Published online Aug 27, 2021. doi: 10.4254/wjh.v13.i8.887
Peer-review started: March 5, 2021
First decision: April 6, 2021
Revised: May 18, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: August 27, 2021
Processing time: 168 Days and 8 Hours
The diagnosis and management of cirrhosis and portal hypertension (PH) with its complications including variceal hemorrhage, ascites, and hepatic encephalopathy continues to evolve. Although there are established “standards of care” in liver biopsy and measurement of PH, gastric varices remain an area without a uni
Core Tip: In this review we familiarize the reader to salient aspects of endoscopic ultrasound (EUS)-guided hepatic interventions including liver biopsy, portal pressure measurements, and treatment of gastric varices, and outline the data supporting their use. We highlight the potential advantages and disadvantages of EUS guided inter
- Citation: Rudnick SR, Conway JD, Russo MW. Current state of endohepatology: Diagnosis and treatment of portal hypertension and its complications with endoscopic ultrasound. World J Hepatol 2021; 13(8): 887-895
- URL: https://www.wjgnet.com/1948-5182/full/v13/i8/887.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i8.887
Chronic liver disease (CLD) continues to represent a substantial healthcare burden, with an estimated 1.5 billion persons affected worldwide. Since 2000 there has been a 13% increase in incidence of CLD and cirrhosis, in addition to increasing prevalence and mortality of cirrhosis in the United States. Moreover, the epidemiology of CLD is shifting from viral hepatitis to an increasing prevalence of liver disease caused by metabolic syndrome and alcohol misuse[1].
Accompanying the increase in cirrhosis is the development of portal hypertension (PH); resulting in the majority of its complications including ascites, variceal hemorrhage, and encephalopathy. Clinically, cirrhosis is often dichotomized into compensated (absence of portal hypertensive complications) and decompensated (presence of portal hypertensive complications), with decompensated cirrhosis portending a poor prognosis[2].
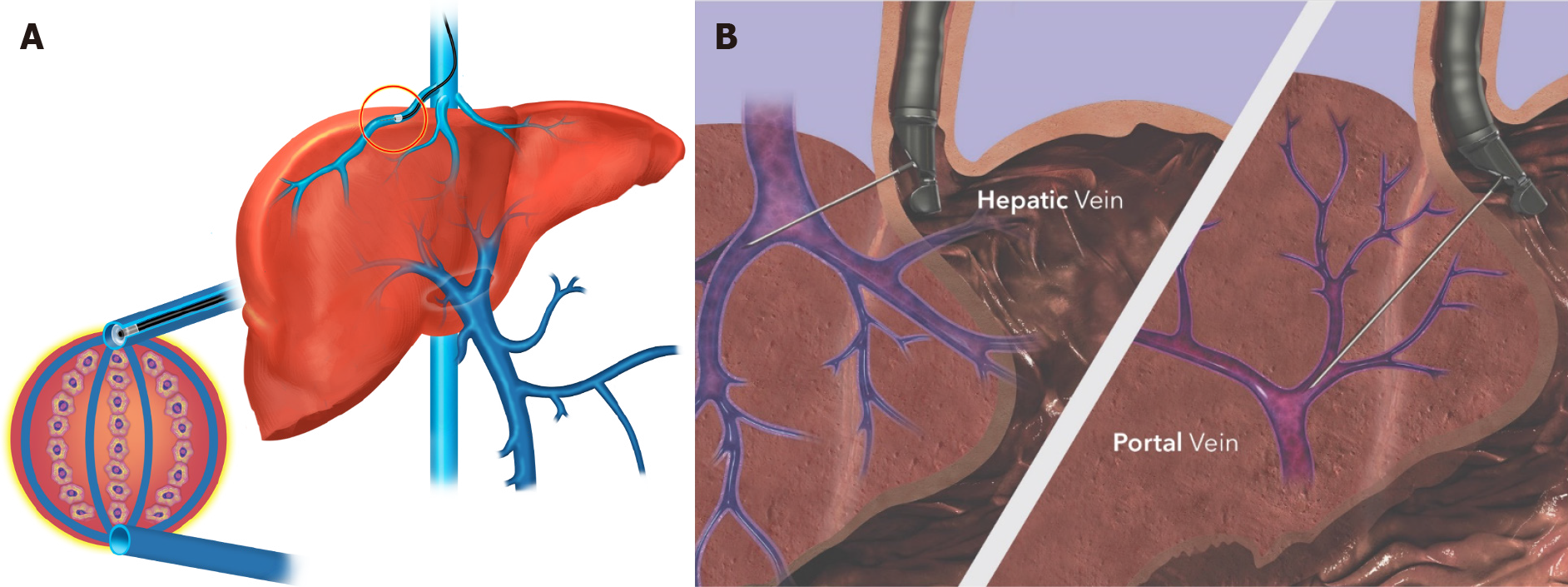
A diagnosis of PH typically requires invasive testing to measure the gradient between the hepatic sinusoids and the hepatic vein (which is the outflow tract of the liver), termed the hepatic venous pressure gradient (HVPG) (Figure 1). PH is present if HVPG > 5 mmHg, with clinically significant PH (CSPH) defined as > 10 mmHg associated with the development of clinical complications (hence its designation) including variceal hemorrhage and ascites. HVPG is an independent prognostic variable, with a 3% increase in mortality risk for each 1 mmHg gradient increase[3].
Accompanying the increasing burden of CLD has been the need for safe, accurate, and cost-effective diagnostic modalities to appropriately classify patients requiring additional therapeutic interventions. Classically liver biopsy; percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB) was utilized to assess the etiology and severity (fibrosis stage) of liver disease by histology. Additionally, invasive measurement of the HVPG via the transjugular venous route in interventional radiology (IR) could be utilized to obtain additional prognostic data in appropriate circumstances. Noninvasive modalities, such as elastography or serologic markers, have been developed as alternatives to liver biopsy[4].
The concept of “Endo-hepatology” was introduced in 2012 as an area of integration or overlap of endoscopic procedures within the practice of Hepatology[5]. In this review we focus on two diagnostic modalities including endoscopic ultrasound (EUS) guided liver biopsy (EUS-LB) and EUS-guided measurement of PH, and one therapeu
Hepatologists should have a fundamental understanding of the similarities and differences in techniques between current clinical standards of practice and EUS-guided modalities, while also recognizing opportunities to appropriately implement EUS-guided diagnostics and therapeutics into their practice. An in depth review of EUS anatomy, devices, and techniques is outside the purview of this review.
Once considered the cornerstone in the evaluation and management of liver disease, the role and modalities of liver biopsy has evolved substantially over the past decade. The evolution of noninvasive testing coupled with concerns regarding the cost and risk of liver biopsy has brought into question the exact role of liver biopsy in the early 21st century[4]. At present, liver biopsy is still considered appropriate for establishing diagnosis, evaluating stage of liver disease (fibrosis), and directing management decisions[6].
Traditionally, liver biopsy has been performed through percutaneous, transjugular, or surgical approaches. At present, image-guided liver biopsy (“real time” or marking) has become the de facto standard of care in most centers, replacing the palpation/ percussion guided technique[7]. Because the diagnosis, grading, and staging of liver disease is dependent upon adequate sample size, it is recommended that the length of the sample is at least 2-3 cm and 16-gauge in caliber (or wider), ideally with ≥ 11 portal tracts for evaluation[6]. Complications related to liver biopsy include pain (30%-50% patients)[8], serious bleeding (0.6%)[9], injury to other organs (0.08%)[10], and rarely death (0.1%)[6].
Since its first description in 2007, publications describing experience with EUS-LB have continued proliferate[11]. Proposed advantages to EUS-LB include more precise localization and characterization of the target tissue, ability to biopsy both lobes of the liver, decreased invasiveness, improved patient tolerance, decreased recovery time, and decreased complications[12]. Acknowledged disadvantages include increased technical difficulty and higher cost compared to other available methods (Table 1).
A single center retrospective study compared the safety and efficacy of “standard of care” [PC-LB (n = 287) & TJ-LB (n = 91)] to EUS-LB (n = 135). There were no statistically significant differences between modalities in regards to rates of adverse events, technical success rate, and diagnostic adequacy. Notably, the number of complete portal tracts for analysis and mean specimen length (two metrics for assessing spe
In 2019 a systematic review and meta-analysis that included eight studies with a total of 437 patients reported the efficacy and safety of EUS-LB biopsy[14]. The primary analysis focused on diagnostic yield; specifically addressing successful histologic diagnosis and frequency of insufficient histologic sample size. A second analysis described pooled rates of all adverse events. A subgroup analysis was performed regarding needle type used for biopsy [core needle vs fine-needle as
The pooled rate of successful histologic diagnosis was 93.9% and the pooled insufficient specimen rate was 10.1%. The pooled rates of adverse events and bleeding were 2.3%, and 1.2%, respectively. In the subgroup analysis, the only statistically significant difference between core needle and FNA needle was obtaining insufficient specimen, which occurred in 20% of patients biopsied with core needle compared to 4% of patients biopsied with FNA needle (P = 0.03). The authors concluded that FNA needles provide better specimens and have improved diagnostic outcomes compared to other core needle biopsies, though they acknowledged significant heterogeneity in the overall analysis.
Despites its limitations, the study by Mohan et al[14] provides robust data de
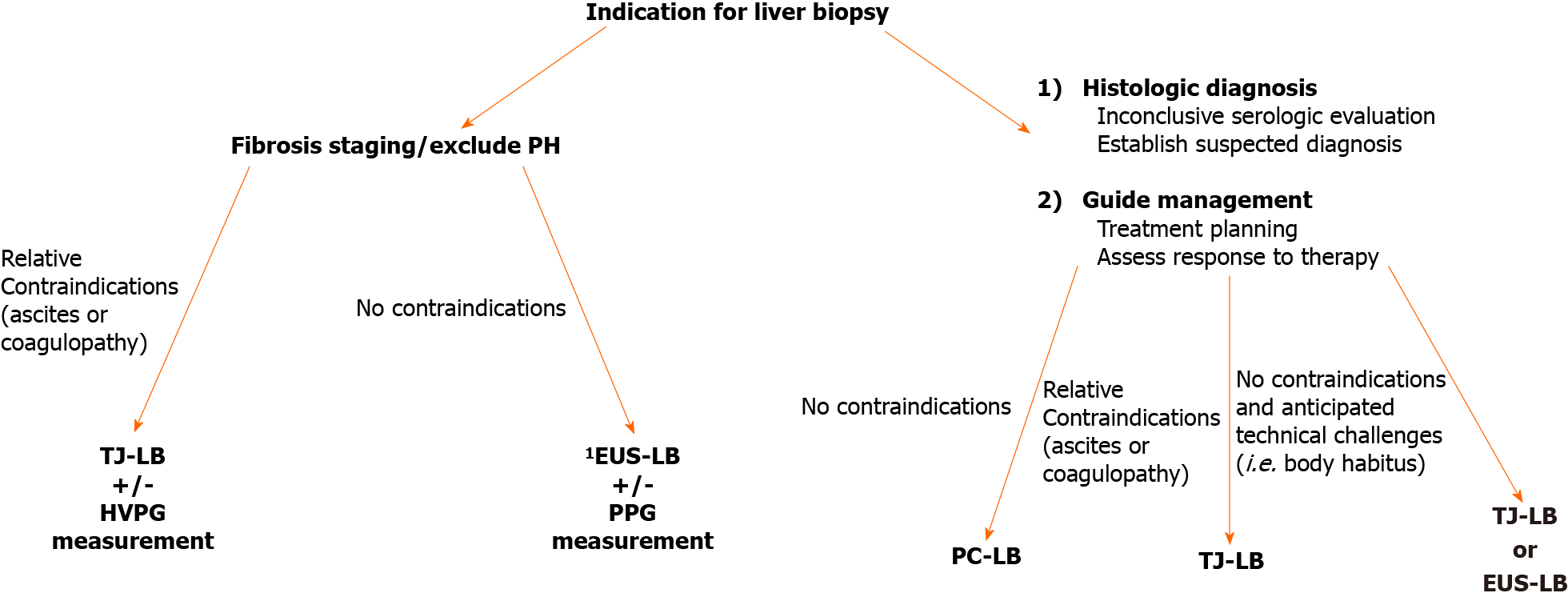
Ultimately, multiple factors influence the choice of liver biopsy modality, and the decision should be made on a case-by-case basis (Figure 2). A seemingly pertinent use of EUS-LB, is in patients with discordant noninvasive testing in whom the goal is to exclude cirrhosis and/or PH, as direct measurements of portal pressures can also be performed simultaneously and biopsies from both lobes can be obtained. With discordant noninvasive testing, accurate fibrosis staging by liver biopsy is paramount. Indeed, it has been demonstrated in patients with NAFLD, biopsies performed on the same day characterized 35% of patients with advanced fibrosis on one sample, while the other sample from the same day did not suggests significant fibrosis[16]. This discordance is of profound significance and directly influences clinical decision-making. As PC-LB and TJ-LB typically sample one hepatic lobe, obtaining “bilobar” biopsies by EUS-LB provides a potential advantage to minimize the risk of misclassifying fibrosis stage.
