Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.551
Peer-review started: August 16, 2021
First decision: November 11, 2021
Revised: November 13, 2021
Accepted: February 24, 2022
Article in press: February 24, 2022
Published online: March 27, 2022
Processing time: 219 Days and 21.2 Hours
Non-alcoholic fatty liver disease (NAFLD) is currently considered as the most common cause of chronic liver disease worldwide. Risk factors for NAFLD have been well-described, including obesity, type 2 diabetes mellites (T2DM), dyslipidemia (DLP) and metabolic syndrome. Hypothyroidism has been identified as an independent risk factor for the development of NAFLD, although the literature is inconsistent
To evaluate the prevalence of hypothyroidism in patients with NAFLD, assess if it is an independent risk factor and explore the effect of thyroxine replacement therapy.
Our cohort’s data was obtained using a validated, large, multicenter database (Explorys Inc, Cleveland, OH, United States) aggregated from pooled outpatient and inpatient records of 26 different healthcare systems, consisting of a total of 360 hospitals in the United States, and utilizing Systematized Nomenclature of Medicine-Clinical Terms for coding. We evaluated a cohort of patients with hypothyroidism and NAFLD. Multivariate analysis was performed to adjust for confounding risk factors including hypertension (HTN), T2DM, DLP, obesity and metabolic syndrome. SPSS version 25, IBM Corp was used for statistical analysis, and for all analyses, a 2-sided P value of < 0.05 was considered statistically significant. Exclusion criteria were limited to age < 18 years.
Among the 37648180 included individuals in this database who are above the age of 18 years, there were a total of 2320 patients with NAFLD (6.16 per 100000) in the last five years (2015-2020), amongst which 520 patients (22.4%) had hypothyroidism. Baseline characteristics of patients in this database are described in Table 1. Patients with NAFLD were also more likely to have obesity, T2DM, DLP, HTN, and metabolic syndrome (Table 2). While males and females were equally affected, patients in the age group 18-65 years as well as Caucasians seem to be at a higher risk. There was an increased risk of NAFLD among patients with hypothyroidism (OR = 1.587). Furthermore, thyroid hormone replacement was not associated with a decreased risk for developing NAFLD (OR = 1.106, C = 0.952-1.285, P = 0.303).
Hypothyroidism seems to be an independent risk factor for the development of NAFLD. Thyroid hormone replacement did not provide a statistically significant risk reduction. Further studies are needed to evaluate the effect of thyroid hormone replacement and assess if being euthyroid while on thyroid replacement therapy affects development and/or progression of NAFLD.
Core Tip: One of the largest population-based case-control studies screening more than 37 million patients to study the inconsistent relationship between hypothyroidism and non-alcoholic fatty liver disease (NAFLD), and -to the best of our knowledge- the first paper investigating the theoretical role of thyroid hormone replacement in preventing NAFLD among hypothyroidism patients.
- Citation: Almomani A, Hitawala AA, Kumar P, Alqaisi S, Alshaikh D, Alkhayyat M, Asaad I. Prevalence of hypothyroidism and effect of thyroid hormone replacement therapy in patients with non-alcoholic fatty liver disease: A population-based study. World J Hepatol 2022; 14(3): 551-558
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/551.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.551
Non-alcoholic fatty liver disease (NAFLD) is currently considered as the most common cause of chronic liver disease worldwide and the second most common indication for liver transplantation in the United States after chronic hepatitis C with a histological disease spectrum ranging from steatosis to non-alcoholic steatohepatitis (NASH) and eventually cirrhosis. Its international prevalence is steadily increasing (15% in 2005 to 25% in 2010), and it is expected to emerge as the leading cause of end-stage liver disease in the near future[1]. Several genetic and environmental risk factors for NAFLD have been described in the literature, including obesity, unhealthy eating habits, low physical activity levels, type 2 diabetes mellitus (T2DM), dyslipidemia (DLP), hypertension (HTN) and metabolic syndrome[1-3].
Thyroid hormone plays a major role in regulating the metabolism of lipids and carbohydrates which are affected in patients with NAFLD. Furthermore, hypothyroidism in particular shares similar risk factors to those of NAFLD including insulin resistance, DLP, obesity and metabolic syndrome[4,5]. Liangpunsakul et al[4] was the first to describe the potential relationship between hypothyroidism and NAFLD, and found a significantly higher hypothyroidism prevalence among patients with NAFLD. This association was further replicated in later retrospective studies[5]. However, these studies were limited by the smaller sample size and the inconsistency of the literature to some degree. Our aim is to conduct a population-based study to estimate the prevalence of hypothyroidism in patients with NAFLD, and statistically adjust for all known confounders to assess whether hypothyroidism is an independent risk factor for NAFLD, and to further assess the effect of thyroid hormone replacement therapy.
Our cohort’s data was obtained using a validated, multicentered and daily-updated database (Explorys Inc, Cleveland, OH, United States) developed by IBM Corporation, Watson Health[6]. Explorys consists of electronic health records of 26 different healthcare systems across the United States and a total of 360 hospitals with more than 50 million patients. Explorys utilizes Systematized Nomenclature of Medicine Clinical Terms (SNOMED-CT) for the definition of the diseases and pools large outpatient and inpatient daintified data that can be formulated into numerous cohorts according to the clinical element being studied. Explorys further allows for the identification of the timeline of events in reference to the index clinical event of interest, and hence the ability to study the temporal relationship between different variables. The Institutional Review Board approval is not required since Explorys is a Health Insurance Portability and Accountability Act-compliant platform.
We retrospectively evaluated an initial cohort of patients with a SNOMED-CT of “Hypothyroidism” between the years 2015 to 2020. Our exclusion criteria were limited to patients less than 18 years old. Baseline characteristics of patients with hypothyroidism are shown in Table 1. A second cohort of patients with a SNOMED-CT of “Non-Alcoholic Fatty Liver” was identified. Age, gender and race-based data were collected. Potential confounders that were analyzed included: hypothyroidism, HTN, T2DM, DLP, obesity and metabolic syndrome. Among those with hypothyroidism, weather the patient was on thyroxine replacement therapy was also analyzed.
| Parameter | Hypothyroidism | ||
| Present (%) | Absent (%) | ||
| Age (yr) | 18-65 | 1335370 (48.3) | 21097850 (60.5) |
| > 65 | 1402550 (50.7) | 6951210 (19.9) | |
| Gender | Female | 2087040 (75.5) | 18562590 (53.2) |
| Race | Caucasian | 2267940 (82.0) | 20165960 (57.8) |
| African-American | 196720 (7.1) | 4120940 (11.8) | |
| Asian | 40710 (1.5) | 539190 (1.5) | |
| Comorbidities | HTN | 1665090 (60.2) | 7441760 (21.3) |
| T2DM | 790680 (28.6) | 3114700 (8.9) | |
| Dyslipidemia | 1716240 (62.1) | 6469880 (18.5) | |
| Obesity | 753060 (27.2) | 3391060 (9.7) | |
| Metabolic syndrome | 54440 (2.0) | 2709750 (7.8) | |
Demographics and related diseases were characterized by descriptive statistics. The overall prevalence of NAFLD was calculated by dividing the total number of individuals with NAFLD by the total number of individuals in the database (2015-2020), hence making sure that all patients in the denominator had an equal opportunity of being diagnosed with NAFLD. Multivariate analysis was performed to adjust for the confounders in the later cohort (Table 2). SPSS version 25, IBM Corp was used for statistical analysis, and for all analyses, a 2-sided P value of < 0.05 was considered statistically significant.
| Parameter | Odds ratio | 95%CI | P value |
| Age (18-65) | 1.658 | 1.524-1.804 | < 0.0001 |
| Male | 1.008 | 0.934-1.088 | 0.841 |
| Caucasian | 1.636 | 1.489-1.799 | < 0.0001 |
| Obesity | 3.616 | 3.318-3.940 | < 0.0001 |
| T2DM | 2.178 | 1.994-2.379 | < 0.0001 |
| Dyslipidemia | 2.346 | 2.121-2.596 | < 0.0001 |
| Hypertension | 1.326 | 1.201-1.465 | < 0.0001 |
| Metabolic syndrome | 4.782 | 4.782-5.460 | < 0.0001 |
| Hypothyroidism | 1.587 | 1.388-1.815 | < 0.0001 |
| Hypothyroidism on Thyroxine replacement therapy | 1.106 | 0.952-1.285 | 0.188 |
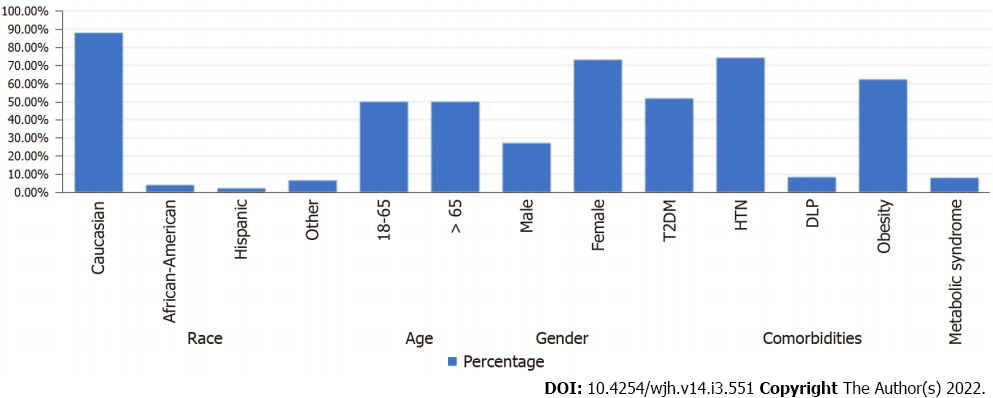
Baseline characteristics of patients in this database are described in Table 1. Among the 37648180 included individuals in this database who are above the age of 18 years, there were a total of 2320 patients with NAFLD in the period from 2015 to 2020. The 5-year period prevalence rate of NAFLD was 6.16 per 100000. Amongst those with NAFLD, 520 patients (22.4%) had hypothyroidism. Patients with NAFLD were also more likely to have obesity (OR, 3.616, 95%CI: 3.318-3.940), type 2 diabetes mellitus (OR, 2.178, 95%CI: 1.994-2.379), dyslipidemia (OR, 2.346, 95%CI: 2.121-2.596), hypertension (OR, 1.326, 95%CI: 1.201-1.465), and metabolic syndrome (OR, 4.782, 95%CI: 4.782-5.460) (Table 2). Males (OR, 1.008, 95%CI: 0.934-1.088) and females were equally affected, but the results were statistically insignificant. Patients in the age group 18-65 years (OR, 1.658, 95%CI: 1.524-1.804) as well as Caucasians (OR, 1.63, 95%CI: 1.489-1.799) seem to be at a higher risk. There was an increased risk of NAFLD among patients with hypothyroidism (OR, 1.587, 95%CI: 1.388-1.815). Furthermore, thyroid hormone replacement was not associated with a decreased risk for developing NAFLD (OR, 1.106, 95%CI: 0.952-1.285, P = 0.303). Characteristics of patients with NAFLD and hypothyroidism are shown in Figure 1.
Over the last couple of decades, NAFLD has emerged as one of the most common causes of chronic liver disease, including cryptogenic cirrhosis across the globe[7-9]. Risk stratification for NAFLD has become a focus of research because of the close relationship with different metabolic syndromes like T2DM, DLP, obesity, polycystic ovarian syndrome, and thyroid disorders. Albeit the overlap of complex metabolic pathophysiology of NAFLD and thyroid function remains controversial, many studies have suggested a strong association between the two[10-12].
The underlying pathophysiological mechanism of NAFLD has not been well explained. Still, the most commonly accepted theory implicates insulin resistance as the central role in developing hepatic steatosis and perhaps steatohepatitis[13,14]. Thyroid hormone has a vital role in cell metabolism and energy hemostasis. Thyroid dysfunction is associated with many diseases, for instance, cardiovascular disease, obesity, dementia, fracture, and recently NAFLD[15]. Thyroid hormones impact various metabolic pathways, and evidence corroborates the association of thyroid dysfunction and the pathogenesis of NAFLD. The two most telltale signs of the NAFLD disease spectrum are insulin resistance and hepatic lipid dysregulation[16]. Thyroid hormones (T3 and T4) use intracellular receptor signaling pathways in the liver to induce lipid metabolism. Even though molecular pathways leading to insulin resistance are complex and have not been completely elucidated, the association between thyroid dysfunction, both overt and subclinical hypothyroidism, and NAFLD has been extensively reported.
For example, a population-based study by Chung et al[12] showed that the prevalence of NAFLD and elevated liver enzymes were higher in a patient with hypothyroidism (OR: 1.38; 95%CI: 1.17-1.62) and confirmed a relevant dose-dependent clinal relationship between NAFLD and thyroid hormones. Moreover, thyroid hormones level has been shown to exert an effect in all the spectrum of steatosis. For instance, the exciting case-control comparative study by Pagadala et al[5] for the prevalence of hypothyroidism in NAFLD and NASH showed that hypothyroidism was more common in patients with NASH than patients with NAFLD (25% vs 12.8%, P = 0.03).
Another study from the western region of India by Parikh et al[17] reported a prevalence of 16.8% hypothyroidism in NAFLD patients with a strong clinically significant association amongst two diseases (OR, 14.94, 95%CI: 3.5-62.6). Authors also concluded that steatohepatitis was found to be more common in hypothyroid individuals as compared to controls (OR 3.9, 95%CI: 1.2-11.1). Ludwig et al[18] did a population-based cross-sectional study of 1276 participants which showed an increased prevalence of hepatic steatosis in subjects with reduced thyroid hormones (P = 0.0143; P ≤ 0.0001).
Since hypothyroidism and NAFLD share numerous characteristics, including weight gain, whether hypothyroidism is a risk factor for NAFLD remains difficult to answer in retrospective studies (AA1). To provide stronger evidence of the causality relationship, Bano et al[19] conducted a prospective cohort study of 9419 patients followed over ten years and observed the effects of hypothyroidism in NAFLD patients, and found a 1.24-fold higher NAFLD risk (95%CI: 1.01-1.53) in patients with hypothyroidism. Another recent descriptive cross-sectional study by Martínez-Escudé et al[20] reported a significantly higher prevalence of NAFLD and liver fibrosis in subjects with TSH ≥ 2.5 (μIU/mL). Also, in a comparative study of 1773 euthyroid participants, both TSH and levels Free T3 Level were found to be positively associated with the risk of NAFLD when diagnosed by ultrasound and fatty liver index, respectively[21]. Finally, a recent metanalysis found that overall hypothyroidism has a positive association with the risk of NAFLD[22].
Along with these well-crafted studies, some substantial evidences have questioned the exact association between NAFLD and thyroid regulation. A recent Spanish study reported no association between hypothyroidism and NAFLD[23]. The authors observed that thyroid hormone level was not associated with a higher prevalence of NAFLD. Similarly, in a study by Lee et al[23], the authors found no relationship of increased incidence of NAFLD in patients with the subclinical or overt types of hypothyroidism.
Many of the studies describing the relationship between these two entities were largely limited by the sample size. To fill this gap, we conducted one of the largest nationwide multicenter studies which screened 37648180 individuals, among which 520 individuals had concomitant NAFLD and hypothyroidism. Our retrospective cohort study has shown that hypothyroidism is an independent risk factor for NAFLD, and that about 1 in every 5 patients with NAFLD have concomitant hypothyroidism (22.4%). Overall, this is one of the highest prevalence rates for NAFLD in hypothyroidism patients. Secondly, the effect of thyroid hormone replacement in hypothyroidism patients and its effect on NAFLD prevention has not been well explored. In a post hoc analysis of a randomized controlled trial for patients with subclinical hypothyroidism, the prevalence of NAFLD was reduced from 48.5% to 24.2% (P = 0.041) after 15 mo of thyroid hormone replacement, whereas the prevalence of NAFLD remained stable in the untreated group[24], however; this trial was limited by the small sample size of ~360 patients (AA2). Moreover, those who received thyroid hormone replacement therapy had higher weight loss, which can itself explain the prevalence change in the treated population. Our study failed to show a statistically significant NAFLD risk reduction among patients with hypothyroidism who are placed on thyroid hormone replacement (OR, 1.106, 95%CI: 0.952-1.285, P = 0.303) but the weight changes were difficult to assess. Without adequately powered prospective trials that also adjusts for weight changes, the question whether thyroid hormone replacement has a direct protective effect against NAFLD remains difficult to answer, and the appropriate duration for effective replacement therapy and the goals of treatment remain unclear (AA3).
One of the limitations in our study is that we could not analyze the diagnostic method used for assessing NAFLD and set cut-off values for diagnosing hypothyroidism, since these are SNOMED-CT coded diagnoses on identified patient’s charts. We also could not specify the exact degree at which hypothyroidism becomes a NAFLD risk factor (AA4). Also, we could not evaluate for how long have these patients with hypothyroidism been on thyroid hormone replacement therapy, and whether they have achieved the euthyroid state or not. More prospective trials are needed to answer this question.
Hypothyroidism seems to be an independent risk factor for the development of NAFLD demonstrated in retrospective and prospective studies. Some studies have suggested that thyroid hormone replacement can potentially prevent or reverse NAFLD, which is potentially caused by weight loss. However, our study showed that thyroid hormone replacement did not provide a statistically significant risk reduction. Further prospective studies are needed to assess the role of thyroid hormone replacement therapy in patients with NAFLD, the duration for effective treatment and the treatment goals.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide, and hypothyroidism has been identified as an independent risk factor. The available data are limited by small sample size and the effect of thyroid hormone replacement therapy is not well studied.
The main topics of this article is to give a focused analysis on the hypothyroidism and to assess whether it is an independent risk factor for the development of NAFLD by filling the small sample size gap in the literature, provide a review of the current medical literature in this field, and -most importantly- to evaluate the role of thyroid hormone replacement therapy in the prevention of the disease.
The objective of this case control study is to assess whether hypothyroidism is an independent risk factor for the development of NAFLD, to review the updated medical literature, and to assess the role of thyroid hormone replacement therapy in the prevention of the disease.
We used a validated multicenter database (Explorys Inc.) from pooled outpatient and inpatient records of 26 different healthcare systems, consisting of a total of 360 hospitals in the United States to collect our data. We evaluated a cohort of patients with hypothyroidism and NAFLD. Multivariate analysis was performed to adjust for confounding risk factors including hypertension (HTN), type 2 diabetes mellites (T2DM), dyslipidemia (DLP), obesity and metabolic syndrome. We evaluated a cohort of patients with hypothyroidism and NAFLD. Multivariate analysis was performed to adjust for confounding risk factors including HTN, T2DM, DLP, obesity and metabolic syndrome.
Among 37648180 in the database who are above the age of 18 years, a total of 2320 patients with NAFLD in the period from 2015 to 2020 were included. NAFLD prevalence was 6.16 per 100000, among which 520 patients (22.4%) had hypothyroidism. Patients with NAFLD were also more likely to have obesity, type 2 diabetes mellitus, dyslipidemia, hypertension, and metabolic syndrome. Males and females were equally affected, but the results were statistically insignificant. Patients in the age group 18-65 years as well as Caucasians seem to be at a higher risk. There was an independent increase in the risk of NAFLD among patients with hypothyroidism, and thyroid hormone replacement was not associated with a decreased risk for developing NAFLD. Prospective studies are needed to better delineate the role of thyroid hormone replacement therapy in these individual.
There was an independent increase in the risk of NAFLD among patients with hypothyroidism, and thyroid hormone replacement is not associated with a decreased risk for developing NAFLD. Other studies have shown a potential protective effect of thyroid hormone replacement therapy. Based on the conflicting results with the existing literature, further studies are needed to better investigate the relationship between thyroid hormone replacement therapy and NAFLD.
Future research should focus on assessing the degree of hypothyroidism that leads to NAFLD, and the role of thyroid hormone replacement therapy including the duration of treatment and the end-point goals.
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4022] [Article Influence: 502.8] [Reference Citation Analysis (2)] |
| 2. | Leslie T, Pawloski L, Kallman-Price J, Escheik C, Hossain N, Fang Y, Gerber LH, Younossi ZM. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann Hepatol. 2014;13:533-540. [PubMed] |
| 3. | Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, Younossi ZM. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (2)] |
| 4. | Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non-alcoholic steatohepatitis? J Clin Gastroenterol. 2003;37:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Pagadala MR, Zein CO, Dasarathy S, Yerian LM, Lopez R, McCullough AJ. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 6. | IBM Corporation. The IBM Explorys Platform: liberate your healthcare data. [Cited 28 Novemeber 2020]. Available from: https://www.ibm.com/downloads/cas/4P0QB9JN. |
| 7. | Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2721] [Article Influence: 123.7] [Reference Citation Analysis (3)] |
| 10. | He W, An X, Li L, Shao X, Li Q, Yao Q, Zhang JA. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front Endocrinol (Lausanne). 2017;8:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Feisa SV, Chopei IV. Subclinical hypothyroidism in patients with non-alcoholic fatty liver disease at the background of carbohydrate metabolism disorders. Wiad Lek. 2018;71:261-264. [PubMed] |
| 12. | Chung GE, Kim D, Kim W, Yim JY, Park MJ, Kim YJ, Yoon JH, Lee HS. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Anstee QM, McPherson S, Day CP. How big a problem is non-alcoholic fatty liver disease? BMJ. 2011;343:d3897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Marchesini G, Babini M. Nonalcoholic fatty liver disease and the metabolic syndrome. Minerva Cardioangiol. 2006;54:229-239. [PubMed] |
| 15. | Rieben C, Segna D, da Costa BR, Collet TH, Chaker L, Aubert CE, Baumgartner C, Almeida OP, Hogervorst E, Trompet S, Masaki K, Mooijaart SP, Gussekloo J, Peeters RP, Bauer DC, Aujesky D, Rodondi N. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: a Meta-Analysis of Prospective Cohort Studies. J Clin Endocrinol Metab. 2016;101:4945-4954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Dongiovanni P, Valenti L, Rametta R, Daly AK, Nobili V, Mozzi E, Leathart JB, Pietrobattista A, Burt AD, Maggioni M, Fracanzani AL, Lattuada E, Zappa MA, Roviaro G, Marchesini G, Day CP, Fargion S. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut. 2010;59:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Parikh P, Phadke A, Sawant P. Prevalence of hypothyroidism in nonalcoholic fatty liver disease in patients attending a tertiary hospital in western India. Indian J Gastroenterol. 2015;34:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ludwig U, Holzner D, Denzer C, Greinert A, Haenle MM, Oeztuerk S, Koenig W, Boehm BO, Mason RA, Kratzer W, Graeter T; EMIL-Study. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord. 2015;15:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Bano A, Chaker L, Plompen EP, Hofman A, Dehghan A, Franco OH, Janssen HL, Darwish Murad S, Peeters RP. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J Clin Endocrinol Metab. 2016;101:3204-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Martínez-Escudé A, Pera G, Costa-Garrido A, Rodríguez L, Arteaga I, Expósito-Martínez C, Torán-Monserrat P, Caballería L. TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Wang W, Yu X, Qi X. Thyroid Function and Risk of Non-Alcoholic Fatty Liver Disease in Euthyroid Subjects. Ann Hepatol. 2018;17:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Zeng X, Li B, Zou Y. The relationship between non-alcoholic fatty liver disease and hypothyroidism: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e25738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Lee KW, Bang KB, Rhee EJ, Kwon HJ, Lee MY, Cho YK. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study. Clin Mol Hepatol. 2015;21:372-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Liu L, Yu Y, Zhao M, Zheng D, Zhang X, Guan Q, Xu C, Gao L, Zhao J, Zhang H. Benefits of Levothyroxine Replacement Therapy on Nonalcoholic Fatty Liver Disease in Subclinical Hypothyroidism Patients. Int J Endocrinol. 2017;2017:5753039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li H, China; Pham TTT, Viet Nam S-Editor: Zhang H L-Editor: A P-Editor: Zhang H