Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.482
Peer-review started: October 12, 2021
First decision: December 2, 2021
Revised: December 15, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: March 27, 2022
Processing time: 162 Days and 23.5 Hours
Hepatitis E virus (HEV) originally identified as a cause of acute icteric hepatitis in developing countries has grown to be a cause of zoonotic viral hepatitis in developed countries such as the United States. While there are eight identified genotypes to date, genotype 1 (HEV1), HEV2, HEV3, HEV4 are the most common to infect humans. HEV1 and HEV2 are most common in developing countries including Latina America, Africa and Asia, and are commonly transmitted through contaminated water supplies leading to regional outbreaks. In contrast HEV3 and HEV4 circulate freely in many mammalian animals and can lead to occasional transmission to humans through fecal contamination or consumption of undercooked meat. The incidence and prevalence of HEV in the United States is undetermined given the absence of FDA approved serological assays and the lack of commercially available testing. In majority of cases, HEV infection is a self-limiting hepatitis requiring only symptomatic treatment. However, this is not the case in immunocompromised individuals, including those that have undergone solid organ or stem cell transplantation. In this subset of patients, chronic infection can be life threatening as hepatic insult can lead to inflammation and fibrosis with subsequent cirrhosis and death. The need for re-transplantation as a result of post-transplant hepatitis is of great concern. In addition, there have been many reported incidents of extrahepatic manifestations, for which the exact mechanisms remain to be elucidated. The cornerstone of treatment in immunocompromised solid organ transplant recipients is reduction of immunosuppressive therapies, while attempting to minimize the risk of organ rejection. Subsequent treatment options include ribavirin, and pegylated interferon alpha in those who have demonstrated ribavirin resistance. Further investigation assessing safety and efficacy of anti-viral therapy is imperative given the rising global health burden. Given this concern, vaccination has been approved in China with other investigations underway throughout the world. In this review we introduce the epidemiology, diagnosis, clinical manifestations, and treatment of HEV, with emphasis on immunocompromised individuals in the United States.
Core Tip: Hepatitis E Virus is a leading cause of acute icteric hepatitis in developing countries. Despite being self- limiting in most cases, immunocompromised individuals are at a risk of chronic hepatitis, which can be life threatening. Hallmark of treatment includes reduction of immunosuppressive therapies followed by possible need of anti-viral therapy, which has shown to be ineffective.
- Citation: Damiris K, Aghaie Meybodi M, Niazi M, Pyrsopoulos N. Hepatitis E in immunocompromised individuals. World J Hepatol 2022; 14(3): 482-494
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/482.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.482
Hepatitis E Virus (HEV) was first reported as a non-A, non-B hepatitis in 1980 (another distinct type from post transfusion non-A, non B hepatitis), causing epidemic water-borne acute hepatitis[1]. Although significant improvements have occurred regarding virology, epidemiology, diagnosis, prevention, and treatment of hepatitis E, it is still the leading cause of acute icteric hepatitis in developing countries[2]. Previously, HEV was known as epidemic viral hepatitis in endemic areas. Currently, it is also identified as a zoonotic viral hepatitis in developed countries[3]. Blood transfusions and tissue transplantation are recognized as new routes for virus transmission worldwide[4].
HEV can cause a wide range of clinical manifestations, including acute hepatitis that can be self-resolving, chronic hepatitis (mostly in immunocompromised patients), and extrahepatic manifestations including renal and neurologic symptoms and complications[3]. Despite the development of several serological tests, screening and diagnosis of HEV is still challenging. In most cases, HEV infection is a self-limiting disease despite any treatment. Treatment of acute HEV at times can be imperative, especially in immunocompromised patients, as it can decrease the risk of chronic hepatitis, cirrhosis, and subsequently death[5]. A potentially effective vaccination strategy has been developed for HEV prevention, altering the incidence in Asian countries[6]. The World Health Organization (WHO) recommends consideration of vaccination for high-risk patients such as pregnant women[7]. In this review we summarize the epidemiology of Hepatitis E in the United States, review the clinical manifestations and treatment options with emphasis on their implications in immunocompromised individuals.
HEV is one of the leading causes of viral-induced acute liver failure worldwide[8]. HEV1, HEV2, HEV3, and HEV4 are the main genotypes which have clinical implications on humans. Genotypes 1 and 2 cause infection in hyperendemic areas such as Asia, Africa, Mexico, and the Middle East (Table 1). Humans are the main reservoir for these genotypes and contamination of drinking water supplies with human feces is the main route of transmission. Consequently, endemics can emerge after heavy rainfall and flooding[9,10]. Transmission through blood transfusion[11] and vertical transmission[12] are well documented for sporadic infection worldwide.
| HEV1, HEV2 | HEV3, HEV4 | |
| Geography | Developing countries (Asia, Africa, and South America) | Developed countries (Europe, United states, Japan, and Hong Kong) |
| Disease pattern | Endemic | Sporadic |
| Seasonal pattern | Yes | No |
| Reservoir | Only human | Animals (Pigs, wild boars, deer) |
| Transmission | Fecal-oral | Food-born, blood products, transplantation |
| Age | More common among young adult | More common among older adults |
| Risk factor | Chronic liver disease, pregnancy | Chronic liver disease, immunocompromised |
| Safety measure | Clean water, sanitation, and hygiene | Avoid contact with high-risk animals, cook meat adequately |
| Chronic infection | Not reported | In immunocompromised patients. |
HEV3 and HEV4 are the most prevalent genotypes in industrialized countries[13]. In contrast to HEV1 and HEV2, genotypes 3 and 4 can infect both humans and animals. Pigs, wild boars, and deer are identified as the reservoirs for these genotypes[14]. Transmission by consuming raw or undercooked meat, or close contact with the infected animal is responsible for autochthonous infection[15,16]. There is only one case report of HEV7 infection in humans who regularly consumed camel meat and milk in the United Arab Emirates[17].
The precise incidence and prevalence of HEV infection in the United States is undetermined. HEV is not amongst the nationally notifiable diseases leaving systematic collection, analysis, and evaluation of HEV data a challenge. The absence of sensitive and specific FDA-approved serology assays poses another obstacle in assessing the incidence of HEV in the United States[18]. The lack of commercially available tests also leads to misdiagnosed HEV infection at alarming rates. Reviewing several national drug-induced liver injuries (DILI) registries revealed HEV infection as the true cause of liver injury in patients initially diagnosed with DILI[19].
National Inpatient Sample (NIS) data from Healthcare Cost and Utilization Project showed the rate of hospitalization due to hepatitis E increased from 3.7 per 10 million in 2010 to 6.4 per 10 million in 2015. Although hospitalization is still low in the United States, the increasing rate is worrisome[20]. The National Health and Nutrition Examination Survey (NHANES) data also demonstrated an increase in HEV seropositivity (IgG/IgM) from 5% in 2013-2014 to 7.7% in 2015-2016. Simultaneously, the rate of IgM seropositivity (recent infection) almost doubled in US-born individuals[21]. The multivariate logistic regression model identified a strong association of HEV seropositivity with aging, female gender, and non-Hispanic Asian ethnicities[21]. Testing serum samples from 681 adult Americans with acute liver failure (ALF) revealed a low rate of acute HEV infection (0.04%) in this population. However, the rate of positive anti-HEV IgG (signifying prior exposure) was significantly higher in the ALF patients than in the general US population[22].
All autochthonous HEV infections detected in the United States are caused by HEV genotype 3. Caitlin reported the risk of anti-HEV seropositivity in people who consumed undercooked meat was 12.9 times higher than the general population. This observation confirmed undercooked meat as a route of zoonotic HEV infection in the US[23]. In one study, serum samples from pigs at 25 slaughterhouses in 10 states were tested for HEV infection. HEV RNA and anti-HEV seropositivity was 6.3% and 40%, respectively. Blood of HEV RNA-positive pigs potentially can contaminate slaughterhouses' supply chains, making it a key source of infection control[24]. A recent study suggested consuming self-grown food as another possible source for zoonotic HEV infection[25].
Ticehurst et al[26] reported the possible HEV transmission through blood transfusion for the first time in the United States. A random sample from 5040 blood donations showed 11.4% and 1.8% positive anti-HEV IgG and anti-HEV IgM, respectively[27]. Stramer et al[28] reported two positive HEV RNA among 18829 samples of blood donated from six geographic regions. Despite low contamination rates, they suggested providing HEV-negative blood for patients at risk of developing hepatitis, such as severely immunosuppressed patients. Among 128,020 samples of plasma from 27 states, the prevalence of HEV RNA positivity was reported at 0.002%. Therefore, routine screening for HEV contamination in plasma donation was not suggested[29]. Several countries are considering HEV screening in blood donors. Delage et al[30]evaluated cost-benefit and the quantitative risk of blood donation screening for HEV infection in the United States. Due to the lower rate of HEV in North America, HEV blood donation screening will be more expensive than in other countries, and have minimal clinical benefits.
For the first time in the US, Kuniholm et al[31] reported a chronic HEV infection in an HIV-positive patient. They also confirmed that chronic infection could persevere even with a CD4+ count > 200 cells/mm3[31]. Assessing 311 patients who received allografts revealed 4% posttransplant HEV infection. Although no chronic infection was reported, developing posttransplant infection was associated with graft rejection[32]. A recent study on 145 post-liver transplant patients with a history of hepatitis C virus (HCV) infection showed 6 (4.1%) patients developing anti-HEV IgM antibodies in 5 years. All samples were negative for HEV RNA. Treatment of HCV with Interferon and Ribavirin may contribute in clearance of HEV infection[33].
The majority of acute HEV infections are asymptomatic or can cause minor nonspecific systemic illness, that is often self-limiting. It has been estimated that approximately 5-30% of patients acutely infected go on to develop acute icteric hepatitis[34]. Acute icteric hepatitis is characterized by malaise, fever, body aches, anorexia, nausea and vomiting, which occurs for about a one-week period of time classified as the prodromal phase. Following the prodromal phase, patients enter the icteric phase characterized by jaundice and dark urine, which can be coupled with a marked increase in aminotransferases (greater than 8-10 time the upper limit of normal) and a variable degree of hyperbilirubinemia[35]. These symptoms collectively resolve over the course of a few days to weeks, marking the convalescent phase.
In a small percentage of patients, the acute icteric phase can progress to acute liver failure (ALF) or acute on chronic liver failure (ACLF) in those with underlying chronic liver disease[36]. Pregnant women are of particular risk to developing ALF during their second and third trimester, with mortality rate of nearly 25% as a result of hepatic failure or obstetric complications[37]. ACLF is defined by the European Association for the Study of Liver Diseases (EASL) as acute deterioration of pre-existing chronic liver disease usually related to a precipitating event and is associated with increased 28-day mortality due to multi-system organ failure[38]. Typical manifestations include acute worsening of liver function with complications such as worsening ascites, hepatic encephalopathy or coagulopathy[36]. The impact of acute HEV infection in patients with chronic liver disease in the United States has been reported. In a study conducted by Kyvernitakis et al[39], 11% of 115 patients with chronic HCV infection diagnosed with cancer were positive for HEV IgG. Seropositivity was significantly associated with older age, place of birth outside the United States, cirrhosis, and history of reused needles/syringes during vaccination[39]. In another study, HEV related ALF was assessed in 681 adults with ALF by testing for anti HEV IgM, IgG and HEV-RNA. A total of three men demonstrated repeatedly detectable anti HEV IgM, but negative HEV RNA, signifying rarity of acute HEV infection in ALF patients (0.4%). 43.4% of ALF patients tested positive for anti HEV IgG, with prevalence being highest from the Midwest and in those of older age[22]. There has also been documentation of a fatal hepatic decompensation caused by HEV4 in an orthotopic liver transplant recipient following a prolonged visit to Hong Kong[40]. In another prospective study in the United States, HEV infection was noted to contribute to a small but important percentage of cases of acute liver injury that was initially suspected to be caused by drug induced liver injury[41].
Chronic HEV infection in solid organ transplant (SOT) recipients can be defined as HEV replication (viremia) present for more than 3 mo after the onset of infection[42]. Chronic infection was initially reported by Kamar et al[43] in 2008, when patients who received kidney or liver transplants developed a persistent increase in aminotransferase levels, evidence of histological activity, and liver fibrosis during follow-up after acute HEV. It has been suggested that up to 66% of SOT recipients exposed to HEV go on to develop chronic infection, which is mostly asymptomatic but can most commonly include fatigue and or mild to moderate aminotransferase rise, diarrhea and arthralgias[44]. Chronic infection has been most commonly reported with HEV3 infection[45] however, there have been reports of persistent hepatitis when infected with HEV4[46].
Prevalence of post liver transplant HEV infection in non-endemic regions has been estimated to be between 1% and 2%[47]. Chronic HEV infection has been shown to cause structural injury to the liver including formation of nodules, fibrotic changes and subsequent cirrhosis[48], with reports that approximately 10% of those who develop chronic infection progress to cirrhosis within 2-5 years[49]. Injury caused by viral infection including inflammation has been shown to regress following the clearance of HEV[50]. In persons with prior liver transplantation, chronic infection can result in post-transplant hepatitis, rapid progression to cirrhosis and liver failure, and even the need for re-transplantation which can lead to recurrence of HEV infection in the newly transplanted liver[51].
The effects of chronic HEV can be seen beyond those with SOT, affecting various immunocompromised individuals. Chronic infection has been reported in an individual with non-Hodgkin’s lymphoma undergoing treatment[52], and in stem cell transplant recipients on immunosuppression[53]. International studies have demonstrated significantly greater seroprevalence of IgG and IgM antibodies in cancer patients[54], and reported self-resolving acute infection, and even the need for ribavirin treatment in patients with gynecological malignancies treated with chemotherapy[55]. Such findings should spark further investigation when treating cancer patients with elevated transaminases. Chronic infection has also been seen in patients with human immunodeficiency virus with low CD4+ cell count of less than 200[56,57]. Rheumatological patients receiving mild immunosuppressive treatments are also at increased risk of chronic infection[58].
Infection with HEV can lead to a variety of extrahepatic manifestations including neurological, hematological, renal, and other immune-mediated manifestations. The exact mechanism remains to be elucidated, and suggestions include cross reactions between viral epitopes and self-antigens in tissues, and possible viral replication in other non-hepatic tissues[59].
Neurological manifestations are the most commonly encountered, and include Guillain- Barré syndrome (GBS), neuralgic amyotrophy (NA), encephalitis, myelitis, myositis, vestibular neuritis, peripheral neuritis, and Bell’s palsy[60]. In a European study, 16.5% of HEV infected patients reported neurological manifestation, which were more common in immunocompetent patients compared to immunosuppressed individuals (22.6% vs 3.2%, P < 0.001)[61]. GBS can occur both after acute or chronic infection with various HEV genotypes and is the most frequently described extrahepatic manifestation[62]. In a case- control study from the Netherlands comparing GBS patients to healthy controls, the prevalence acute HEV was higher in GBS patients compared to controls (5% vs 0.5%)[63]. Similar manifestations were seen in a study from the United Kingdom and France, in which more than 5% of those infected with HEV3 developed neurological complications during follow-up[64]. NA is an acute and painful neuropathy in the upper extremity characterized by rapid multifocal motor weakness and sensory loss, followed by atrophy[65]. A cohort study from the United Kingdom and the Netherlands demonstrated that 10% of patients with NA had acute hepatitis E[66]. Central nervous system infections including encephalitis and meningitis have been described, with HEV RNA being present in the serum and cerebrospinal fluid in immunosuppressed individuals after SOT[62]. It remains unknown if these neurological manifestations are a result of immune mediated molecular mimicry or direct cytopathic effects of the virus[36]. Based on findings from a variety of studies, it is recommended that clinicians consider infection with HEV as a culprit when encountering patients with neurological disorders and concomitant elevations in liver enzymes[67].
Renal manifestations of HEV include kidney injury, membranoproliferative glomerulonephritis and cryoglobulinemia. In a retrospective study assessing kidney function and histology in SOT recipients with HEV3 infection, there was a statistically significant decrease in glomerular filtration rate during infection (-5L/min, P = 0.04). Histological examination of those with high proteinuria and decreased GFR during both the acute and chronic phase of infection demonstrated relapse of IgA nephropathy, membranoproliferative glomerulonephritis, and the majority of patients having cryoglobulinemia that resolved after clearance of HEV[68]. The relationship between cryoglobulinemia and HEV infection remains unclear. In a study assessing SOT recipients infected with HEV, the prevalence of cryoglobulinemia was increased during chronic infection (52.9%) compared to the acute phase of infection (36.4%) and HEV negative SOT recipients (23.6%, P < 0.01); also identifying HEV as a predictive factor for cryoglobulinemia (odds ratio 2.3)[69]. Although the exact mechanism is unknown, it is possible that immune complex deposits may play a critical role, as seen in Hepatitis C infection where HCV antigen, anti-HCV IgG antibodies and rheumatoid factor deposit in glomeruli[70]
Over the years many hematological manifestations from HEV infection have been reported. One such manifestation is hemolytic anemia secondary to glucose-6-phosphate dehydrogenase deficiency, leading to oxidative stress in red blood cells during viral hepatitis infection. Several cases have been docu
| Organ/System | Manifestation |
| Neurological | Guillain- Barré syndrome, Bell’s palsy, myelitis, peripheral neuropathy, neuralgic amyotrophy, encephalitis, meningitis vestibular neuritis, mononeuritis multiplex, seizure, pseudotumor cerebri, oculomotor palsy, polyradiculoneuropathy |
| Hematological | Thrombocytopenia, hemolytic anemia, aplastic anemia, hemophagocytic syndrome, thrombotic thrombocytopenic purpura, Cutaneous T cell lymphoproliferative disorder, monoclonal gammopathy of uncertain significance |
| Cardiovascular | Myocarditis, Henoch-Schönlein purpura |
| Renal | Reduction in glomerular filtration rate, IgA nephropathy, cryoglobulinemia, membranoproliferative glomerulonephritis, membranous glomerulonephritis |
| Musculoskeletal | Myositis, polyarthritis |
| Thyroid | Autoimmune thyroiditis, subacute thyroiditis |
| Pancreas | Acute pancreatitis |
The incubation period of HEV is approximately 2 to 6 wk and precedes the IgM response detected during the same time that liver enzyme abnormalities arise. Diagnosis of HEV can be accomplished either directly by detecting the HEV RNA or capsid antigen in the blood and other body fluids or indirectly by detecting anti-HEV antibodies in infected individuals’ serum[79]. The detection of anti- HEV IgM antibody is an important marker of acute viral infection, and has a short positivity mostly ranging from 3-4 mo, but can be present for up to one year[36]. When testing for anti-HEV IgM with conventional assays and commercially available immunohistochemistry assays, sensitivity has been reported to be > 97% in immunocompetent patients and 80-85% for immunocompromised patients with > 99.5% specificity[80,81]. It is important to consider additional testing for RNA presence in immunocompromised individuals due to the poor antibody response exhibited by this population[13]. IgG antibody response is delayed and long lasting with persistence of several years, although the exact duration remains uncertain. In order to detect these antibodies, enzyme immunoassays are utilized with recombinant ORF2 and/or ORF3 proteins from HEV1 strains, which also cross react with other genotypes, however assay detection varies considerably[82]. Use of commercially available assays have limited detection which vary between 0.25 and 2.5 WHO units per ml, and the determination of anti-HEV IgG concentration can be used to estimate reinfection after natural infection or immunization[79]. It has been suggested that immunocompromised patients with anti-HEV IgG concentration < 7 WHO units per ml can become reinfected with increased risk of developing chronic hepatitis[83]. In addition, it has been suggested that anti-HEV IgG titers > 2.5 units per ml are protective following vaccination[84].
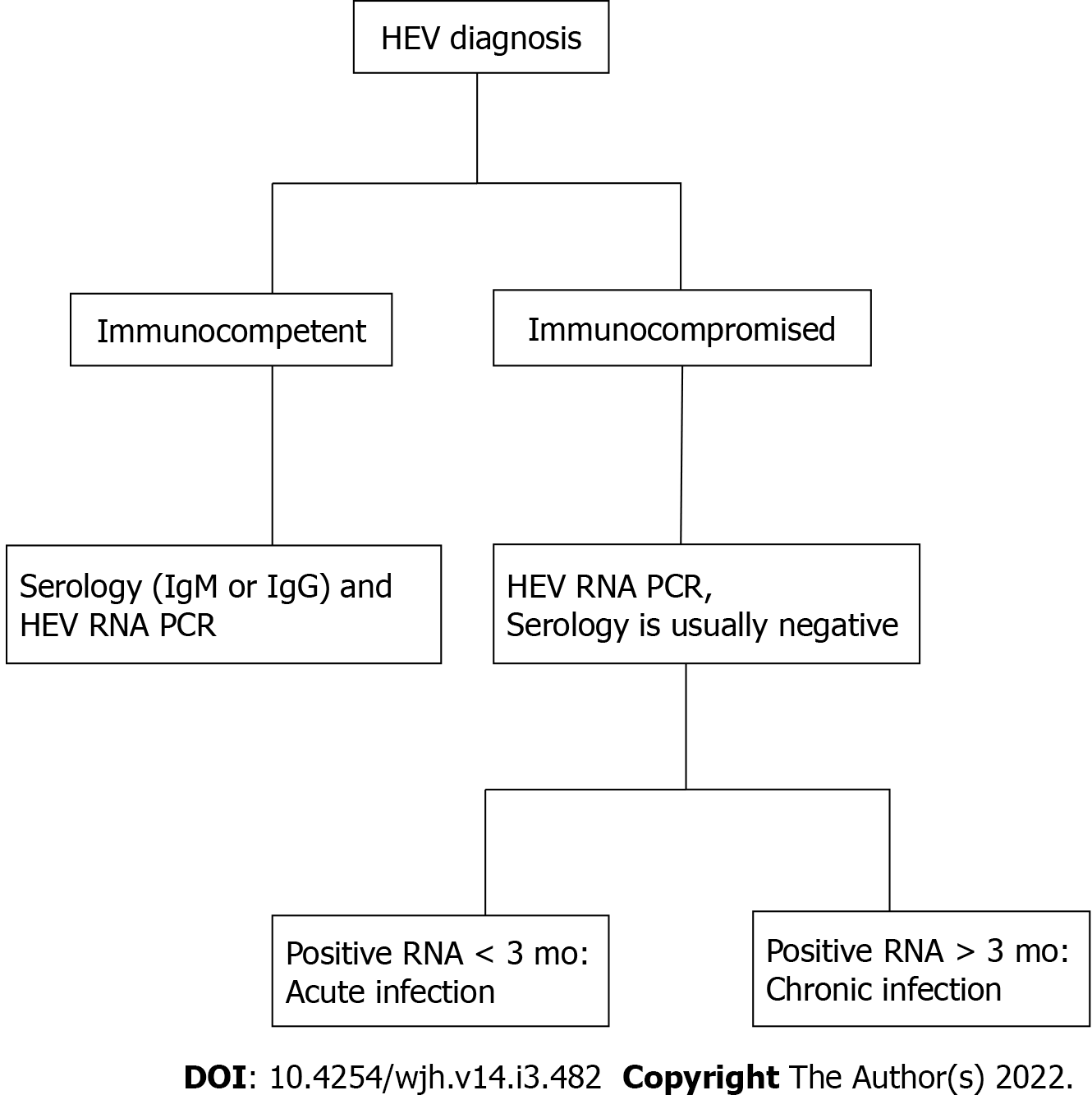
The detection and quantification of HEV RNA in blood and other bodily secretions is the gold standard of detecting both acute and chronic active HEV infection, adding benefit to diagnosis of infection in immunocompromised individuals with inherent poor immunologic response[36] (Figure 1). Other situations in which RNA detection is of great utility includes donor screening, diagnosis of chronic HEV infection, and assessing response to antiviral therapy[85]. HEV RNA becomes detectable during the incubation period and can be present in the blood for about 4 wk and 6 wk in feces[81]. Given the narrow window of detectable RNA, an undetectable HEV RNA does not exclude recent infection, particularly when patients present late in their illness[86]. Persistence of RNA for at least 3 mo defines chronic infection[42]. Available types of nucleic acid amplification tests (NAATs) include reverse transcription polymerase chain reaction (RT-PCR), real time RT-PCR, and reverse transcription loop-mediated isothermal amplification, with varying sensitivity in HEV RNA detection[36]. In response to varying sensitivities the World Health Organization (WHO) has developed the international standard and international reference panel for HEV1, HEV2, HEV3, and HEV4, allowing comparison of results obtained from different NAATs with reports using a common unit, the international unit (IU). NAATs detect HEV RNA targets, particularly conserved domains (ORF2 and ORF3 overlap region), of HEV genotypes 1-4[87].
Viral antigens are present in the blood and liver during the early phase of acute hepatitis persisting longer in chronic infection and can be diagnosed using sandwich enzyme immunoassays detecting HEV capsid antigen derived from ORF2[88]. HEV antigen assays have excellent specificity, however sensitivity is a major concern ranging from 40% to 91%[89]. It has been shown that HEV antigen may remain present for months following clearance of chronic HEV infection, suggesting the presence of antigen does not necessarily indicate presence of virions[90]. Given the simplicity, lesser cost and faster results when compared to HEV RNA detection, HEV capsid testing may become an alternative in diagnosis, however the role of HEV antigen diagnosis is yet to be determined[36,79].
Unlike in most immunocompetent individuals who require no specific treatment for acute HEV infection, chronic infection in immunocompromised hosts (i.e., solid organ transplant recipients) requires treatment to avoid rapid progression to cirrhosis or even death[5]. In SOT recipients, reduction of immunosuppressive therapies is considered the first line therapeutic option, with approximately one third of patients achieving viral clearance after dose reduction[44,50]. However, it is important to remember that reducing immunosuppression can lead to increased risk of organ rejection.
In a large retrospective multicenter case series, Kamar et al[42] assessed the efficacy of ribavirin in SOT recipients diagnosed with chronic hepatitis E and HEV viremia. A total of 59 (54 confirmed HEV genotype 3) patients were included of which 37 had received kidney transplants, 10 had liver transplants, 5 heart transplants, 5 combined kidney and pancreas transplants, and 2 patients had undergone lung transplantation. Median dosing of ribavirin was 600 mg/day for a median duration of 3 mo. Following treatment 95% of patients exhibited clearance of HEV and 78% exhibited sustained virological response (SVR). Although 60% of patients unfortunately developed recurrence, 40% of these individuals were able reach SVR following a prolonged treatment course of an additional 6 mo. Adverse events included anemia, requiring dose reduction in 29% of patients, and the use of erythropoietin and blood transfusion[91]. A more recent study conducted by Kamar et al[42], retrospectively investigated 30 European centers to assess outcomes of ribavirin therapy in 255 SOT recipients with chronic HEV3. 81% of patients achieved SVR with initial ribavirin treatment (median 600 mg/day for 3 mo), while 90% were able to achieve SVR following an additional course of treatment after initially failing to meet SVR. Interestingly it was also noted that an increased lymphocyte count at the initiation of treatment was a positive predictive factor of SVR, while poor hematological tolerance requiring dose reduction was associated with relapse after completion of therapy[92].
Treatment of chronic HEV in immunosuppressed individuals who have received SOT poses a challenge following lack of response to ribavirin. A final option includes treatment with pegylated interferon alpha (PEG-IFNa), which has been shown to be effective following liver transplantation. In a study of three post liver transplant patients, a three-month course of PEG-IFNa resulted in an antiviral response with HEV clearance was obtained in two of the study participants[93]. Similar findings were noted by Haagsma et al[94] who demonstrated efficacy of PEG-IFNa when reduction of immunosuppressive medications was not adequate. However, it is important to note that PEG-IFNa is contraindicated in lung, heart, renal and pancreas transplant recipients due to the risk of organ rejection[95].
Treatment of HEV in ribavirin resistant infections can be a challenge. Approval of sofosbuvir revolutionized the treatment of chronic hepatitis C and the role of sofosbuvir in the treatment of HEV has also been investigated. Based on in vitro studies, sofosbuvir has been considered as a treatment for ribavirin resistant HEV alone or synergistically with ribavirin[96]. Effectiveness of sofosbuvir has been shown to lead to viral clearance in acute HEV when used in combination with ribavirin[97] and for the treatment of refractory HEV in an individual following kidney transplantation[98]. However, other studies have demonstrated inability to reach SVR when treated with combination therapy in a patient with chronic HEV (genotype 3) following multivisceral organ transplantation[99]. A recent case series of 3 SOT recipients treated with combination of sofosbuvir and ribavirin following failed ribavirin monotherapy (inability to achieve SVR) displayed failure of complete elimination of HEV. RNA plasma levels returned to pretreatment levels following cessation of therapy, suggesting antiviral activity of combination therapy[100]. Monotherapy with sofosbuvir has also been shown to be ineffective with high rates of relapse following only partial response in individuals with chronic HEV[101]. To date none of the mentioned drugs have been approved in the treatment of HEV, and further large-scale studies are indicated to assess safety and efficacy, alone or in combination. Although many clinical trials are actively investigating efficacy of vaccine prevention, there is limited investigation on HEV treatment (clinicaltrials.gov).
Development of a safe and efficacious vaccine has shined light on the prevention of HEV and subsequent worldwide morbidity and mortality. Zhu et al[102] published results of a randomized, double blind phase 3 trial of recombinant HEV vaccine (HEV 239: Hecolin®) administered in 3 doses at 0,1 and 6 mo in China. Results demonstrated a near 100% efficacy, with no serious adverse effects at 12 mo follow-up after vaccine administration[102]. Long term efficacy of up to 4.5 years displayed continuous efficacy of 87%, and cross protective efficacy between genotype HEV1 and HEV4 which are prevalent in China[103]. Currently a large, cluster-randomized, blinded trial (NCT02759991) is investigating the effectiveness of Hecolin in pregnant women in Bangladesh[104]. It has been recommended that vaccination against HEV in certain high-risk individuals such as those who are immunocompromised, have chronic liver disease, pregnant women in endemic areas, and those in hyperendemic parts of the world[81]. Further studies are urgently needed to investigate vaccine efficacy toward other prevalent genotypes and to assess safety and efficacy in those with aforementioned underlying chronic medical conditions prior to being garnered approval beyond China. Recently a single investigation was completed in the United States, assessing Hecolin® safety, reactogenicity and immunogenicity in healthy adults (NCT03827395), for which we eagerly await results.
Hepatitis E infection is a major global health burden that leads to extensive morbidity and mortality, particularly in developing countries. While most cases of acute HEV infection are self-limiting and only require symptomatic treatment, progression to chronic disease can be fatal. Individuals particularly at risk for chronic infection include solid organ transplant recipients and those with other immunosuppressive conditions such as HIV and rheumatological conditions. Elevation in liver enzymes in the immunosuppressed should prompt urgent serological testing coupled with HEV RNA detection, given inherent poor immunological response. The initial hallmark to treatment is the reduction of immunosuppressive therapies to allow physiological defense and viral clearance. Subsequent treatment options include ribavirin; however, resistance poses a challenge as other treatment options can be harmful to SOT recipients. While vaccine development has proven to be effective, it is imperative that we continue to assure clean drinking water and safe food practices worldwide. Further clinical investigations are essential in order to help develop safe and efficacious viral treatments that can save millions of lives worldwide.
| 1. | Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 429] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9742] [Article Influence: 695.9] [Reference Citation Analysis (0)] |
| 3. | Pischke S, Hartl J, Pas SD, Lohse AW, Jacobs BC, Van der Eijk AA. Hepatitis E virus: Infection beyond the liver? J Hepatol. 2017;66:1082-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 499] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, Teo CG. Transfusion-transmitted hepatitis E in a 'nonhyperendemic' country. Transfus Med. 2006;16:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Guerra JAAA, Kampa KC, Morsoletto DGB, Junior AP, Ivantes CAP. Hepatitis E: A Literature Review. J Clin Transl Hepatol. 2017;5:376-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Koyuncu A, Mapemba D, Ciglenecki I, Gurley ES, Azman AS. Setting a Course for Preventing Hepatitis E in Low and Lower-Middle-Income Countries: A Systematic Review of Burden and Risk Factors. Open Forum Infect Dis. 2021;8:ofab178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | WHO. Hepatitis E vaccine: WHO position paper, May 2015--Recommendations. Vaccine. 2016;34:304-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Patterson J, Hussey HS, Silal S, Goddard L, Setshedi M, Spearman W, Hussey GD, Kagina BM, Muloiwa R. Systematic review of the global epidemiology of viral-induced acute liver failure. BMJ Open. 2020;10:e037473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597-604. [PubMed] |
| 10. | VISWANATHAN R. A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res. 1957;45:145-155. [PubMed] |
| 11. | Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat. 2009;16:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Khuroo MS, Khuroo MS, Khuroo NS. Hepatitis E: Discovery, global impact, control and cure. World J Gastroenterol. 2016;22:7030-7045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (3)] |
| 14. | Doceul V, Bagdassarian E, Demange A, Pavio N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Chaussade H, Rigaud E, Allix A, Carpentier A, Touzé A, Delzescaux D, Choutet P, Garcia-Bonnet N, Coursaget P. Hepatitis E virus seroprevalence and risk factors for individuals in working contact with animals. J Clin Virol. 2013;58:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Said B, Ijaz S, Chand MA, Kafatos G, Tedder R, Morgan D. Hepatitis E virus in England and Wales: indigenous infection is associated with the consumption of processed pork products. Epidemiol Infect. 2014;142:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK, Purdy MA, Teo CG. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355-7.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 18. | Hofmeister MG, Foster MA, Teshale EH. Epidemiology and Transmission of Hepatitis A Virus and Hepatitis E Virus Infections in the United States. Cold Spring Harb Perspect Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Grewal P, Ahmad J. Beware of HCV and HEV in Patients with Suspected Drug-Induced Liver Injury. Curr Hepatol Rep. 2018;17:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Wasuwanich P, Ingviya T, Thawillarp S, Teshale EH, Kamili S, Crino JP, Scheimann AO, Argani C, Karnsakul W. Hepatitis E-Associated Hospitalizations in the United States: 2010-2015 and 2015-2017. J Viral Hepat. 2021;28:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Cangin C, Focht B, Harris R, Strunk JA. Hepatitis E seroprevalence in the United States: Results for immunoglobulins IGG and IGM. J Med Virol. 2019;91:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Fontana RJ, Engle RE, Scaglione S, Araya V, Shaikh O, Tillman H, Attar N, Purcell RH, Lee WM; US Acute Liver Failure Study Group. The role of hepatitis E virus infection in adult Americans with acute liver failure. Hepatology. 2016;64:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Cossaboom CM, Heffron CL, Cao D, Yugo DM, Houk-Miles AE, Lindsay DS, Zajac AM, Bertke AS, Elvinger F, Meng XJ. Risk factors and sources of foodborne hepatitis E virus infection in the United States. J Med Virol. 2016;88:1641-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Sooryanarain H, Heffron CL, Hill DE, Fredericks J, Rosenthal BM, Werre SR, Opriessnig T, Meng XJ. Hepatitis E Virus in Pigs from Slaughterhouses, United States, 2017-2019. Emerg Infect Dis. 2020;26:354-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Diehl TM, Adams DJ, Nylund CM. Ingesting Self-Grown Produce and Seropositivity for Hepatitis E in the United States. Gastroenterol Res Pract. 2018;2018:7980413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Ticehurst JR, Pisanic N, Forman MS, Ordak C, Heaney CD, Ong E, Linnen JM, Ness PM, Guo N, Shan H, Nelson KE. Probable transmission of hepatitis E virus (HEV) via transfusion in the United States. Transfusion. 2019;59:1024-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Zafrullah M, Zhang X, Tran C, Nguyen M, Kamili S, Purdy MA, Stramer SL. Disparities in detection of antibodies against hepatitis E virus in US blood donor samples using commercial assays. Transfusion. 2018;58:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Stramer SL, Moritz ED, Foster GA, Ong E, Linnen JM, Hogema BM, Mak M, Chia CP, Dodd RY. Hepatitis E virus: seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion. 2016;56:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Roth NJ, Schäfer W, Alexander R, Elliott K, Elliott-Browne W, Knowles J, Wenzel JJ, Simon TL. Low hepatitis E virus RNA prevalence in a large-scale survey of United States source plasma donors. Transfusion. 2017;57:2958-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Delage G, Fearon M, Gregoire Y, Hogema BM, Custer B, Scalia V, Hawes G, Bernier F, Nguyen ML, Stramer SL. Hepatitis E Virus Infection in Blood Donors and Risk to Patients in the United States and Canada. Transfus Med Rev. 2019;33:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 31. | Kuniholm MH, Ong E, Hogema BM, Koppelman M, Anastos K, Peters MG, Seaberg EC, Chen Y, Nelson KE, Linnen JM. Acute and Chronic Hepatitis E Virus Infection in Human Immunodeficiency Virus-Infected U.S. Women. Hepatology. 2016;63:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Sue PK, Pisanic N, Heaney CD, Forman M, Valsamakis A, Jackson AM, Ticehurst JR, Montgomery RA, Schwarz KB, Nelson KE, Karnsakul W. Hepatitis E Virus Infection Among Solid Organ Transplant Recipients at a North American Transplant Center. Open Forum Infect Dis. 2016;3:ofw006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Koning L, Charlton MR, Pas SD, Heimbach JK, Osterhaus AD, Watt KD, Janssen HL, de Knegt RJ, van der Eijk AA. Prevalence and clinical consequences of Hepatitis E in patients who underwent liver transplantation for chronic Hepatitis C in the United States. BMC Infect Dis. 2015;15:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Lhomme S, Marion O, Abravanel F, Izopet J, Kamar N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 35. | Goel A, Aggarwal R. Hepatitis E: Epidemiology, Clinical Course, Prevention, and Treatment. Gastroenterol Clin North Am. 2020;49:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Aslan AT, Balaban HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26:5543-5560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (4)] |
| 37. | Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2279] [Article Influence: 175.3] [Reference Citation Analysis (6)] |
| 39. | Kyvernitakis A, Taremi M, Blechacz B, Hwang J, Jiang Y, Mahale P, Torres HA. Impact of hepatitis E virus seropositivity on chronic liver disease in cancer patients with hepatitis C virus infection. Hepatol Res. 2015;45:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Perumpail RB, Ahmed A, Higgins JP, So SK, Cochran JL, Drobeniuc J, Mixson-Hayden TR, Teo CG. Fatal Accelerated Cirrhosis after Imported HEV Genotype 4 Infection. Emerg Infect Dis. 2015;21:1679-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH, Tillmann HL, Gu J, Serrano J, Hoofnagle JH; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665-72.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 42. | Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am J Transplant. 2013;13:1935-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 44. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 45. | Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 46. | Geng Y, Zhang H, Huang W, J Harrison T, Geng K, Li Z, Wang Y. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat Mon. 2014;14:e15618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Haagsma EB, Niesters HG, van den Berg AP, Riezebos-Brilman A, Porte RJ, Vennema H, Reimerink JH, Koopmans MP. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2009;15:1225-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 49. | Kamar N, Mansuy JM, Cointault O, Selves J, Abravanel F, Danjoux M, Otal P, Esposito L, Durand D, Izopet J, Rostaing L. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant. 2008;8:1744-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssière L, Cointault O, Ribes D, Cardeau I, Nogier MB, Mansuy JM, Muscari F, Peron JM, Izopet J, Rostaing L. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Haagsma EB, van den Berg AP, Porte RJ, Benne CA, Vennema H, Reimerink JH, Koopmans MP. Chronic hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2008;14:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 52. | Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150:430-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | le Coutre P, Meisel H, Hofmann J, Röcken C, Vuong GL, Neuburger S, Hemmati PG, Dörken B, Arnold R. Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation. Gut. 2009;58:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Bai MJ, Zhou N, Dong W, Li GX, Cong W, Zhu XQ. Seroprevalence and risk factors of hepatitis E virus infection in cancer patients in eastern China. Int J Infect Dis. 2018;71:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Bettinger D, Schlabe S, Pischke S, Mallmann MR, Keyver-Paik MD, Kuhn W, Strassburg CP, Thimme R, Spengler U. Ribavirin in Acute Hepatitis E Infection in Patients with Gynecological Cancer: A Case Series. J Clin Transl Hepatol. 2018;6:237-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Jagjit Singh GK, Ijaz S, Rockwood N, Farnworth SP, Devitt E, Atkins M, Tedder R, Nelson M. Chronic Hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect. 2013;66:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Colson P, Dhiver C, Poizot-Martin I, Tamalet C, Gérolami R. Acute and chronic hepatitis E in patients infected with human immunodeficiency virus. J Viral Hepat. 2011;18:227-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Pischke S, Peron JM, von Wulffen M, von Felden J, Höner Zu Siederdissen C, Fournier S, Lütgehetmann M, Iking-Konert C, Bettinger D, Par G, Thimme R, Cantagrel A, Lohse AW, Wedemeyer H, de Man R, Mallet V. Chronic Hepatitis E in Rheumatology and Internal Medicine Patients: A Retrospective Multicenter European Cohort Study. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Fousekis FS, Mitselos IV, Christodoulou DK. Extrahepatic manifestations of hepatitis E virus: An overview. Clin Mol Hepatol. 2020;26:16-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Mclean BN, Gulliver J, Dalton HR. Hepatitis E virus and neurological disorders. Pract Neurol. 2017;17:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Abravanel F, Pique J, Couturier E, Nicot F, Dimeglio C, Lhomme S, Chiabrando J, Saune K, Péron JM, Kamar N, Evrard S, de Valk H, Cintas P, Izopet J; HEV study group. Acute hepatitis E in French patients and neurological manifestations. J Infect. 2018;77:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Dalton HR, Kamar N, van Eijk JJ, Mclean BN, Cintas P, Bendall RP, Jacobs BC. Hepatitis E virus and neurological injury. Nat Rev Neurol. 2016;12:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 63. | van den Berg B, van der Eijk AA, Pas SD, Hunter JG, Madden RG, Tio-Gillen AP, Dalton HR, Jacobs BC. Guillain-Barré syndrome associated with preceding hepatitis E virus infection. Neurology. 2014;82:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 64. | Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, Rostaing L, Keane F, Ijaz S, Izopet J, Dalton HR. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 65. | Seror P. Neuralgic amyotrophy. An update. Joint Bone Spine. 2017;84:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | van Eijk JJ, Madden RG, van der Eijk AA, Hunter JG, Reimerink JH, Bendall RP, Pas SD, Ellis V, van Alfen N, Beynon L, Southwell L, McLean B, Jacobs BC, van Engelen BG, Dalton HR. Neuralgic amyotrophy and hepatitis E virus infection. Neurology. 2014;82:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 67. | Bazerbachi F, Haffar S, Garg SK, Lake JR. Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature. Gastroenterol Rep (Oxf). 2016;4:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Kamar N, Weclawiak H, Guilbeau-Frugier C, Legrand-Abravanel F, Cointault O, Ribes D, Esposito L, Cardeau-Desangles I, Guitard J, Sallusto F, Muscari F, Peron JM, Alric L, Izopet J, Rostaing L. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 69. | Marion O, Abravanel F, Del Bello A, Esposito L, Lhomme S, Puissant-Lubrano B, Alric L, Faguer S, Izopet J, Kamar N. Hepatitis E virus-associated cryoglobulinemia in solid-organ-transplant recipients. Liver Int. 2018;38:2178-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | D'Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 176] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Ahmad BS, Ahmad A, Jamil S, Abubakar Mohsin Ehsanullah SA, Munir A. Severe haemolysis and renal failure precipitated by hepatitis E virus in G6PD Deficient patient: A case report. J Pak Med Assoc. 2018;68:1397-1399. [PubMed] |
| 72. | Leaf RK, O'Brien KL, Leaf DE, Drews RE. Autoimmune hemolytic anemia in a young man with acute hepatitis E infection. Am J Hematol. 2017;92:E77-E79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Rauff B, Idrees M, Shah SA, Butt S, Butt AM, Ali L, Hussain A, Irshad-Ur-Rehman, Ali M. Hepatitis associated aplastic anemia: a review. Virol J. 2011;8:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 74. | Zylberman M, Turdó K, Odzak A, Arcondo F, Altabert N, Munné S. [Hepatitis E virus-associated aplastic anemia. Report of a case]. Medicina (B Aires). 2015;75:175-177. [PubMed] |
| 75. | Shah SA, Lal A, Idrees M, Hussain A, Jeet C, Malik FA, Iqbal Z, Rehman Hu. Hepatitis E virus-associated aplastic anaemia: the first case of its kind. J Clin Virol. 2012;54:96-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Stasi R, Chia LW, Kalkur P, Lowe R, Shannon MS. Pathobiology and treatment of hepatitis virus-related thrombocytopenia. Mediterr J Hematol Infect Dis. 2009;1:e2009023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Masood I, Rafiq A, Majid Z. Hepatitis E presenting with thrombocytopaenia. Trop Doct. 2014;44:219-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Singh NK, Gangappa M. Acute immune thrombocytopenia associated with hepatitis E in an adult. Am J Hematol. 2007;82:942-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, Dalton HR. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 443] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 80. | Abravanel F, Chapuy-Regaud S, Lhomme S, Miedougé M, Peron JM, Alric L, Rostaing L, Kamar N, Izopet J. Performance of anti-HEV assays for diagnosing acute hepatitis E in immunocompromised patients. J Clin Virol. 2013;58:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Legrand-Abravanel F, Thevenet I, Mansuy JM, Saune K, Vischi F, Peron JM, Kamar N, Rostaing L, Izopet J. Good performance of immunoglobulin M assays in diagnosing genotype 3 hepatitis E virus infections. Clin Vaccine Immunol. 2009;16:772-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Norder H, Karlsson M, Mellgren Å, Konar J, Sandberg E, Lasson A, Castedal M, Magnius L, Lagging M. Diagnostic Performance of Five Assays for Anti-Hepatitis E Virus IgG and IgM in a Large Cohort Study. J Clin Microbiol. 2016;54:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Abravanel F, Lhomme S, Chapuy-Regaud S, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Kamar N, Izopet J. Hepatitis E virus reinfections in solid-organ-transplant recipients can evolve into chronic infections. J Infect Dis. 2014;209:1900-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 84. | Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 475] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 85. | Zhao ZY, Ruan B, Shao H, Chen ZJ, Liu SL. Detection of hepatitis E virus RNA in sera of patients with hepatitis E by polymerase chain reaction. Hepatobiliary Pancreat Dis Int. 2007;6:38-42. [PubMed] |
| 86. | Webb GW, Dalton HR. Hepatitis E: an expanding epidemic with a range of complications. Clin Microbiol Infect. 2020;26:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 689] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 88. | Al-Sadeq DW, Majdalawieh AF, Mesleh AG, Abdalla OM, Nasrallah GK. Laboratory challenges in the diagnosis of hepatitis E virus. J Med Microbiol. 2018;67:466-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Vollmer T, Knabbe C, Dreier J. Comparison of real-time PCR and antigen assays for detection of hepatitis E virus in blood donors. J Clin Microbiol. 2014;52:2150-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Behrendt P, Bremer B, Todt D, Brown RJ, Heim A, Manns MP, Steinmann E, Wedemeyer H. Hepatitis E Virus (HEV) ORF2 Antigen Levels Differentiate Between Acute and Chronic HEV Infection. J Infect Dis. 2016;214:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 91. | Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 383] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 92. | Kamar N, Abravanel F, Behrendt P, Hofmann J, Pageaux GP, Barbet C, Moal V, Couzi L, Horvatits T, De Man RA, Cassuto E, Elsharkawy AM, Riezebos-Brilman A, Scemla A, Hillaire S, Donnelly MC, Radenne S, Sayegh J, Garrouste C, Dumortier J, Glowaki F, Matignon M, Coilly A, Figueres L, Mousson C, Minello A, Dharancy S, Rerolle JP, Lebray P, Etienne I, Perrin P, Choi M, Marion O, Izopet J; Hepatitis E Virus Ribavirin Study Group. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin Infect Dis. 2020;71:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 93. | Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P, Muscari F, Izopet J. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat. 2015;22:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82-85.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 97. | Biliotti E, Franchi C, Spaziante M, Garbuglia AR, Volpicelli L, Palazzo D, De Angelis M, Esvan R, Taliani G. Autochthonous acute hepatitis E: treatment with sofosbuvir and ribavirin. Infection. 2018;46:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Drinane M, Jing Wang X, Watt K. Sofosbuvir and Ribavirin Eradication of Refractory Hepatitis E in an Immunosuppressed Kidney Transplant Recipient. Hepatology. 2019;69:2297-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Schulz M, Papp CP, Bock CT, Hofmann J, Gerlach UA, Maurer MM, Eurich D, Mueller T. Combination therapy of sofosbuvir and ribavirin fails to clear chronic hepatitis E infection in a multivisceral transplanted patient. J Hepatol. 2019;71:225-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 100. | van Wezel EM, de Bruijne J, Damman K, Bijmolen M, van den Berg AP, Verschuuren EAM, Ruigrok GA, Riezebos-Brilman A, Knoester M. Sofosbuvir Add-on to Ribavirin Treatment for Chronic Hepatitis E Virus Infection in Solid Organ Transplant Recipients Does Not Result in Sustained Virological Response. Open Forum Infect Dis. 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Horvatits T, Schulze Zur Wiesch J, Lütgehetmann M, Lohse AW, Pischke S. The Clinical Perspective on Hepatitis E. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 102. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 570] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 103. | Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, Guo M, Liu XH, Li JX, Yang CL, Tang Q, Jiang RJ, Pan HR, Li YM, Shih JW, Ng MH, Zhu FC, Xia NS. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 104. | Zaman K, Dudman S, Stene-Johansen K, Qadri F, Yunus M, Sandbu S, Gurley ES, Overbo J, Julin CH, Dembinski JL, Nahar Q, Rahman A, Bhuiyan TR, Rahman M, Haque W, Khan J, Aziz A, Khanam M, Streatfield PK, Clemens JD. HEV study protocol : design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open. 2020;10:e033702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Janczewska E S-Editor: Wang LL L-Editor: A P-Editor: Wang LL