Although invasive and considered the gold standard in assessment of PH, HVPG is in fact an indirect method of measurement[17]. Calculation of the HVPG includes measuring the free hepatic venous pressure (FHVP) and wedged hepatic venous pressure (WHVP; typically wedged pressure in the right hepatic vein). The transduced wedged hepatic venous pressure estimates sinusoidal pressure. The difference between the WHVP and FHVP is the estimated portosystemic gradient[18]. Conceptually, this is analogous to Swan-Ganz catheterization in the pulmonary artery.
In the absence of fibrosis/nodules (i.e. cirrhosis), the pressure equalizes throughout the interconnected sinusoidal network, and results in minimal gradient (i.e., normal; up to 4 mmHg). Thus, it does not provide useful information regarding prehepatic or presinusoidal PH (i.e., non-cirrhotic causes of PH). In the presence of cirrhosis, the WHVP is an accurate surrogate for portal vein pressure, allowing calculation of the gradient by the equation: WHVP-FHVP = HVPG. As previously outlined, HVPG has significant prognostic value in predicting poor outcomes in patients with PH[3].
In comparison, EUS-guided portal pressure gradient (PPG) measurements employ a direct sampling technique. Thus, the direct measurement of the portal vein pressure could be considered the gold standard because it is not an estimate of sinusoidal pressure as is WHVP. The difference in the mean measurement of these pressures is termed the PPG which is analogous to the HVPG, with the caveat that direct portal vein measurement also allows for the assessment of prehepatic/presinusoidal PH; a limitation of the transjugular approach.
In 2016, Huang et al[19] published their experience in a porcine animal model with a novel EUS-guided system which included a manometer attached to a 25-gauge FNA needle for directly measuring pressures in the hepatic and portal veins. The purpose of this animal study was to assess clinical feasibility and assess correlation with the standard of care; HVPG measurement through transjugular approach[19].
In a pilot study, 28 patients between the age of 18-75 years with a history of liver disease or suspected cirrhosis underwent EUS-PPG measurements utilizing the technique and equipment in the animal study. The portal vein and hepatic vein were targeted via a transgastric–transduodenal approach (IVC was substituted for hepatic vein when not technically feasible). Feasibility was defined as the technical success of obtaining pertinent measurements. Safety was assessed by postprocedural interview and telephone call 48 h following procedure. As correlation to the standard of care (transjugular HVPG) was obtained in animal studies, clinical parameters of PH were evaluated in each patient. Exclusion criteria included pregnancy, international normalized ratio (INR) > 1.5, platelet count < 50000, active GI bleeding, and post sinusoidal PH[20].
Technical success rate of EUS-PPG measurement was 100% without any adverse events. PPG measurements had excellent correlation with clinical parameters of PH. Mean PPG in patients with varices was 14.37 mmHg, compared to 4.26 mmHg in patients without varices (P = 0.0002); which is consistent with criteria that gradients ≥ 10 mmHg (i.e., CSPH) are associated with the development of varices. The authors concluded that EUS-PPG measurement was a safe and feasible alternative to currently available diagnostics[20].
There are obvious limitations of this pilot study which may limit widespread generalizability of this technique. The exclusion of patients with INR > 1.5 and inclusion of only 4 patients with INR > 1.2 (especially with the knowledge that INR is a poor predictor of procedural bleeding risk in patients with cirrhosis) is a major limitation of this small pilot study[21].
Results of this pilot study ultimately led to the Food and Drug Administration approval of the EchoTip Insight portosystemic pressure gradient measurement system (Cook Medical, Winston-Salem, NC, United States) in 2019 (Figure 1). Following approval, multiple centers have begun utilizing this method. Registry data are eagerly anticipated to assess the feasibility, utility, and safety profile of this method outside the realm of small pilot study/clinical trials.
One of the challenges facing any new technology, including EUS-PPG measurement is identifying the appropriate clinical application. Despite the useful prognostic information it provides, in current clinical practice, obtaining the HVPG is not considered standard of care in many areas due to its invasiveness, cost, and limited availability[2]. With the exception of Transjugular intrahepatic portosystemic shunt (TIPS) and TJ-LB in the authors’ experience, HVPG measurements are not routinely obtained.
A potential role of EUS–PPG measurements in current practice would be to supplant the transjugular approach for HVPG/biopsy, and reserve the latter approach for patients undergoing TIPS and in those with more severe coagulopathy. Further
There is significant heterogeneity in the location, vascular anatomy, bleeding risk, and response to treatment of GV. The Sarin classification has been the most commonly used for risk stratification and management, however it is limited to describing endoscopic anatomy, and does not necessarily reflect the underlying vascular anatomy of GV; which has significant treatment implications[22,23].
A proposed algorithm for the treatment of acute GV bleeding suggests utilizing variceal band ligation for treatment of gastroesophageal varices (GOV) 1 (i.e., treat as esophageal varices), while utilizing injection therapies (i.e., tissue adhesives such as cyanoacrylate) in the management of GOV2 and isolated gastric varices 1 (IGV1) (together known as “cardiofundal varics”)[24]. At present, therapeutic options for treatment of GV hemorrhage include endoscopic injection of tissue adhesives (via EGD or EUS), TIPS, and balloon-occluded retrograde transvenous obliteration) (BRTO). It has been suggested that EUS-guided therapy of GV is superior to endoscopic injection as it decreases the rate of rebleeding[25].
In 2000, Lee et al[26] published their results of a prospective study utilizing cyanoacrylate and lipiodol injection in the management of bleeding GV[26]. In this study 38% of patients had GOV2 and 27% patients had IGV1. After initial bleeding was controlled, 47 patients received “on demand” therapy if bleeding recurred, while 54 patients underwent biweekly EUS with injection until obliteration of varices was confirmed. Although early rebleeding rates (defined ≤ 48 h) were similar between both groups, the recurrence of late bleeding (> 48 h) was significantly reduced in the repeat injection group (18.5% vs 44.7%, P = 0.0053).
A randomized trial evaluated prevention of first GV bleed (primary prophylaxis)[27]. In a study of 89 patients with large (≥ 10 mm) GOV2 and IGV1, patients were randomized to endoscopic cyanoacrylate glue injection, nonselective beta blocker (NSBB), and observation. Overall, cyanoacrylate injection was associated with lower bleeding rates (10%) than NSBB (38%), and observation (53%). Survival was similar in the cyanoacrylate (93%), and NSBB group (83%), but higher compared to the ob
The management of active hemorrhage from GV remains a significant clinical challenge. A meta-analysis comparing cyanoacrylate glue injection to endoscopic band ligation demonstrated similar results for initial hemostasis, but favored cyanoacrylate injection for prevention of rebleeding[28]. Limitations of this meta-analysis included variable quality of evidence, and heterogeneity in type of varices treated.
The addition of endovascular coils to cyanoacrylate glue injection has been proposed to reduce the risk of systemic embolization, a rare but potentially fatal complication[29,30]. A single center retrospective study of 152 patients specifically addressed the use of coil injection and cyanoacrylate glue in patients with cardiofundal varices; 94% of whom had IGV1. Over a 6-year period, 5% of patients treated had active hemorrhage, while 69% had evidence of recent bleeding (i.e., treatment constituted secondary prophylaxis). Technical success rate was 99%. Follow-up EUS examinations were available for 100/152 patients. Complete obliteration of varices based on Doppler was confirmed in 93%, and bleeding from obliterated varices occurred in 3% of patients. The authors concluded that combination of therapy with cyanoacrylate and coil embolization is highly effective for hemostasis and active bleeding, and for primary and secondary prophylaxis with minimal adverse effects.
A systematic review and meta-analysis compared combination therapy (cyanoacrylate + coils) to monotherapy with (cyanoacrylate alone vs coil alone or non-cyanoacrylate treatment)[31]. Eleven studies were included (n = 536) which included 2 randomized control trials, one prospective study, and 8 retrospective studies. Measured outcomes included technical success, clinical success, adverse events, and rate of rebleeding/or intervention. Subgroup analysis compared 3 treatment cohorts; EUS- guided cyanoacrylate injection/EUS-guided coil embolization + cyanoacrylate injection/EUS-guided coil injection alone) (Table 2).
| Modality | EUS-LB | PC-LB | TJ-LB |
| Advantages | Ability to obtain simultaneous bi-lobar biopsies | Familiarity | Circumvent challenging body habitus |
| Circumvent challenging body habitus | Less technical expertise | Ability to perform other diagnostics simultaneously | |
| Improved patient tolerance | Lower cost | Fewer contraindications (i.e., ascites and coagulopathy) | |
| Decreased recovery time | |||
| Ability to perform other diagnostics simultaneously (i.e., PPG measurement) | |||
| Disadvantages | Higher cost | Poorer patient tolerance | Higher cost |
| Need for technical expertise | May be limited by patient body habitus | Need for technical expertise | |
| More prone to sampling error | More prone to sampling error |
Overall technical success of EUS-guided therapies was 100%, clinical success was 97%, and adverse events were 14%. In the subgroup analysis, combination therapy resulted in better technical success (100%) and clinical success (98%) compared to monotherapy with cyanoacrylate alone (97% and 96%, respectively) or coil em
| Treatment | CYA+ coil (combination therapy) | CYA alone | Coil alone | P value (combination vs CYA alone/combination vs coil alone) |
| Outcome rate (%) | ||||
| Technical success | 100 | 97 | 99 | < 0.001/< 0.001 |
| Clinical success | 98 | 96 | 90 | < 0.001/< 0.001 |
| Adverse event | 10 | 21 | 3 | < 0.001/0.057 |
| Adverse event | 14 | 30 | 17 | < 0.001/1.00 |
| Re-intervention | 15 | 26 | 25 | < 0.001/0.047 |
Based upon current treatment algorithms, and understanding the limitations of currently available data, EUS-guided treatment for GV should be reserved for cardiofundal varices. The main advantages of this approach include acute hemostasis and prevention of rebleeding. Furthermore, the use of EUS allows delineation of the vascular anatomy of the variceal complex, which can enable precise delivery of therapy into the varix lumen or afferent vessel (potentially decreasing the risk of embolization) and allow confirmation of vessel obliteration via Doppler examination[32-34]. Cyanoacrylate is off-label for the treatment of GV hemorrhage in the United States, so its use should be limited to centers with appropriately trained endoscopists and experience[2,35].
EUS-guided interventions for the diagnosis and management of PH and its complications have evolved from a novel innovation into a useful clinical tool with a growing evidence-base supporting its role.
Available data suggests that EUS-LB results in comparable diagnostic adequacy (i.e., tissue specimen) to currently available options with similar low rates of adverse events[14]. Measurements of PPG correlate with HVPG measurements and have a similar safety profile[19,20]. An additional benefit is the direct measurement of the portal vein pressure, allowing diagnosis of prehepatic/presinusoidal PH that is not obtained during HVPG measurements as well as the ability to perform liver biopsy. EUS- treatment for GV bleeding may be more effective than current endoscopic therapies, and offers several potential advantages[25,31].
EUS-guided interventions have demonstrated similar efficacy and safety to current standards of care, and should be viewed as a complement (not a replacement) to current diagnostic and therapeutic modalities. A multidisciplinary approach between Hepatologists and EUS-trained endoscopists is vital to ensure appropriate patient selection, ensure accurate and useful data are generated from diagnostic procedures, and that maximal therapeutic benefit is derived from EUS-guided treatments.
The authors wish to thank Cook Medical Endoscopy (Winston-Salem, NC) for their generous sharing of medical illustrations used in the preparation of this manuscript.
| 1. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 789] [Article Influence: 131.5] [Reference Citation Analysis (1)] |
| 2. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1499] [Article Influence: 166.6] [Reference Citation Analysis (3)] |
| 3. | Ripoll C, Bañares R, Rincón D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, Núñez O, Matilla A, Molinero LM. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 5. | Chang KJ, Samarasena JB, Iwashita T, Nakai Y, Lee JG. Endo-hepatology: a new paradigm. Gastrointest Endosc Clin N Am. 2012;22:379-385, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1632] [Article Influence: 96.0] [Reference Citation Analysis (2)] |
| 7. | Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol. 2020;36:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Castéra L, Nègre I, Samii K, Buffet C. Patient-administered nitrous oxide/oxygen inhalation provides safe and effective analgesia for percutaneous liver biopsy: a randomized placebo-controlled trial. Am J Gastroenterol. 2001;96:1553-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL, Dienstag JL; HALT–C Trial Group. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 10. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 807] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 11. | Mathew A. EUS-guided routine liver biopsy in selected patients. Am J Gastroenterol. 2007;102:2354-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Shah AR, Al-Hanayneh M, Chowdhry M, Bilal M, Singh S. Endoscopic ultrasound guided liver biopsy for parenchymal liver disease. World J Hepatol. 2019;11:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Bhogal N, Lamb B, Arbeiter B, Malik S, Sayles H, Lazenby AJ, Chandan S, Dhaliwal A, Singh S, Bhat I. Safety and adequacy of endoscopic ultrasound-guided random liver biopsy in comparison with transjugular and percutaneous approaches. Endosc Int Open. 2020;8:E1850-E1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:238-246.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Lavian JD, Thornton LM, Zybulewski A, Kim E, Nowakowski SF, Ranade M, Patel RS, Lookstein RA, Fischman A, Bishay V. Safety of percutaneous versus transjugular liver biopsy: A propensity score matched analysis. Eur J Radiol. 2020;133:109399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1589] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 17. | Turco L, Garcia-Tsao G. Portal Hypertension: Pathogenesis and Diagnosis. Clin Liver Dis. 2019;23:573-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 19. | Huang JY, Samarasena JB, Tsujino T, Chang KJ. EUS-guided portal pressure gradient measurement with a novel 25-gauge needle device versus standard transjugular approach: a comparison animal study. Gastrointest Endosc. 2016;84:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, Chen WP, Chang KJ. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 872] [Article Influence: 25.6] [Reference Citation Analysis (42)] |
| 23. | Henry Z, Uppal D, Saad W, Caldwell S. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol. 2014;12:919-28.e1; quiz e51-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Bick BL, Al-Haddad M, Liangpunsakul S, Ghabril MS, DeWitt JM. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc. 2019;33:1837-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, Chung SC, Sung JJ. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 27. | Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Ríos Castellanos E, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev. 2015;CD010180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, Binmoeller KF. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc. 2016;83:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 30. | Cheng LF, Wang ZQ, Li CZ, Lin W, Yeo AE, Jin B. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol. 2010;8:760-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Ryou M. Combination therapy versus monotherapy for EUS-guided management of gastric varices: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 32. | Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, Marcos-Sanchez F, Caunedo-Alvarez A, Ortiz-Moyano C, Gomez-Parra M, Herrerias-Gutierrez JM. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc. 2007;66:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Gubler C, Bauerfeind P. Safe and successful endoscopic initial treatment and long-term eradication of gastric varices by endoscopic ultrasound-guided Histoacryl (N-butyl-2-cyanoacrylate) injection. Scand J Gastroenterol. 2014;49:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Iwase H, Suga S, Morise K, Kuroiwa A, Yamaguchi T, Horiuchi Y. Color Doppler endoscopic ultrasonography for the evaluation of gastric varices and endoscopic obliteration with cyanoacrylate glue. Gastrointest Endosc. 1995;41:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | ASGE Technology Committee; Bhat YM, Banerjee S, Barth BA, Chauhan SS, Gottlieb KT, Konda V, Maple JT, Murad FM, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL, Wang A, Rodriguez SA. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc. 2013;78:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LW S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY