Published online Aug 27, 2021. doi: 10.4254/wjh.v13.i8.939
Peer-review started: May 11, 2021
First decision: June 23, 2021
Revised: July 7, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: August 27, 2021
Processing time: 100 Days and 23.5 Hours
Clearly, infection with severe acute respiratory syndrome coronavirus 2 is not limited to the lung but also affects other organs. We need predictive models to determine patients’ prognoses and to improve health care resource allocation during the coronavirus disease 2019 (COVID-19) pandemic. While treating COVID-19, we observed differential outcome prediction weights for markers of hepatocellular injury among hospitalized patients.
To investigate the association between hepatocellular injury and all-cause in-hospital mortality among patients with COVID-19.
This multicentre study employed a retrospective cohort design. All adult patients admitted to Al-Azhar University Hospital, Assiut, Egypt and Abo Teeg General Hospital, Assiut, Egypt with confirmed COVID-19 from June 1, 2020, to July 30, 2020 were eligible. We categorized our cohort into three groups of (1) patients with COVID-19 presenting normal aminotransferase levels; (2) patients with COVID-19 presenting one-fold higher aminotransferase levels; and (3) patients with COVID-19 presenting two-fold higher aminotransferase levels. We analysed the association between elevated aminotransferase levels and all-cause in-hospital mortality. The survival analysis was performed using the Kaplan–Meier method and tested by log-rank analysis.
In total, 376 of 419 patients met the inclusion criteria, while 29 (8%) patients in our cohort died during the hospital stay. The median age was 40 years (range: 28-56 years), and 51% were males (n = 194). At admission, 54% of the study cohort had liver injury. The pattern of liver injury was hepatocellular injury with an aspartate aminotransferase (AST) predominance. Admission AST levels were independently associated with all-cause in-hospital mortality in the logistic regression analysis. A one-fold increase in serum AST levels among patients with COVID-19 led to an eleven-fold increase in in-hospital mortality (P < 0.001). Admission AST levels correlated with C-reactive protein (r = 0.2; P < 0.003) and serum ferritin (r = 0.2; P < 0.0002) levels. Admission alanine aminotransferase levels correlated with serum ferritin levels (r = 0.1; P < 0.04). Serum total bilirubin levels were independently associated with in-hospital mortality in the binary logistic regression analysis after adjusting for age and sex but lost its statistical significance in the fully adjusted model. Serum ferritin levels were significantly associated with in-hospital mortality (P < 0.01). The probability of survival was significantly different between the AST groups and showed the following order: a two-fold increase in AST levels > a one-fold increase in in AST levels > normal AST levels (P < 0.0001).
Liver injury with an AST-dominant pattern predicts the severity of COVID-19. Elevated serum ferritin levels are associated with fatal outcomes.
Core Tip: Liver injury with an aspartate aminotransferase (AST)-dominant pattern can predict the severity of coronavirus disease 2019 (COVID-19). A one-fold and two-fold increase in serum AST levels increased the odds of in-hospital mortality by eleven-fold and thirteen-fold, respectively, compared with individuals with normal AST levels. Our study confirmed an elevated level of ferritin in patients with COVID-19 that was associated with fatal outcomes. Meticulous monitoring is highly recommended for patients with COVID-19 presenting AST-dominant hepatocellular injury, especially those older than 60 years, those with elevated ferritin levels or those with diabetes mellitus.
- Citation: Madian A, Eliwa A, Abdalla H, Aly HAA. Hepatocellular injury and the mortality risk among patients with COVID-19: A retrospective cohort study. World J Hepatol 2021; 13(8): 939-948
- URL: https://www.wjgnet.com/1948-5182/full/v13/i8/939.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i8.939
Globally, coronavirus disease 2019 (COVID-19) has a substantial impact on the healthcare system. Over time, clinicians have clearly determined that the infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is not limited to the lung but also affects the nervous system, gastrointestinal tract and hepatobiliary system[1].
The entry of SARS-CoV-2 into target cells is facilitated by angiotensin-converting enzyme 2 (ACE2) receptors. ACE2 receptors are expressed at high levels in lung alveolar epithelial cells, vascular endothelium and epithelium of the small intestine[2]. This pattern might explain the pathophysiology of gastrointestinal manifestations associated with COVID-19, such as vomiting and diarrhoea. Moreover, ACE2 receptors are expressed at high levels on cholangiocytes (60% of cells) and to a lesser extent on hepatocytes (3% of cells)[3]. Therefore, the hepatobiliary system may be at increased risk of SARS-CoV-2 infection. The liver appears to be the second most affected organ after the lung[4].
In fact, many case series identified abnormal elevations in the levels of aminotransferases and hypoalbuminemia early and during the progression of COVID-19[5,6]. However, substantial variability in the reported prevalence of liver injury among patients with COVID-19 was noted (14% to 50%)[3]. Moreover, the clinical effect of de novo liver injury on the prognosis of patients with COVID-19 has not been investigated in detail. In addition, multiple clinical comorbidities that might confound liver injury-associated mortality should be studied[7].
Ideally, clinicians should be able to identify patient outcomes to improve health care resource allocation.
The aim of the present study was to investigate whether biomarkers of hepatocellular injury have prognostic value in predicting all-cause in-hospital mortality among patients with COVID-19.
This multicentre study employed a retrospective cohort design. The medical records of all consecutive adult patients admitted to Al-Azhar University Hospital, Assiut, Egypt and Abo Teeg General Hospital, Assiut, Egypt with confirmed COVID-19 from June 1, 2020, to July 30, 2020 were retrieved and analysed. Both hospitals were designated to treat patients with confirmed COVID-19 by the Egyptian Ministry of Health. The inclusion criteria were hospitalization and adult patients > 18-years-old with confirmed COVID-19 based on a positive nasopharyngeal swab for SARS-CoV-2. Exclusion criteria were non-hospitalized patients, pregnant females, patients with chronic liver disease (in accordance with institutional clinical guidelines, patients with elevated aminotransferase levels were screened for markers of viral hepatitis and markers of autoimmune hepatitis), patients who refused to participate in the study and patients with incomplete data. The institutional review board granted approval for the study protocol.
The exposure of interest was the serum levels of aminotransferases at the time of hospital admission. We measured the levels of aminotransferases within 24 h of hospital admission. Elevated aminotransferase levels were defined as either an increase in the levels of aspartate aminotransferase (AST) (> 40 U/L), alanine aminotransferase (ALT) (> 40 U/L) or both proteins compared with the upper limit of normal. We classified our cohort into three groups based on serum levels of aminotransferases: (1) Patients with COVID-19 presenting normal aminotransferase levels; (2) Patients with COVID-19 presenting a one-fold increase in aminotransferase levels; and (3) Patients with COVID-19 presenting a two-fold increase in aminotransferase levels.
In every enrolled participant, covariates analysed included baseline patient characteristics, such as age, sex and smoking status, comorbidities, such as diabetes mellitus, hypertension, ischaemic heart disease, chronic kidney disease and chronic obstructive pulmonary disease, Deyo–Charlson index, obesity and initial laboratory investigations and AST/ALT, serum total bilirubin, serum albumin, blood urea, serum creatinine, C-reactive protein (CRP), creatine kinase (CK), serum ferritin and D-dimer levels.
The predefined primary outcome of the study was all-cause in-hospital mortality. The secondary outcome was the length of the hospital stay. The vital status of the study participants was obtained from hospital records. The censoring date for follow-up of the outcome was August 15, 2020.
Continuous variables were presented as medians and interquartile ranges, and categorical variables were presented as absolute numbers and percentages. For the statistical analysis of group differences, we performed unadjusted binary logistic regression analyses. We utilized a stepwise analysis adjusted for sex and age as well as for clinically relevant confounders listed above to investigate confounding factors. Spearman’s correlation coefficients were calculated to analyse the relationships between variables. All P values presented were two-tailed; values less than 0.05 were considered statistically significant. We used Stata Software (Stata Statistical Software: Release 16. College Station, TX: Stata Corp LP) for data visualization and analysis.
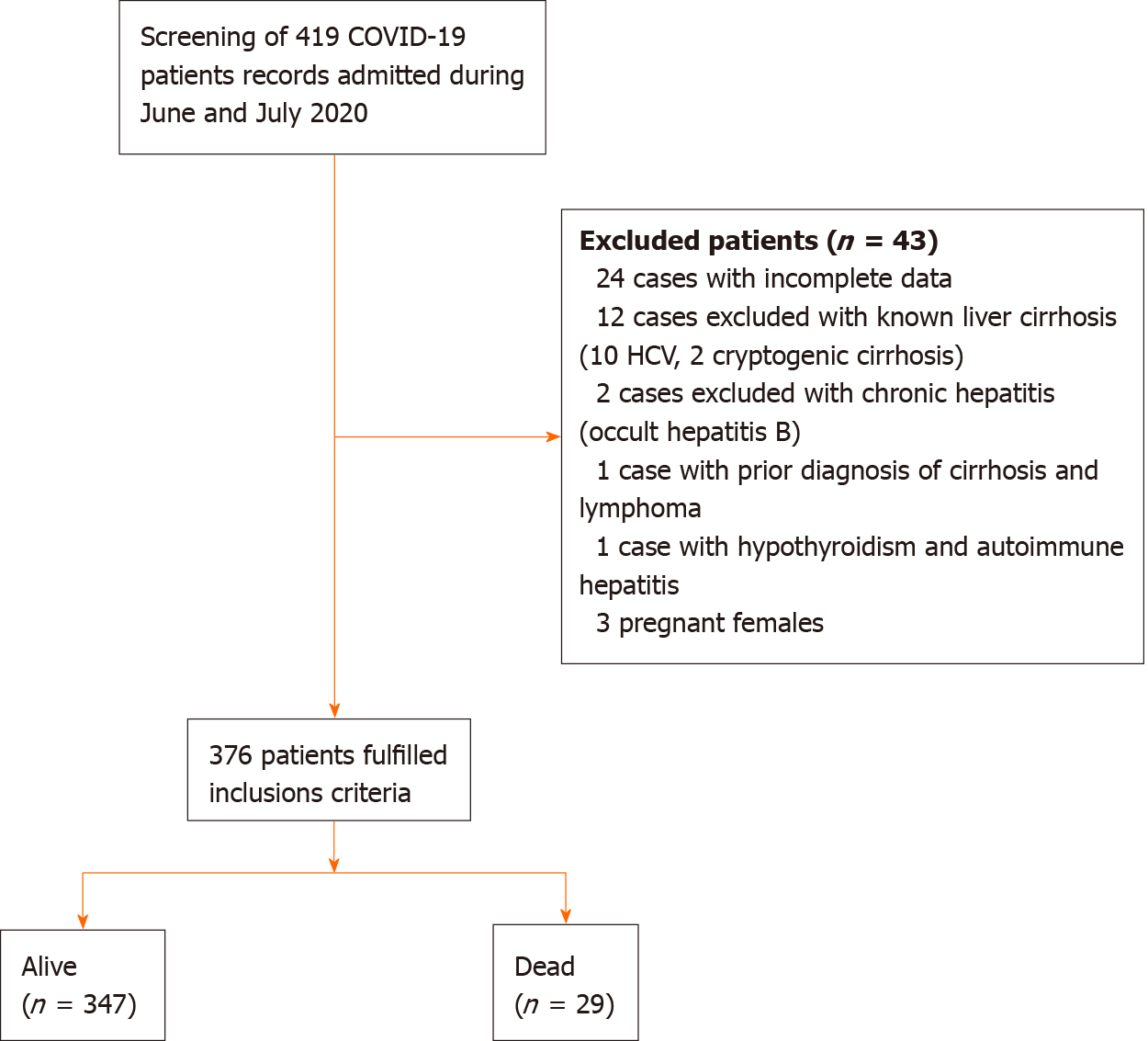
In total, 376 of 419 patients met the inclusion criteria, and 8% of these patients died during the hospital stay (n = 29) (Figure 1). The median age was 40 years (range: 28-56 years), and 51% were males (n = 194) (Table 1). Patients in our study cohort were stratified according to all-cause in-hospital mortality into alive and dead groups, and their characteristics are presented in Table 1.
| Characteristics | Alive, n = 347 | Dead, n = 29 | Unadjusted odds ratio | P value |
| Age years median (IQR) | 1.08 (1.05 to 1.11) | 0.0001 | ||
| < 40 | 186 (98.4) | 3 (1.6) | Ref | |
| 40-60 | 118 (93.6) | 8 (6.3) | 4.8 (1.7 to 13.4) | 0.003 |
| > 60 | 43 (70.5) | 18 (29.5) | 17.6 (6.5 to 48.0) | 0.0001 |
| Male sex, n (%) | 179 (92.2) | 15 (7.7) | 1.1 (0.6 to 2.1) | 0.98 |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 60 (77.0) | 18 (23.0) | 5.4 (2.9 to 10.2) | 0.0001 |
| Hypertension, n (%) | 88 (83.8) | 17 (16.2) | 2.8 (1.5 to 5.2) | 0.001 |
| Ischaemic heart disease, n (%) | 7 (50.0) | 7 (50.0) | 16.1 (5.2 to 50.2) | 0.0001 |
| Chronic kidney disease, n (%) | 5 (45.5) | 6 (54.5) | 18.5 (5.2 to 65.5) | 0.0001 |
| Chronic respiratory disease, n (%) | 57 (89.1) | 7 (10.9) | 1.7 (0.7 to 4.1) | 0.25 |
| Deyo–Charlson index, n (%) | ||||
| 0-1 | 281 (96.6) | 10 (3.4) | Ref | |
| 2-3 | 64 (80.0) | 16 (20.0) | 7.8 (3.2 to 18.3) | 0.0001 |
| > 3 | 2 (40.0) | 3 (60.0) | 46.5 (6.9 to 313.5) | 0.0001 |
| Biochemical results on admission | ||||
| Serum ALT, n (%) | ||||
| < 40 U/L | 225 (93.4) | 16 (6.6) | Ref | |
| 40-80 U/L | 98 (89.9) | 11 (10.1) | 1.5 (0.6 to 3.3) | 0.36 |
| > 80 U/L | 24 (92.3) | 2 (7.7) | 1.1 (0.3 to 5.4) | 0.83 |
| Serum AST, n (%) | ||||
| < 40 U/L | 246 (96.8) | 8 (3.2) | Ref | |
| 40-80 U/L | 87 (82.3) | 18 (17.1) | 6.1 (2.5 to 14.7) | 0.0001 |
| > 80 U/L | 14 (82.3) | 3 (17.6) | 6.1 (1.5 to 25.5) | 0.01 |
| AST/ALT | ||||
| 1.2-1.5 | 41 (87.2) | 6 (12.7) | 2.6 (1.0 to 7.2) | 0.05 |
| > 1.5 | 35 (81.4) | 8 (18.6) | 4.1 (1.6 to 10.4) | 0.003 |
| Serum albumin < 3.5 g/dL, n (%) | 125 (85.6) | 21 (14.4) | 4.5 (1.9 to 10.5) | 0.001 |
| Serum total bilirubin > 1.5 mg/dL, n (%) | 2 (33.3) | 4 (66.7) | 8.0 (2.2 to 29.4) | 0.002 |
| Serum creatinine > 1.1 mg/dL for males; > 0.95 mg/dL for females, n (%) | 66 (77.7) | 19 (22.3) | 8.9 (3.9 to 20.6) | 0.0001 |
| C-reactive protein ≥ 1 mg/L, n (%) | 338 (92.1) | 29 (7.9) | 1.0 | |
| Serum ferritin > 400 μg/L for males; > 150 μg/L for females | 183 (86.7) | 28 (13.3) | 24.7 (3.3 to 184.1) | 0.002 |
| D-dimer > 0.5 μg/mL | 335 (92.0) | 29 (8.0) | 1.0 | |
| Obesity (body mass index > 30) | 106 (86.2) | 17 (13.8) | 3.2 (1.5 to 7.0) | 0.003 |
| Creatin kinase > 117 IU/L | 11 (91.7) | 1 (8.3) | 1.1 (0.1 to 8.7) | 0.9 |
Regression analysis: (1) Unadjusted analysis. The unadjusted binary logistic regression analysis revealed that age, diabetes, hypertension, ischaemic heart disease, chronic kidney disease, obesity and Deyo-Charlson index were significant clinical factors associated with all-cause in-hospital mortality. In addition, AST, serum total bilirubin, serum albumin, serum creatinine and serum ferritin levels were the laboratory biomarkers significantly associated with all-cause in-hospital mortality. However, sex, chronic obstructive pulmonary disease and ALT, CRP, CK and D-dimer levels were not associated with all-cause in-hospital mortality; and (2) Adjusted analysis. The AST level at admission was the only biomarker of liver injury that was independently associated with all-cause in-hospital mortality in the unadjusted binary logistic regression analysis and model 1 adjusted for age and sex (Table 2). In addition, in model 2, after stepwise adjustment for several clinically relevant confounders, AST levels were still significantly associated with all-cause in-hospital mortality. Serum total bilirubin levels were independently associated with in-hospital mortality in the binary logistic regression after adjusting for age and sex but lost its statistical significance in the fully adjusted model.
| Unadjusted | Model 1 (adjusted for age and sex) | Model 2 | ||||||||
| AST | OR | CI | P value | OR | CI | P value | OR | CI | P value | |
| < 40 | Ref | Ref | Ref | |||||||
| 40-80 | 6.1 | 2.5-14.7 | 0.0001 | 4.8 | 1.9-12.1 | 0.001 | 10.8 | 2.5-40.9 | 0.001 | |
| > 80 | 6.1 | 1.5-25.5 | 0.01 | 2.9 | 0.6-13.7 | 0.18 | 12.8 | 1.5-93.4 | 0.02 | |
| Covariates | ||||||||||
| Age | ||||||||||
| < 40 | Ref | |||||||||
| 40-60 | 1.1 | 0.2-6.2 | 0.9 | |||||||
| > 60 | 6.3 | 1.2-33.1 | 0.03 | |||||||
| Sex | 1.1 | 0.4-3.7 | 0.7 | |||||||
| DM | 5.7 | 1.0-31.7 | 0.04 | |||||||
| HTN | 0.3 | 0.1-2.0 | 0.2 | |||||||
| IHD | 5.0 | 0.7-37.3 | 0.1 | |||||||
| COPD | 0.7 | 0.1-3.6 | 0.6 | |||||||
| DCI | ||||||||||
| 0-1 | ||||||||||
| 2-3 | 0.90 | 0.090-9.600 | 0.07 | |||||||
| > 3 | 0.200 | 0.001-25.600 | 0.5 | |||||||
| ALT | ||||||||||
| < 40 | Ref | |||||||||
| 40-80 | 0.30 | 0.09-1.20 | 0.1 | |||||||
| > 80 | 0.10 | 0.02-1.20 | 0.07 | |||||||
| Albumin | 0.8 | 0.2-2.5 | 0.7 | |||||||
| Bilirubin | 17.2 | 0.9-312.8 | 0.05 | |||||||
| Ferritin | 20.7 | 1.7-247.0 | 0.01 | |||||||
| Creatinine | 1.8 | 0.6-5.8 | 0.3 | |||||||
| Obesity | 3.3 | 0.9-11.4 | 0.06 | |||||||
CK was studied as a marker of muscle injury. At admission, AST levels did not correlate with CK levels (r = -0.006; P = 0.9). In addition, admission ALT levels did not correlate with CK levels (r = -0.02; P = 0.6).
Serum ferritin and CRP levels were examined as markers of inflammation. At admission, AST levels correlated with CRP (r = 0.2; P < 0.003) and serum ferritin (r = 0.2; P < 0.0002) levels. Admission ALT levels correlated with serum ferritin levels (r = 0.1; P < 0.04) but not with CRP (r = 0.09; P = 0.08).
Admission serum ferritin levels correlated with CRP levels (r = 0.4; P < 0.0001).
Among our cohort, we identified 6 patients with biphasic hyperbilirubinemia. ALT levels correlated with serum total bilirubin levels (r = 0.2; P < 0.003). Therefore, hyperbilirubinemia was due to liver injury and not haemolysis.
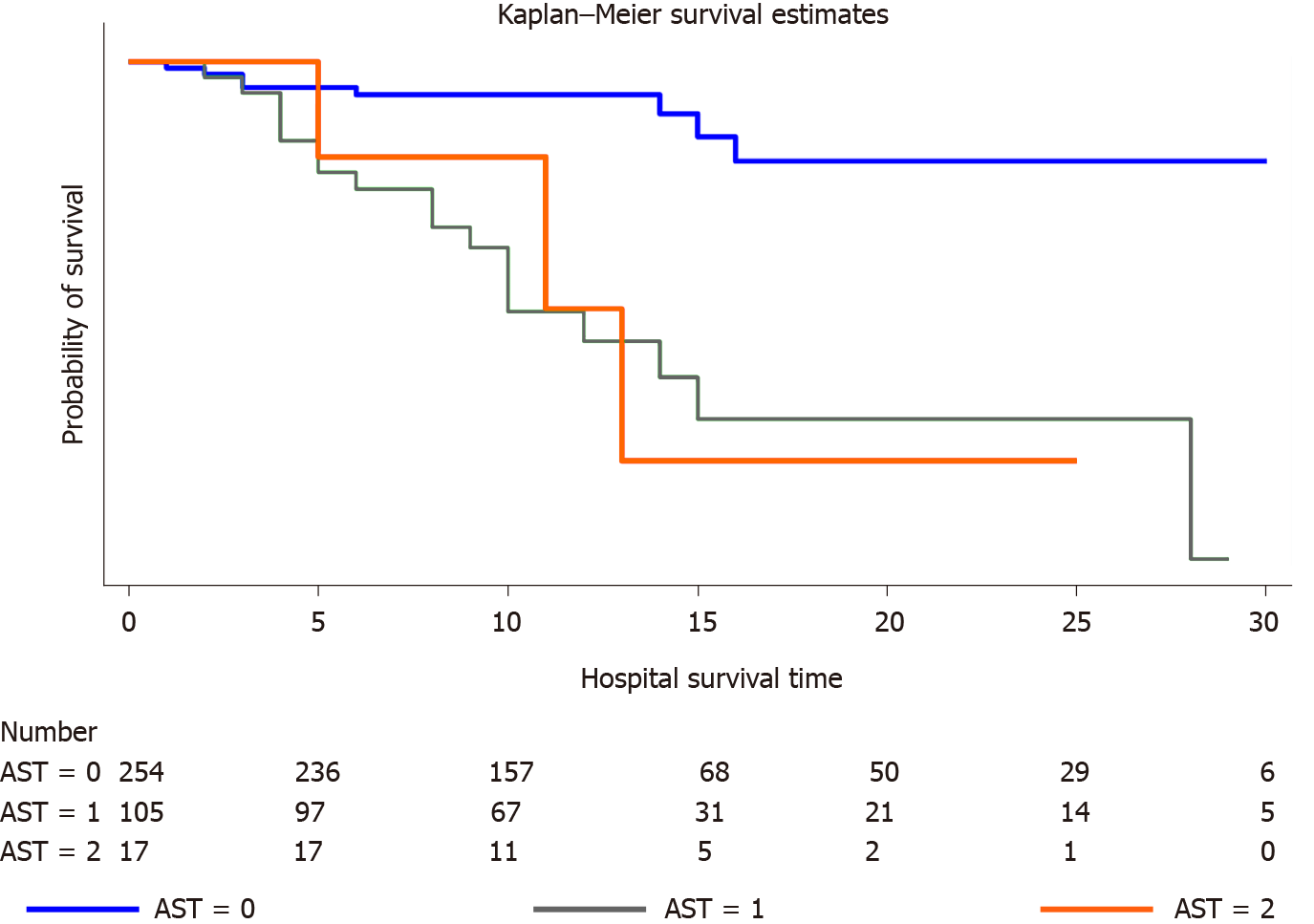
The probability of survival was significantly different between AST groups. As shown in the Kaplan-Meier curves (Figure 2), the probability of mortality progressively increased as the serum level of AST increased in the following order: two-fold increase in AST levels > a one-fold increase in AST levels > normal AST levels.
Numerous studies have reported the effect of liver injury on the outcomes of hospitalized patients with COVID-19[8]. However, a growing concern is that many demographic, clinical and laboratory markers might confound this association. These potential confounders should be recognized, and their effects on the association between biomarkers of hepatocellular injury and patient outcomes should be investigated. In fact, while treating COVID-19, we observed differential outcome prediction weights for markers of hepatocellular injury among hospitalized patients.
At admission, 54.0% of patients in our cohort had liver injury. AST levels were elevated in 32.5% (n = 122), ALT levels were elevated in 36.0% (n = 135), and both ALT and AST levels were increased in 23.0% (n = 87). Among nonsurvivors, the pattern of liver injury was hepatocellular injury with an AST predominance. Both ALT and AST levels were elevated in 45.0% of nonsurvivors. An isolated elevation of AST levels was detected in 31.0% of nonsurvivors, while an isolated elevation of ALT levels was detected in 3.0%. Notably, 48.0% of nonsurvivors presented an AST/ALT ratio > 1.2, and serum total bilirubin levels were increased in 1.6% of patients in our study cohort.
We observed an obvious increase in mortality among patients with COVID-19 presenting elevated serum AST levels at the time of admission. A one-fold increase in the serum level of AST increased the odds of in-hospital mortality eleven-fold compared to those with normal AST levels at admission. Moreover, a two-fold increase in serum AST levels predicated a thirteen-fold increase in mortality. We can thus postulate from the findings of the present study that elevated AST levels at admission are a harbinger of a worse prognosis for patients with COVID-19. On the other hand, serum ALT, bilirubin and albumin levels did not alter mortality after correction for age, sex and other relevant clinical factors. Our findings are consistent with recent reports investigating progressive liver injury and the risk of mortality among patients with COVID-19 where AST levels but not ALT levels at admission were a strong predictor of mortality[9-11].
In our cohort, AST levels did not correlate with the levels of CK, a marker of muscle injury, at admission. Moreover, AST levels correlated moderately with inflammatory markers at admission. Based on these findings, liver injury in patients with COVID-19 may be related to the proinflammatory state associated with cytokine release.
In healthy individuals, plasma levels of ALT and AST represent the balance between normal turnover of hepatocytes by apoptosis and the clearance rates of these enzymes from hepatic sinusoids. Normally, ALT is present in the cytoplasm of hepatocytes, whereas AST is present in the cytoplasm and mitochondria of hepatocytes. Although the ratio of hepatic AST/ALT is 2.5:1, the serum levels of AST and ALT are similar after hepatocyte turnover because the clearance rate of AST is two times faster than the clearance rate of ALT[12,13].
In individuals with hepatocellular injury, serum levels of AST and ALT reflect the time course of hepatic injury and prognosis of hepatic insult. Early hepatocyte injury results in the release of cytosolic AST and ALT. If hepatocyte injury is severe, mitochondrial damage will result in increased release of mitochondrial AST in serum. Therefore, the predominant increase in the admission AST levels in our cohort might reflect early and severe hepatocyte injury. Furthermore, SARS-CoV-2 may induce endothelial cell injury in the hepatic microcirculation and promote portal or sinusoidal microthrombosis. In individuals with ischaemic liver injury (due to microthrombosis), serum AST levels peak before ALT levels, a pattern that was observed in our cohort[9,11,13].
In practice, an isolated and predominant elevation of AST levels indicates a nonhepatic source of AST, e.g., muscle, and haemolysis.
Myositis results in increased levels of AST and, to a lesser extent, ALT; however, the increased serum levels of muscular aminotransferases should be associated with increased serum levels of CK. In our cohort, no significant correlation between AST and CK levels at admission was observed. This finding suggests true hepatic injury as the main source of elevated AST levels.
Haemolysis results in increased levels of AST and unconjugated bilirubin. In our cohort, we identified 6 patients with biphasic hyperbilirubinemia. ALT levels correlated with serum total bilirubin levels. Therefore, hyperbilirubinemia was due to liver injury and not haemolysis.
Among our cohort, 33.0% of patients were obese, perhaps with underdiagnosed nonalcoholic fatty liver disease. In addition, 20.0% of patients in our cohort were diagnosed with diabetes. Both diabetes mellitus and obesity increase serum levels of AST and ALT, but this change is more prominent for ALT than for AST[14].
The mechanism of liver injury among patients with COVID-19 is unclear and possibly multifactorial. The entry of SARS-CoV-2 into hepatocytes and cholangiocytes is mediated by ACE2 receptors. Liver biopsies obtained from deceased patients diagnosed with COVID-19 revealed focal degeneration and necrosis. In addition, SARS-CoV-2 particles were detected in hepatocytes[4]. Focal hepatic degeneration and necrosis may be due to the direct cytopathic effect of viral entry or could be an immune-mediated process. Entry of SARS-CoV-2 into hepatocytes triggers an innate and adaptive immune response that results in clearing of virus-infected cells. However, if the mounted immune response is exaggerated and uncontrolled, this aberrant immune response may contribute to the development of a cytokine storm and multisystem dysfunction[4,15]. Moreover, hyperinflammatory syndrome can induce disseminated intravascular coagulation with ischaemic hepatocellular injury by microvascular thrombosis in the hepatic microcirculation. In addition, direct endothelial cell damage in the hepatic microcirculation induced by SARS-CoV-2 may promote microvascular thrombosis and ischaemic liver injury[11].
In addition, our findings indicated that an age > 60 years, diabetes mellitus and increased serum ferritin levels were independent strong predictors of mortality among patients with COVID-19 presenting liver injury. These observations are consistent with recent studies[10].
Our study provides evidence that serum ferritin levels were associated with all-cause in-hospital mortality. Of our cohort, 56.0% of patients presented elevated serum ferritin levels. Moreover, 97.0% of nonsurvivors had elevated serum ferritin levels. In addition, logistic regression analysis showed that the serum ferritin level was an independent risk biomarker for in-hospital mortality among patients with COVID-19. Furthermore, admission serum ferritin levels correlated with CRP levels. These results suggest that elevated serum ferritin levels at admission may reflect disease severity. Our findings are consistent with a recent report confirming that increased ferritin levels are associated with in-hospital mortality in patients with COVID-19[16].
Inflammatory cytokines stimulate hepatocytes and macrophages to release ferritin, which plays a vital role in many autoimmune diseases and inflammatory disorders. A vicious loop exists between ferritin and inflammatory cytokines, i.e. activated hepatocytes and macrophages release ferritin, which in turn stimulates the production of various inflammatory cytokines. Serum ferritin is an inflammatory cytokine that indirectly stimulates proinflammatory pathways through the activation of the transcription factor nuclear factor kappa-B. Moreover, the heavy subunit of ferritin directly increases the mRNA expression of many inflammatory cytokines, such as interleukin-1, interleukin-6, tumour necrosis factor and NOD-like receptor 3, indicating the proinflammatory properties of ferritin[16,17].
Patients with diabetes have elevated serum ferritin levels, and these patients are at increased risk of serious complications from COVID-19. Therefore, ferritin may be a key mediator of immune dysregulation that contributes to the cytokine storm in patients with diabetes mellitus and COVID-19[16,17].
This retrospective study revealed an association between AST levels and mortality in patients with COVID-19 but did not reveal causality. Numerous medications and clinical and biological conditions injure hepatocytes but were only partially considered in the regression analysis. We used liver enzyme level at the time of admission for group categorization without knowing whether they were episodic or progressive changes. We did not consider concurrent medication use, such as angiotensin converting enzyme inhibitors or angiotensin receptor blockers, in our analysis. Underdiagnosed nonalcoholic fatty liver disease and occult consumption of alcohol were not considered.
Our study revealed that liver injury is highly prevalent among patients with COVID-19 at admission. Liver injury with an AST-dominant pattern can be used to predict the severity of COVID-19. This study confirmed an elevated level of ferritin in patients with COVID-19. Admission serum ferritin levels are associated with fatal outcomes. Meticulous monitoring is highly recommended for patients with COVID-19 presenting AST-dominant hepatocellular injury, especially those older than 60 years, those with elevated levels of ferritin and those with diabetes mellitus.
Clearly, infection with severe acute respiratory syndrome coronavirus 2 is not limited to the lung but also affects other organs.
Predictive models are needed to determine patients’ prognoses and to improve health care resource allocation during the coronavirus disease 2019 (COVID-19) pandemic.
To investigate whether biomarkers of hepatocellular injury at admission have prognostic value in predicting all-cause in-hospital mortality in patients with COVID-19.
A retrospective cohort study was conducted on 376 consecutive adult patients admitted to Al-Azhar University Hospital, Assiut, Egypt and Abo Teeg General Hospital, Assiut, Egypt with confirmed COVID-19 from June 1, 2020 to July 30, 2020.
High-risk populations, especially patients aged ≥ 60 years, patients with aspartate aminotransferase (AST)-dominant liver injury or those with diabetes, should be intensively monitored. Admission serum AST and serum ferritin levels have the strongest association with the prognosis of patients with COVID-19 and can be used to monitor patients with COVID-19 at risk of liver injury.
Liver injury with an AST-dominant pattern can predict the severity of COVID-19. This study confirmed an elevated level of ferritin in patients with COVID-19. Elevated serum ferritin levels are associated with in-hospital mortality.
Meticulous monitoring is highly recommended for patients with COVID-19 presenting AST-dominant hepatocellular injury, especially those older than 60 years, patients with elevated serum ferritin levels or those with diabetes mellitus.
We appreciate the effort of all medical staff and technicians who agreed to participate in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Maheshwarappa RP, Papadopoulos KI, Sivanand N S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li X
| 1. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 467] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 2. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4184] [Article Influence: 190.2] [Reference Citation Analysis (0)] |
| 3. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (3)] |
| 5. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 576] [Article Influence: 96.0] [Reference Citation Analysis (1)] |
| 6. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 872] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 7. | Chen P, Zhou B. Clinical Characteristics of COVID-19 in Patients With Liver Injury. Clin Gastroenterol Hepatol. 2020;18:2846-2847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Pawlowska J, Lebensztejn DM, Jankowska I. Coronavirus Disease 2019-Liver Injury-Literature Review and Guidelines Based on the Recommendations of Hepatological Societies. Pediatr Gastroenterol Hepatol Nutr. 2021;24:119-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 10. | Zhang SS, Dong L, Wang GM, Tian Y, Ye XF, Zhao Y, Liu ZY, Zhai JY, Zhao ZL, Wang JH, Zhang HM, Li XL, Wu CX, Yang CT, Yang LJ, Du HX, Wang H, Ge QG, Xiu DR, Shen N. Progressive liver injury and increased mortality risk in COVID-19 patients: A retrospective cohort study in China. World J Gastroenterol. 2021;27:835-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Herta T, Berg T. COVID-19 and the liver - Lessons learned. Liver Int. 2021;41 Suppl 1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34:117-130. [PubMed] |
| 13. | Yazar H, Kayacan Y, Ozdin M. De Ritis ratio and biochemical parameters in COVID-19 patients. Arch Physiol Biochem. 2020;1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Zhao L, Cheng J, Chen Y, Li Q, Han B, Xia F, Chen C, Lin D, Yu X, Wang N, Lu Y. Serum alanine aminotransferase/aspartate aminotransferase ratio is one of the best markers of insulin resistance in the Chinese population. Nutr Metab (Lond). 2017;14:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020;11:827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1687] [Cited by in RCA: 1784] [Article Influence: 297.3] [Reference Citation Analysis (0)] |
| 16. | Deng F, Zhang L, Lyu L, Lu Z, Gao D, Ma X, Guo Y, Wang R, Gong S, Jiang W. [Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19]. Med Clin (Barc). 2021;156:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |