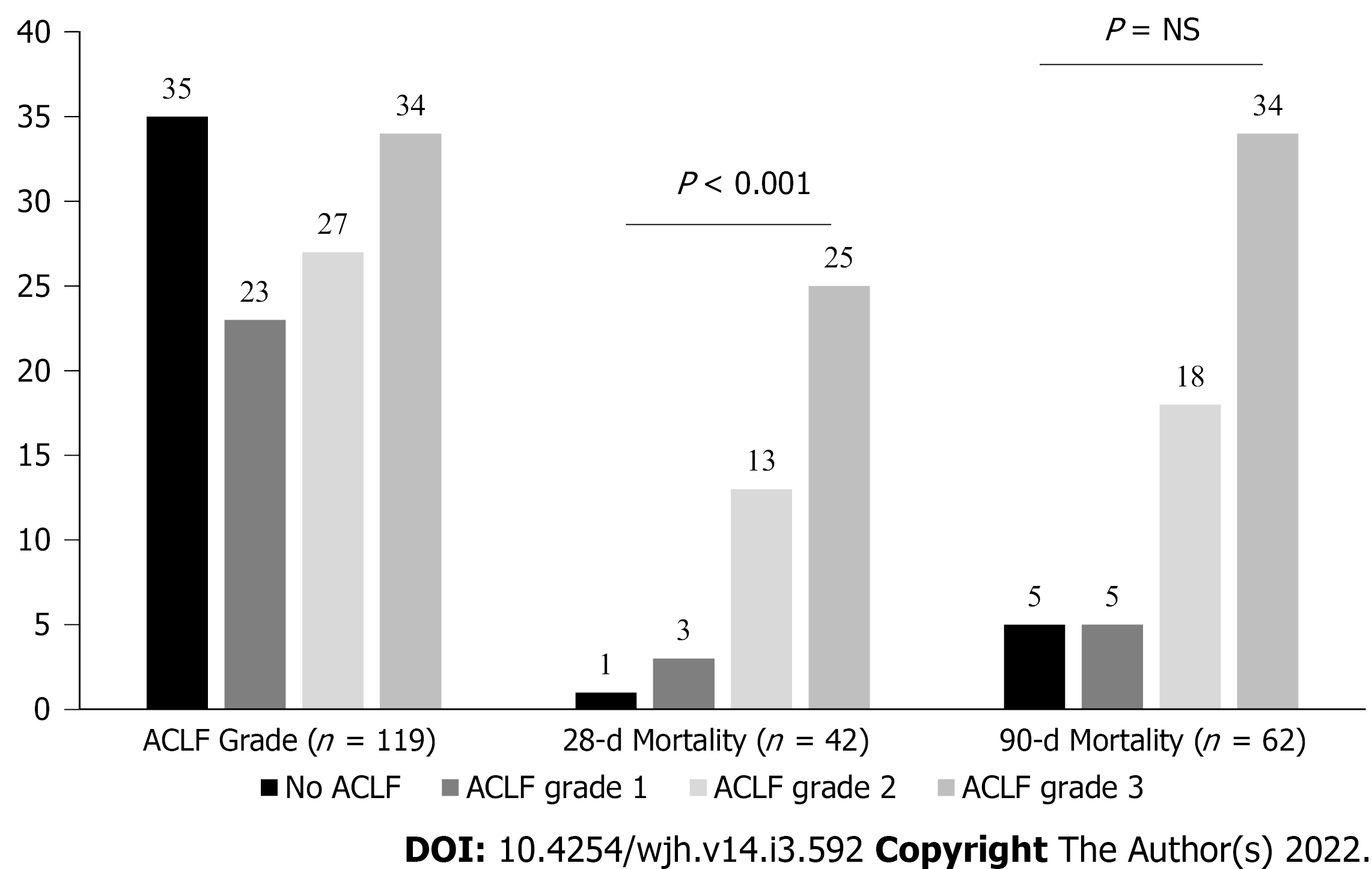
As per CANONIC grading of ACLF, there were 35 patients in no ACLF, 23 in ACLF grade-1, 27 in ACLF grade-2, 34 in ACLF grade-3. At 28-d, there was mortality of one patient with no ACLF, three in ACLF grade-1, 13 in ACLF grade-2, and 25 in ACLF grade-3. At 90-d, there was a mortality of five patients in no ACLF, five in ACLF grade-1, 18 in ACLF grade-2, and 34 in ACLF grade-3 (Figure 2). In infection AKI group (n = 67), 55 patients had ACLF and 12 had no ACLF.
The study gives three important findings in relation to infection AKI in cirrhosis of liver: (1) prevalence of infection AKI in cirrhosis; (2) One-and three-mo mortality; and (3) predictors of mortality at 28-d in infection AKI group. There was a 56% prevalence of infection AKI in patients with cirrhosis presenting with AKI. Remaining patients had AKI due to pre-renal and diuretic-related causes. This study had SBP as the cause of infection in 45% followed by pneumonia (13%) and cellulitis (10%) in acute decompensation of cirrhosis. The CANONIC series had SBP in 25% of all infections and source of infection was undefined in 13%[9]. The International Club of Ascites Global Study Group also showed higher prevalence of SBP (35% vs 27%) and pneumonia (28% vs 19%) in Asia compared to Europe respectively[2]. The Global study group showed higher rates of ACLF in Asia compared to global data (46% vs 35%; P < 0.01) in patients with cirrhosis with infection respectively[10]. Our study had ACLF in 82% of patients in infection AKI group. We had selectively included patients of cirrhosis with AKI and as renal dysfunction is a late manifestation in the course of cirrhosis, this may be the reason behind the higher rates of ACLF in our study population as compared to previous studies which included all patients with acute decompensation of cirrhosis.
The previous data from India suggest higher rates of acute viral hepatitis A and E as a cause for acute insult in acute decompensation of cirrhosis and ACLF[11]. However, recent studies show a trend towards increasing rates of infection with multidrug resistant (MDR) and extremely drug resistant (XDR) bacteria in Asia[12]. The Global study showed higher prevalence of MDR (76% vs 16%) and XDR bacteria (33% vs 1%-16%) in Indian centers, as compared to Western centers respectively[10].
Presence of infections in cirrhosis activates systemic inflammation and results in multi-organ dysfunction[13]. The pathogen associated molecular patterns (PAMPs) arising from the gut and damage associated molecular patterns (DAMPs) released from necrotic hepatocytes stimulate toll like receptors (TLRs) on hepatocytes and cause release of Interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-alpha, etc. The cytokine storm, PAMPs and DAMPs in the circulation increase the expression of TLR4 receptors in the kidneys leading to increased permeability, proteinuria, and alteration in vascular tone. Through various molecular pathways, oxidative stress and apoptosis in tubular epithelial cells increases and results in sepsis induced AKI[14,15]. In animal studies, pharmacological interventions targeting TLR receptors in the kidney have shown reduced injury in sepsis induced AKI[16]. Shah et al[17] showed that there is already increased expression of TNF-alpha, TLR4, etc. in kidneys in cirrhosis, making them susceptible to inflammatory insult and gut decontamination with norfloxacin prevents renal dysfunction after LPS stimulation. The present study revealed that 1-mo mortality in the infection AKI group was significantly higher than non-infection AKI (38 vs 4; P < 0.0001) respectively. It is likely that the greatest impact of infection as an acute insult is on short term mortality, and if aggressive and appropriate management is given timely, it may improve renal function also.
Various studies on histopathology of renal tissues in cirrhosis have shown direct renal damage due to high bilirubin levels. There is formation of bile casts in the tubular lumen and accumulation of conjugated bilirubin in tubular epithelial cells, which leads to mitochondrial damage with defective oxidative phosphorylation. All above changes predispose patients of cirrhosis with jaundice to cholemic nephropathy[18]. Nazar et al[19] evaluated response of terlipressin in treatment of hepatorenal syndrome type-1 and found serum bilirubin level > 10 mg/dL as a predictor of poor response to therapy. The response rate in patients with bilirubin > 10 mg/dL was 13% as compared to 67% in bilirubin values < 10 mg/dL (P = 0.001). We reported higher mean bilirubin values in the infection AKI group than non-infection AKI group (11.3 vs 4.4 mg/dL) respectively. Possibly with increasing severity of chronic liver disease as assessed by MELD score, the immune function worsens and there is propensity to get infection in these patients. Our study also revealed significantly higher serum bilirubin values (15.7 vs 4.2 mg/dL) in non-survivors than survivors at 28-d, respectively.
In ACLF, HE is multifactorial. Sepsis, metabolic disturbances like hypokalemia, hyponatremia secondary to diuretic use or volume loss, liver dysfunction with hyperammonemia can precipitate HE. We have shown previously that there is increasing cerebral edema in patients with increasing grades of ACLF[20]. Therefore, HE also marks a poor prognosis in patients with infection AKI.
Our study has some limitations. The sample size could be higher, due to which subgroup analysis could not be done as the number of patients were small in individual groups. Second, being a tertiary care institute, most of the patients were referred from primary and secondary care centers after receiving antibiotics, therefore, culture reports were not available in all the patients. Also, the data on beta blockers was not available at baseline for all the patients and could not be analyzed.
In ACLF, renal dysfunction is multifactorial with the presence of sepsis, circulatory dysfunction either due to volume loss or sepsis and higher bilirubin levels. We showed that pre-renal, upper GI bleed and diuretic-induced AKI is less severe with favorable outcomes after successful management with very low rate of recurrence. On the other hand, in patients with infections, it is not only the control of infection, but also the number of organ failures which is crucial to determine the final outcome of these patients. Finally, higher grades of ACLF in patients with infection, AKI having liver dysfunction and cerebral failure has worst prognosis with high 28-d mortality.
Infections lead to worsening hemodynamics in cirrhosis which results in organ failures. Renal dysfunction in these patients further complicates the clinical scenario. We noted that higher bilirubin levels and Hepatic encephalopathy in patients with infection associated AKI portends a dismal prognosis. The present study emphasizes the worse prognosis with infection and need of early identification and aggressive management on admission to improve short-term mortality.
Acute kidney injury (AKI) in cirrhosis has dismal outcomes. Recent data suggests infections being most common insult for acute decompensation of cirrhosis. Infections lead to acute deterioration of already compromised hemodynamics in cirrhosis.
Infections in cirrhosis is a precursor towards multi-organ dysfunction. Kidney failure is one of the early manifestation in cirrhosis which has a potential for reversibility. Identifying high risk of mortality in patients with AKI in cirrhosis may warrant early institution of treatment, especially in presence of infection. This may help to develop new protocols to salvage kidney in presence of infections in cirrhosis.
To compare infection and non-infection AKI in cirrhosis, and to determine predictors of mortality at 28-d in patients with infection associated AKI.
It was a prospective, observational study conducted at a tertiary care hospital for a period of 1 year. After written, informed consent total 119 patients with AKI in cirrhosis were included into the study. AKI was defined as per International Club of Ascites-AKI 2015 criteria. Patients were divided into infection and non-infection AKI groups. Non-infection AKI included patients with pre-renal and diuretic induced AKI. Infection and non-infection AKI groups were compared for clinical and laboratory data. In infection AKI group logistic regression analysis was performed to determine 28-d predictors of mortality.
There were 119 patients of cirrhosis with AKI. Alcohol (n = 104) was most common etiology of cirrhosis. The infection AKI group had 67 (56%) patients and non-infection AKI had 52 (44%) patients which included pre-renal AKI in 36 (30%) and diuretic-induced AKI in 16 (14%). Infection AKI patients had higher progression of AKI (19/67 vs 2/52; P = 0.01) and 28-d mortality (38/67 vs 4/5; P ≤ 0.01) as compared to non-infection AKI patients. On subgroup analysis of Infection AKI group, on multivariate analysis, serum bilirubin as well as presence of HE were independent predictors of 28-d mortality. There was no significant difference of mortality at 90-d between two groups.
This study says that AKI in cirrhosis with infection has high short term mortality. High bilirubin and presence of hepatic encephalopathy predicts high 28-d mortality in infection associated AKI. Probably AKI in patients with cirrhosis is multifactorial with sepsis, volume depletion, bilirubin as important factors.
High bilirubin levels can contribute to nephropathy as well as encephalopathy. Still, we do not have effective therapies for high bilirubin values. Future research should focus on drugs to lower bilirubin levels. And probably more data is needed on infections in cirrhosis.
We thank Prof Rakesh Mittal, Department of Pharmacology, Pt B D Sharma Institute of Medical Sciences for reviewing outcomes of statistical analysis.
| 1. | Asrani SK, Kamath PS. Natural history of cirrhosis. Curr Gastroenterol Rep. 2013;15:308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 393] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 3. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 401] [Article Influence: 50.1] [Reference Citation Analysis (1)] |
| 4. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (2)] |
| 5. | Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 993] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 6. | Dirchwolf M, Ruf AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J Hepatol. 2015;7:1974-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 7. | Mehta G, Gustot T, Mookerjee RP, Garcia-Pagan JC, Fallon MB, Shah VH, Moreau R, Jalan R. Inflammation and portal hypertension - the undiscovered country. J Hepatol. 2014;61:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Trebicka J, Reiberger T, Laleman W. Gut-Liver Axis Links Portal Hypertension to Acute-on-Chronic Liver Failure. Visc Med. 2018;34:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2276] [Article Influence: 175.1] [Reference Citation Analysis (6)] |
| 10. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 360] [Article Influence: 51.4] [Reference Citation Analysis (4)] |
| 11. | Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Gupta T, Lochan D, Verma N, Rathi S, Agrawal S, Duseja A, Taneja S, Chawla YK, Dhiman RK. Prediction of 28-day mortality in acute decompensation of cirrhosis through the presence of multidrug-resistant infections at admission. J Gastroenterol Hepatol. 2020;35:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 328] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 14. | Vázquez-Carballo C, Guerrero-Hue M, García-Caballero C, Rayego-Mateos S, Opazo-Ríos L, Morgado-Pascual JL, Herencia-Bellido C, Vallejo-Mudarra M, Cortegano I, Gaspar ML, de Andrés B, Egido J, Moreno JA. Toll-Like Receptors in Acute Kidney Injury. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 581] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 16. | Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, Macnaughtan J, Sharma V, Olde Damink SWM, Mookerjee RP, Jalan R. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Krones E, Pollheimer MJ, Rosenkranz AR, Fickert P. Cholemic nephropathy - Historical notes and novel perspectives. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1356-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, Solá E, Baccaro ME, Terra C, Arroyo V, Ginès P. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (6)] |
| 20. | Gupta T, Dhiman RK, Ahuja CK, Agrawal S, Chopra M, Kalra N, Duseja A, Taneja S, Khandelwal N, Chawla Y. Characterization of Cerebral Edema in Acute-on-Chronic Liver Failure. J Clin Exp Hepatol. 2017;7:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He Z, Wang YH S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL