Published online Apr 27, 2021. doi: 10.4254/wjh.v13.i4.456
Peer-review started: January 3, 2021
First decision: January 25, 2021
Revised: February 6, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: April 27, 2021
Processing time: 102 Days and 17.3 Hours
Acute cholangitis (AC) is a disease spectrum with varying extent of severity. Age ≥ 75 years forms part of the criteria for moderate (Grade II) severity in both the Tokyo Guidelines (TG13 and TG18). Aging is associated with reduced physiological reserves, frailty, and sarcopenia. However, there is evidence that age itself is not the determinant of inferior outcomes in elective and emergency biliary diseases. There is a paucity of reports comparing clinical outcomes amongst elderly patients vs non-elderly patients with AC.
To investigate the effect of age (≥ 80 years) on AC's morbidity and mortality using propensity score matching (PSM).
This is a single-center retrospective cohort study of all patients diagnosed with calculous AC (January 2016 to December 2016) and ≥ 80 years old (January 2012 to December 2016) at a tertiary university-affiliated teaching hospital. Inclusion criteria were patients who were treated for suspected or confirmed AC secondary to biliary stones. Patients with AC on a background of hepatobiliary malignancy, indwelling permanent metallic biliary stents, or concomitant pancreatitis were excluded. Elderly patients were defined as ≥ 80 years old in our study. A 1:1 PSM analysis was performed to reduce selection bias and address confounding factors. Study variables include comorbidities, vital parameters, laboratory and radiological investigations, and type of biliary decompression, including the time for endoscopic retrograde cholangiopancreatography (ERCP). Primary outcomes include in-hospital mortality, 30-d and 90-d mortality. Length of hospital stay (LOS) was the secondary outcome.
Four hundred fifty-seven patients with AC were included in this study (318 elderly, 139 non-elderly). PSM analysis resulted in a total of 224 patients (112 elderly, 112 non-elderly). The adoption of ERCP between elderly and non-elderly was similar in both the unmatched (elderly 64.8%, non-elderly 61.9%, P = 0.551) and matched cohorts (elderly 68.8% and non-elderly 58%, P = 0.096). The overall in-hospital mortality, 30-d mortality and 90-d mortality was 4.6%, 7.4% and 8.5% respectively, with no statistically significant differences between the elderly and non-elderly in both the unmatched and matched cohorts. LOS was longer in the unmatched cohort [elderly 8 d, interquartile range (IQR) 6-13, vs non-elderly 8 d, IQR 5-11, P = 0.040], but was comparable in the matched cohort (elderly 7.5 d, IQR 5-11, vs non-elderly 8 d, IQR 5-11, P = 0.982). Subgroup analysis of patients who underwent ERCP demonstrated the majority of the patients (n = 159/292, 54.5%) had delayed ERCP (> 72 h from presentation). There was no significant difference in LOS, 30-d mortality, 90-d mortality, and in-hospital mortality in patients who had delayed ERCP in both the unmatched and matched cohort (matched cohort: in-hospital mortality [n = 1/42 (2.4%) vs 1/26 (3.8%), P = 0.728], 30-d mortality [n = 2/42 (4.8%) vs 2/26 (7.7%), P = 0.618], 90-d mortality [n = 2/42 (4.8%) vs 2/26 (7.7%), P = 0.618], and LOS (median 8.5 d, IQR 6-11.3, vs 8.5 d, IQR 6-15.3, P = 0.929).
Mortality is indifferent in the elderly (≥ 80 years old) and non-elderly patients (< 80 years old) with AC.
Core Tip: There is a paucity of data on mortality outcomes amongst elderly vs non-elderly patients with acute cholangitis. The overall in-hospital mortality, 30-d mortality and 90-d mortality was 4.6%, 7.4% and 8.5% respectively, with no significant differences in both the unmatched and matched cohorts. Mortality was comparable in patients with delayed endoscopic retrograde cholangiopancreatography.
- Citation: Chan KS, Mohan R, Low JK, Junnarkar SP, Huey CWT, Shelat VG. Elderly patients (≥ 80 years) with acute calculous cholangitis have similar outcomes as non-elderly patients (< 80 years): Propensity score-matched analysis. World J Hepatol 2021; 13(4): 456-471
- URL: https://www.wjgnet.com/1948-5182/full/v13/i4/456.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i4.456
Gallstones are widely prevalent in the community, and patients with gallstones are at risk of complications like acute cholecystitis, acute pancreatitis, and acute cholangitis (AC). AC results from an obstructed biliary system with sepsis, and resulting endotoxic shock is associated with a mortality risk of up to 20%[1]. AC is a disease spectrum ranging from mild AC, which may respond to conservative management with medical therapy, to severe AC, which requires urgent biliary decompression in addition to fluid resuscitation and antibiotics[2]. Tokyo Guidelines (TG13 and TG18) are widely accepted internationally and form the basis for diagnosis, severity stratification, and management of patients with AC[3]. In AC, age determines the severity stratification, and age ≥ 75 years is a criterion for moderate (Grade II) severity in both the TG13 and TG18 guidelines[3]. Aging is associated with reduced cardiac output, impaired gas exchange, reduction in vital capacity, decline in lean body mass, creatinine clearance reduction, hepatic drug metabolism impairment, frailty, and sarcopenia[4]. Due to functional metabolic decline, multiple comorbidities, and atypical presentation with potential diagnostic delays, age contributes to inferior outcomes[5]. Age is an independent predictor of mortality in lower respiratory tract infections, urinary tract infections, gastrointestinal infections and biliary infections[5-8]. Age is also a predictor of disease severity with higher morbidity and mortality risk[9].
However, there is evidence that age itself is not the determinant of inferior outcomes in elective and emergency biliary diseases[10,11]. Endoscopic retrograde cholangiopancreatography (ERCP) have been demonstrated to be safe with good outcomes in elderly patients[11,12]. In a study including 149 acute cholecystitis patients treated with emergency laparoscopic cholecystectomy (LC), Amirthalingam et al[13] showed that patient comorbidities and not age determine outcomes. In a study reporting 85 patients with a median age of 83 years (interquartile range 80-89) and admitted to intensive care unit (ICU) with a diagnosis of AC, Novy et al[14] reported malnutrition [odds ratio (OR) = 34.5, 95% confidence interval (CI): 1.4-817.9] and sequential organ failure assessment (SOFA) score at 48 h (OR by unit 0.7, 95%CI: 0.5-0.9) were associated with higher 6-mo mortality. Further, aging may impact other clinically relevant non-mortality outcomes such as length of hospital stay (LOS). In a prospective study including 124 patients with acute hepatobiliary sepsis and a median age of 64.5 years, Mak et al[15] have reported that age predicts LOS. There is a paucity of comparative data reporting mortality and LOS amongst elderly and non-elderly patients with AC. Also, aging is associated with the confounding effect of comorbidity. This, along with heterogeneity of evidence reporting outcomes in patients with diverse etiology of AC, leaves a lacuna in the scientific literature on the real impact of age on patients with AC due to stone disease. Our hypothesis is, age ≥ 80 years old is associated with higher mortality in patients with AC. This propensity score-matched study aims to investigate if mortality is higher in the elderly (≥ 80 years old) patients with AC as compared to non-elderly (< 80 years old).
This is a single-center retrospective cohort study of all patients diagnosed with calculous AC (January 2016 to December 2016) and ≥ 80 years old AC patients (January 2012 to December 2016) at a tertiary university-affiliated teaching hospital. We included patients treated for a suspected or confirmed AC diagnosis due to biliary stones[16]. Patients with AC on a background of hepatobiliary malignancy, indwelling permanent metallic biliary stents, or concomitant pancreatitis were excluded. The severity grading of AC in the TG13 included age greater than 75 years as a risk factor, which was retained in TG18[17]. Due to a higher sample of elderly patients, the overall cohort's median age was > 80 years, so we defined elderly as ≥ 80 years old. Non-elderly was defined as patients < 80 years old. Our local institutional review board (National Healthcare Group Domain Specific Review Board, No. 2017/00200) approved this study. This study's conduct is per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for retrospective cohort studies[18].
The patient demographics and clinical outcomes were studied. Patient demographics included age, gender, and comorbidities. Comorbidities included diabetes mellitus, ischemic heart disease, chronic obstructive pulmonary disease, asthma, chronic renal failure, and biliary disease history. Previous history of biliary colic, acute cholecystitis, AC, and acute biliary pancreatitis were collectively defined as history of biliary disease. Presenting symptoms at admission included abdominal pain, fever, vomiting, jaundice, and hypotension. Hypotension was defined as admission systolic blood pressure < 90 mmHg. Laboratory data included white blood cell count, platelet count, creatinine, prothrombin time, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, albumin, gamma-glutamyl transferase, and total bilirubin levels. The shock index (SI) was defined as heart rate divided by the respective systolic blood pressure on arrival in triage[19,20]. Abnormal SI was defined as SI < 0.5 or > 0.7). In patients undergoing ERCP and cholecystectomy, procedure-related data and outcomes were collected. Delayed ERCP was defined as ERCP > 72 h from admission. The primary outcomes of this study were in-hospital mortality, 30-d mortality and 90-d mortality. In-hospital mortality was defined as any deaths which occurred during the same hospital admission, regardless of the duration from admission. The 30-d and 90-d mortality were defined as any deaths (including both patients who were still inpatient and those who were discharged) within 30 d and 90 d from admission. The secondary clinical outcome was LOS.
Patients who presented with septic shock were managed according to the Surviving Sepsis Campaign Guidelines for Management of Severe Sepsis and Septic Shock, 2012[21]. The definite diagnosis of AC was based on the TG13 Guidelines, namely, evidence of systemic inflammation (fever, chills, or laboratory data), cholestasis (jaundice or laboratory data), and imaging of the biliary tree (dilatation, stricture, stone, or stent)[16]. The severity was graded as mild, moderate, or severe as per TG13 guidelines[16]. Out unit was involved in TG07 classification, and we were early adopters of the TG13 system. Thus, the majority of patients had TG13 stratification done prospectively. Patients that were included before the TG13 publication were retrospectively assigned TG13 diagnosis and severity stratification. Blood cultures were taken for all patients included in our study. Broad-spectrum empiric intravenous antibiotics were administered based on local antibiogram and in compliance with the World Society of Emergency Surgery guidelines for optimal and rational use of antibiotics in intra-abdominal sepsis[22,23]. Patients with mild AC, patients who declined invasive intervention, and patients who were responsive to antibiotics alone were managed conservatively. Urgent biliary drainage was performed for patients with moderate and severe AC. The endoscopists’ discretion and resources determined the timing of biliary drainage. ERCP was the first-line modality for biliary drainage. A diclofenac suppository is inserted routinely for post-ERCP acute pancreatitis prophylaxis. Percutaneous transhepatic biliary drainage (PTBD) was offered when ERCP was not feasible or contraindicated. Complete stone removal or temporary placement of biliary stents was performed at the endoscopists’ discretion. Index admission cholecystectomy was reserved for patients with mild AC and subject to surgeon preference.
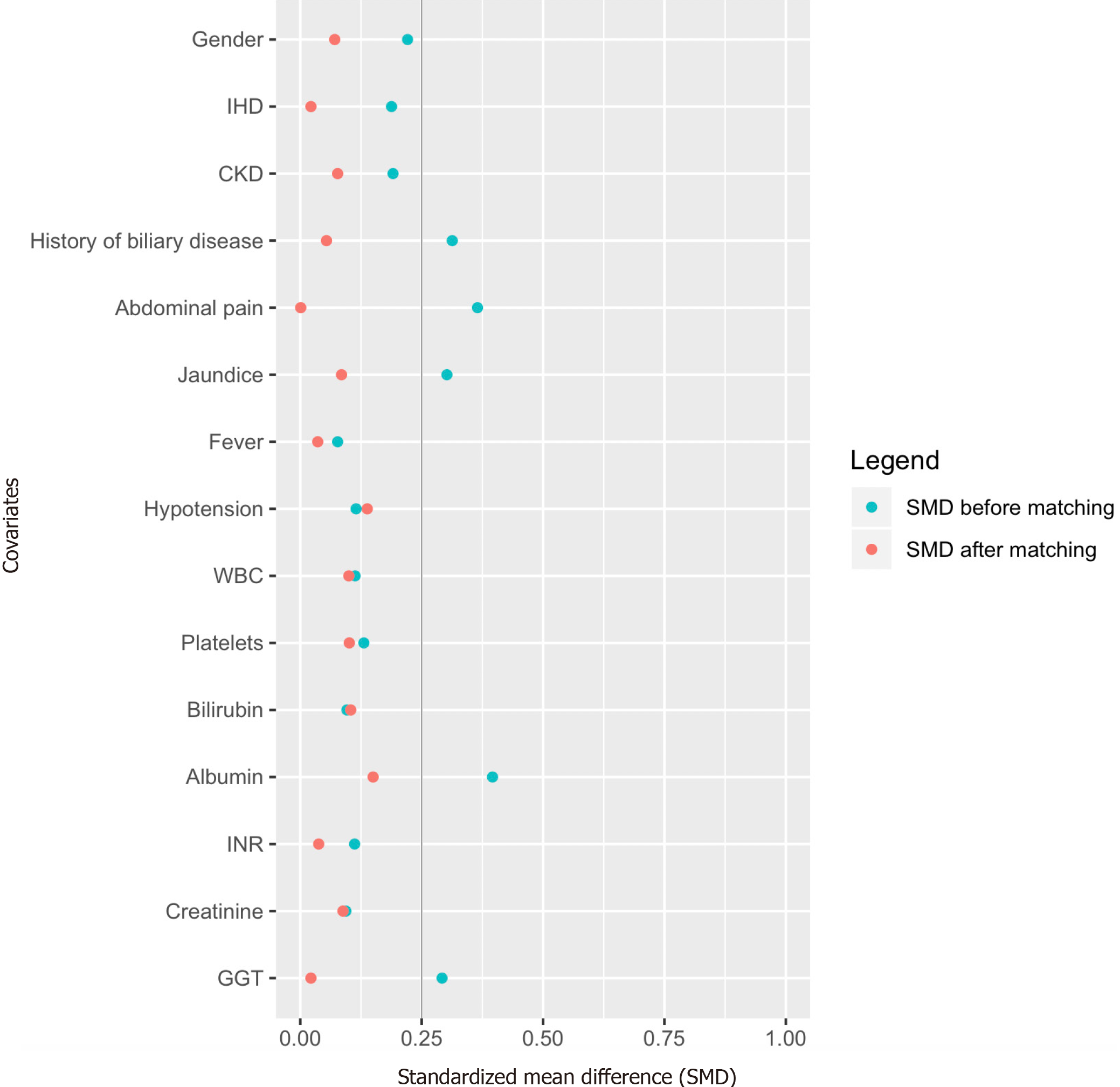
A 1:1 propensity score matching (PSM)[24] was performed by the first author (Chan KS). PSM was performed at a ratio of 1:1 using a caliper width of 0.2 of the standard deviation of the logit of the propensity score[25]. Patients were adjusted for 15 factors. Seven factors: clinical presentation (fever and hypotension) and laboratory investigations (white blood cell count, platelets, bilirubin, international normalized ratio, and albumin) impact clinical outcomes and thus were adjusted[16,26]. Eight factors were statistically significant (P < 0.1) during comparison of the initial demographics between the elderly and non-elderly: gender, comorbidities (ischemic heart disease, chronic renal impairment, and history of biliary disease), clinical presentation (abdominal pain, jaundice), and laboratory investigations (gamma-glutamyl transferase and creatinine), and thus were adjusted. Standardized mean difference (SMD) and Hansen and Bowers were used to assess for covariate and global imbalance, respectively[27].
Categorical values were described as percentages and analyzed by the chi-square test. Continuous variables were expressed as median (interquartile range, IQR) and analyzed by the Mann-Whitney U test, respectively. Statistical significance was determined by P < 0.05. All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, III., United States) and R software (R-3.3.3). The statistical review was performed by one of the co-authors qualified in biomedical statistics (Shelat VG).
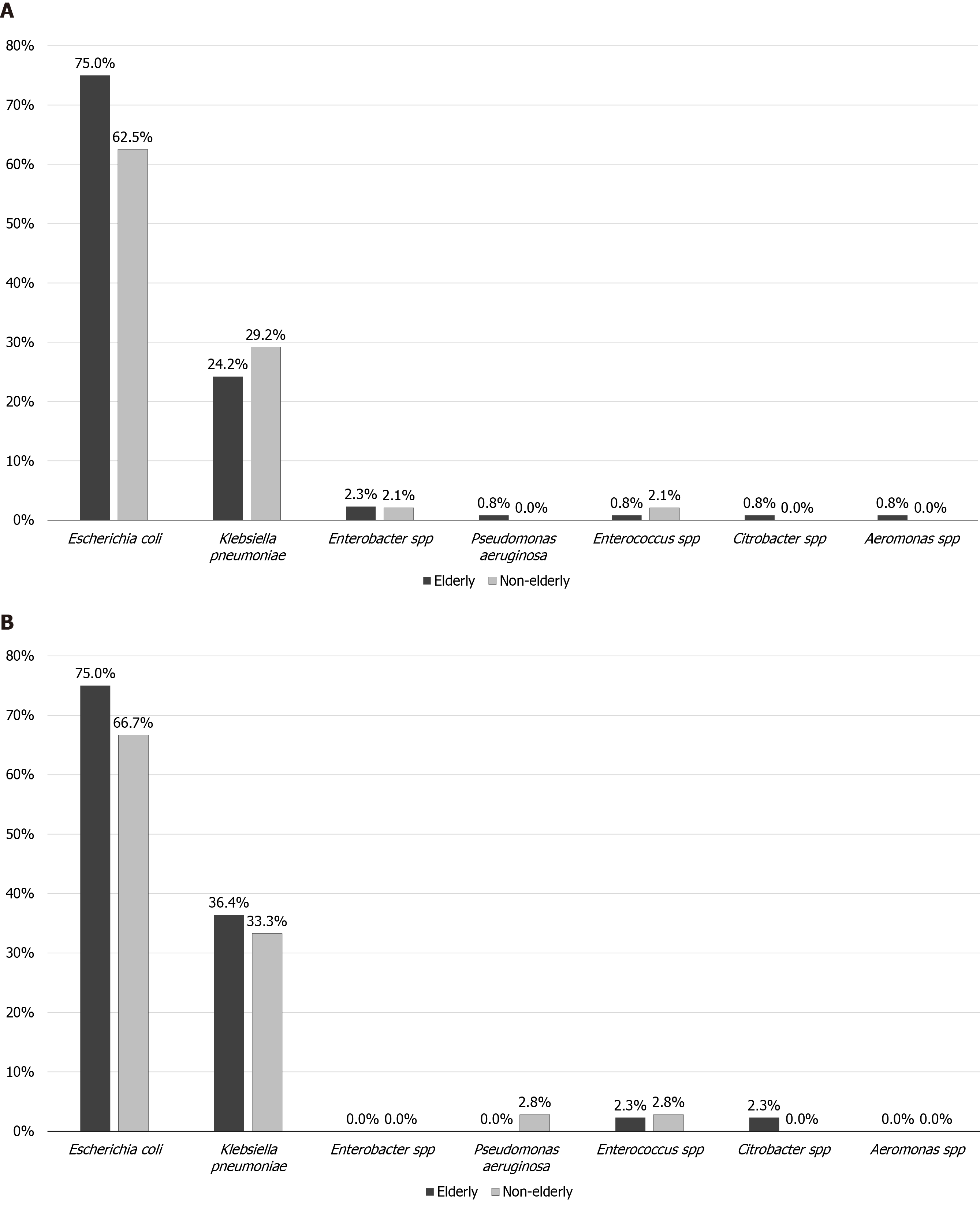
Five hundred fifty-six patients were managed for AC during the study period. Ninety-nine AC patients were excluded due to underlying malignancy. Four hundred fifty-seven patients met the inclusion: 318 (69.6%) elderly vs 139 (30.4%) non-elderly. The overall cohort's median age was 82.4 years (IQR 77.6-85.3), with female predominance (n = 252/457, 55.1%). About half (n = 240/457, 52.5%) of patients had a biliary disease history. One hundred and eighty (39.4%) patients had positive blood cultures, and Escherichia coli was the most common pathogen (n = 129/180, 71.7%). Figure 1 summarizes the microbiology of patients who had positive blood cultures. One hundred and ninety-eight (43.3%) and 126 (27.6%) patients had Grade II and Grade III AC, respectively. When the data of overall cohort was analyzed according to the timing of ERCP (≤ 72 h vs > 72 h from admission), there was no difference in the ERCP timing for patients with at least Grade II AC [≤ 72 h, n = 88/201 (43.8%) vs > 72 h, n = 113/201 (52.2%), P = 0.368].
PSM with a 1:1 ratio resulted in 224 patients (elderly 112, non-elderly 112). Before PSM, 5 of 15 unmatched variables had SMD > 0.25; following PSM, all of the variables reached an SMD < 0.25 (Table 1 and Figure 2), suggesting an adequate and improved balance. Hansen and Bowers test for global significance also did not demonstrate statistical significance in the matched cohort (matched cohort: χ2: 4.73, P = 0.994; unmatched cohort: χ2: 67.4, P < 0.001). Baseline demographics in both the unmatched and matched cohorts are summarized in Table 1. The adoption of biliary drainage procedures was similar between elderly and non-elderly patients in the unmatched cohort. Eleven (3.5%) and 2 (1.4%) elderly and non-elderly respectively received urgent biliary drainage. However, in the matched cohort, elderly patients were more likely to undergo PTBD than non-elderly patients (11.6% vs 4.5%, OR 2.81, P = 0.049). Incidence of index admission cholecystectomy and interval cholecystectomy was also comparable between elderly and non-elderly patients in the unmatched cohort. However, in the matched cohort, elderly patients were less likely to undergo index admission cholecystectomy (1.8% vs 10.7%, OR 0.15, P = 0.006).
| Overall cohort, n = 457 | PSM cohort, n = 224 | |||||||
| Elderly, n = 318 | Non-elderly, n = 139 | P value | SMD | Elderly, n = 112 | Non-elderly, n = 112 | P value | SMD | |
| Age, yr | 84.0 (82.1, 86.6) | 67.9 (57.1, 77.2) | < 0.001 | 84.3 (82.1, 87.3) | 66.6 (55.6, 76.4) | < 0.001 | ||
| Gender1, male (%) | 132 (41.5) | 73 (52.5) | 0.029 | 0.221 | 57 (50.9) | 53 (47.3) | 0.593 | 0.071 |
| Co-morbidities, n (%) | ||||||||
| Diabetes mellitus | 124 (39) | 55 (39.6) | 0.908 | 44 (39.3) | 47 (42) | 0.683 | ||
| Ischemic heart disease1 | 87 (27.4) | 27 (19.4) | 0.071 | 0.188 | 22 (19.6) | 23 (20.5) | 0.868 | 0.022 |
| Chronic renal impairment1 | 61 (19.2) | 17 (12.2) | 0.069 | 0.191 | 17 (15.2) | 14 (12.5) | 0.562 | 0.077 |
| COPD and/or asthma | 18 (5.7) | 4 (2.9) | 0.201 | 8 (7.1) | 3 (2.7) | 0.122 | ||
| History of biliary disease1 | 182 (57.2) | 58 (41.7) | 0.002 | 0.313 | 50 (44.6) | 53 (47.3) | 0.688 | 0.054 |
| Clinical presentation | ||||||||
| Abdominal pain1 | 197 (61.9) | 109 (78.4) | 0.001 | 0.365 | 85 (75.9) | 85 (75.9) | 1.000 | < 0.001 |
| Fever1 | 141 (44.3) | 67 (48.2) | 0.446 | 0.077 | 50 (44.6) | 52 (46.4) | 0.788 | 0.036 |
| Vomiting | 142 (44.7) | 63 (45.3) | 0.895 | 51 (45.5) | 50 (44.6) | 0.893 | ||
| Jaundice1 | 48 (15.1) | 38 (27.3) | 0.002 | 0.302 | 27 (24.1) | 23 (20.5) | 0.521 | 0.085 |
| Hypotension1,2 | 18 (5.7) | 12 (8.6) | 0.238 | 0.115 | 10 (8.9) | 6 (5.4) | 0.299 | 0.138 |
| Laboratory investigations | ||||||||
| WBC1 (109/L) | 12.4 (8.9, 16.1) | 12.1 (8.3, 15.9) | 0.551 | 0.113 | 12.2 (8.6, 15.4) | 12.1 (8.0, 16.2) | 0.745 | 0.100 |
| Platelets1 (109/L) | 192 (150, 250) | 216 (166, 280) | 0.047 | 0.131 | 193 (160, 252) | 209 (162, 280) | 0.308 | 0.101 |
| Creatinine1 (μmol/L) | 103 (81, 138) | 89 (68, 116) | < 0.001 | 0.094 | 103 (80, 136) | 86 (67, 119) | 0.003 | 0.088 |
| Albumin1 (g/L) | 32 (28, 35) | 35 (29, 38) | < 0.001 | 0.396 | 33 (29, 36) | 34 (29, 38) | 0.186 | 0.150 |
| Bilirubin1 (μmol/L) | 54 (33, 84) | 60 (34, 96) | 0.226 | 0.096 | 65 (42, 93) | 58 (33, 93) | 0.287 | 0.104 |
| ALT (IU/L) | 133 (61, 247) | 143 (68, 295) | 0.330 | 142 (82, 244) | 123 (59, 263) | 0.294 | ||
| AST (IU/L) | 160 (78, 366) | 140 (72, 314) | 0.165 | 176 (93, 365) | 150 (74, 345) | 0.149 | ||
| ALP (IU/L) | 209 (130, 346) | 188 (117, 314) | 0.149 | 208 (137, 346) | 184 (111, 291) | 0.136 | ||
| GGT1 (IU/L) | 242 (129, 435) | 327 (158, 562) | 0.006 | 0.292 | 286 (165, 504) | 286 (133, 523) | 0.591 | 0.022 |
| INR1 | 1.13 (1.02, 1.30) | 1.20 (1.10, 1.30) | 0.890 | 0.112 | 1.15 (1.00, 1.30) | 1.20 (1.10, 1.30) | 0.506 | 0.038 |
| Microbiology, positive (%) | 132 (41.5) | 48 (34.5) | 0.160 | 44 (39.3) | 36 (32.1) | 0.265 | ||
| Escherichia coli | 99 (75) | 30 (62.5) | 0.100 | 33 (75) | 24 (66.7) | 0.413 | ||
| Klebsiella pneumoniae | 32 (24.2) | 14 (29.2) | 0.503 | 16 (36.4) | 12 (33.3) | 0.777 | ||
| Enterobacter spp | 3 (2.3) | 1 (2.1) | 0.939 | 0 (0) | 1 (2.8) | 0.266 | ||
| Pseudomonas aeruginosa | 1 (0.8) | 0 (0) | 0.550 | 0 (0) | 0 (0) | - | ||
| Enterococcus spp | 1 (0.8) | 1 (2.1) | 0.453 | 1 (2.3) | 1 (2.8) | 0.886 | ||
| Citrobacter spp | 1 (0.8) | 0 (0) | 0.545 | 1 (2.3) | 0 (0) | 0.357 | ||
| Aeromonas spp | 1 (0.8) | 0 (0) | 0.545 | 0 (0) | 0 (0) | - | ||
| CT scan, n (%) | 108 (34) | 52 (37.4) | 0.477 | 43 (38.4) | 45 (40.2) | 0.784 | ||
| Cholelithiasis | 75 (69.4) | 31 (59.6) | 0.218 | 21 (48.8) | 14 (31.1) | 0.089 | ||
| Biliary dilation | 47 (43.5) | 18 (34.6) | 0.283 | 30 (69.8) | 26 (57.8) | 0.243 | ||
| Choledocholithiasis | 63 (58.3) | 18 (34.6) | 0.005 | 27 (62.8) | 14 (31.1) | 0.003 | ||
| MRCP, n (%) | 157 (49.4) | 73 (52.5) | 0.536 | 61 (54.5) | 55 (49.1) | 0.422 | ||
| Cholelithiasis | 113 (72) | 38 (52.1) | 0.003 | 37 (60.7) | 35 (63.6) | 0.741 | ||
| Biliary dilation | 93 (59.2) | 50 (68.5) | 0.178 | 41 (67.2) | 26 (47.3) | 0.030 | ||
| Choledocholithiasis | 103 (65.6) | 39 (53.4) | 0.077 | 41 (67.2) | 23 (41.8) | 0.006 | ||
| Shock Index, abnormal3 | 194 (61) | 88 (63.3) | 0.641 | 66 (58.9) | 73 (65.2) | 0.335 | ||
| TG13 severity grading | 2 (2, 3) | 2 (1, 2) | < 0.001 | 2 (1, 3) | 2 (1, 2) | 0.016 | ||
| Grade I | 67 (21.1) | 66 (47.5) | 31 (27.7) | 46 (41.1) | ||||
| Grade II | 152 (47.8) | 46 (33.1) | 49 (43.8) | 46 (41.1) | ||||
| Grade III | 99 (31.1) | 27 (19.4) | 32 (28.6) | 20 (17.9) | ||||
The overall in-hospital mortality, 30-d mortality and 90-d mortality was 4.6%, 7.4% and 8.5% respectively; this was comparable between elderly vs non-elderly in both unmatched and matched cohorts. Peri-operative outcomes are summarized in Table 2. In the unmatched cohort, elderly patients had a statistically significant longer LOS (median 8 d, IQR 6-13 vs 8 d, IQR 5-11, P = 0.040). However, after matching, LOS was similar (median 7.5 d, IQR 5-11 vs 8 d, IQR 5-11, P = 0.982).
| Overall cohort, n = 457 | PSM cohort, n = 224 | |||||||
| Elderly, n = 318 | Non-elderly, n = 139 | OR, 95%CI | P value | Elderly, n = 112 | Non-elderly, n = 112 | OR, 95%CI | P value | |
| Initial management | ||||||||
| ERCP | 206 (64.8) | 86 (61.9) | 1.13 (0.75, 1.71) | 0.551 | 77 (68.8) | 65 (58) | 1.59 (0.02, 2.75) | 0.096 |
| Percutaneous transhepatic biliary drainage | 25 (7.9) | 6 (4.3) | 1.89 (0.76, 4.72) | 0.166 | 13 (11.6) | 5 (4.5) | 2.81 (0.97, 8.17) | 0.049 |
| Conservative | 98 (30.8) | 49 (35.3) | 0.82 (0.54, 1.25) | 0.351 | 29 (25.9) | 43 (38.4) | 0.56 (0.32, 0.99) | 0.045 |
| Subsequent management | ||||||||
| Index admission cholecystectomy | 16 (5.0) | 13 (9.4) | 0.51 (0.24, 1.10) | 0.081 | 2 (1.8) | 12 (10.7) | 0.15 (0.03, 0.69) | 0.006 |
| Interval cholecystectomy | 20 (6.3) | 11 (7.9) | 0.78 (0.36, 1.68) | 0.525 | 7 (6.3) | 10 (8.9) | 0.68 (0.25, 1.86) | 0.449 |
| Length of hospital stay, days | 8 (6, 13) | 8 (5, 11) | - | 0.040 | 7.5 (5, 11) | 8 (5, 11) | - | 0.982 |
| In-hospital mortality | 16 (5.0) | 5 (3.6) | 1.42 (0.51, 3.96) | 0.500 | 6 (5.4) | 5 (4.5) | 1.21 (0.36, 4.09) | 0.757 |
| 30-d mortality | 27 (8.5) | 7 (5) | 1.75 (0.74, 4.12) | 0.195 | 8 (7.1) | 7 (6.3) | 1.15 (0.40, 3.30) | 0.789 |
| 90-d mortality | 31 (9.7) | 8 (5.8) | 1.77 (0.79, 3.95) | 0.160 | 8 (7.1) | 8 (7.1) | 1.00 (0.36, 2.77) | 1.000 |
Table 3 summarizes the outcomes of patients who underwent ERCP. In the unmatched subgroup of patients who underwent ERCP and had delayed ERCP (> 72 h from admission) (elderly n = 121, non-elderly n = 38), the primary and secondary outcomes were indifferent between elderly and non-elderly patients respectively: in-hospital mortality [n = 2/121 (1.7%) vs 1/38 (2.6%), P = 0.699], 30-d mortality [n = 9/121 (7.4%) vs 2/38 (5.3%), P = 0.645], 90-d mortality [n = 11/121 (9.1%) vs 2/38 (5.3%), P = 0.453], and LOS (median 10 d, IQR 7-15 vs 8 d, IQR 6-12, P = 0.103). These outcomes remain indifferent after PSM matching: in-hospital mortality [n = 1/42 (2.4%) vs 1/26 (3.8%), P = 0.728], 30-d mortality [n = 2/42 (4.8%) vs 2/26 (7.7%), P = 0.618], 90-d mortality [n = 2/42 (4.8%) vs 2/26 (7.7%), P = 0.618], and LOS (median 8.5 d, IQR 6-11.3 vs 8.5 d, IQR 6-15.3, P = 0.929).
| Overall cohort, n = 292 | PSM cohort, n = 142 | |||||||
| Elderly, n = 206 | Non-elderly, n = 86 | OR, 95%CI | P value | Elderly, n = 77 | Non-elderly, n = 65 | OR, 95%CI | P value | |
| Timing of ERCP from presentation | 0.012 | - | 0.247 | |||||
| Within 24 h | 15 (7.3) | 16 (18.6) | 9 (11.7) | 12 (18.5) | ||||
| 24-48 h | 36 (17.5) | 13 (15.1) | 14 (18.2) | 11 (16.9) | ||||
| 48-72 h | 34 (16.5) | 19 (22.1) | 12 (15.6) | 16 (24.6) | ||||
| >72 h | 121 (58.7) | 38 (44.2) | 42 (54.6) | 26 (40) | ||||
| Stone(s) removed | 102 (49.5) | 44 (51.2) | 0.94 (0.57, 1.55) | 0.797 | 36 (46.8) | 30 (46.2) | 1.02 (0.53, 1.99) | 0.943 |
| Stent placed | 89 (43.2) | 38 (44.2) | 0.96 (0.58, 1.60) | 0.877 | 35 (45.5) | 30 (46.2) | 0.97 (0.50, 1.89) | 0.934 |
| Length of hospital stay, d | 9 (7, 13) | 8 (5, 11) | - | 0.016 | 8 (5, 12) | 8 (5, 12) | - | 0.546 |
| In-hospital mortality | 2 (1) | 1 (1.2) | 0.83 (0.08, 9.31) | 0.882 | 1 (1.3) | 1 (1.5) | 0.84 (0.05, 13.73) | 0.904 |
| 30-d mortality | 13 (6.3) | 4 (4.7) | 1.38 (0.44, 4.36) | 0.581 | 3 (3.9) | 4 (6.2) | 0.62 (0.13, 2.87) | 0.536 |
| 90-d mortality | 16 (7.8) | 4 (4.7) | 1.73 (0.56, 5.32) | 0.337 | 3 (3.9) | 4 (6.2) | 0.62 (0.13, 2.87) | 0.536 |
In the unmatched cohort, an abnormal SI was not associated with ERCP [abnormal SI: 178/282 (63.1%) vs normal SI: 114/175 (65.1%), P = 0.662]. This was observed in both the elderly [abnormal SI: 125/194 (64.4%) vs normal SI: 81/124 (65.3%), P = 0.871] and the non-elderly [abnormal SI: 53/88 (60.2%) vs normal SI: 33/51 (64.7%), P = 0.600]. There was no difference after PSM matching on the association of abnormal SI with ERCP: abnormal SI: 90/139 (64.7%) vs normal SI: 52/85 (61.2%), P = 0.590. This was true in both the elderly [abnormal SI: 48/66 (72.7%) vs normal SI: 29/46 (63%), P = 0.277] and the non-elderly [abnormal SI: 42/73 (57.5%) vs normal SI: 23/39 (59%), P = 0.883]. Subgroup analysis of patients with an abnormal SI on triage did not show any significant differences in outcomes between elderly and non-elderly patients (Table 4).
| Overall cohort, n = 282 | PSM cohort, n = 139 | |||||||
| Elderly, n = 194 | Non-elderly, n = 88 | OR, 95%CI | P value | Elderly, n = 66 | Non-elderly, n = 73 | OR, 95%CI | P value | |
| Length of hospital stay, d | 8 (6-13) | 8 (6-10.8) | 0.379 | 8 (5-12) | 6 (5-10) | 0.217 | ||
| In-hospital mortality | 10 (5.2) | 3 (3.4) | 1.54 (0.41, 5.74) | 0.517 | 3 (4.5) | 3 (4.1) | 1.11 (0.22, 5.71) | 0.900 |
| 30-d mortality | 19 (9.8) | 4 (4.5) | 2.28 (0.75, 6.91) | 0.136 | 5 (7.6) | 4 (5.5) | 1.41 (0.36, 5.50) | 0.616 |
| 90-d mortality | 20 (10.3) | 5 (5.7) | 1.91 (0.62, 5.26) | 0.205 | 5 (7.6) | 5 (6.8) | 1.12 (0.31, 4.04) | 0.869 |
In this single-center propensity score-matched study, patients ≥ 80 years old with AC due to biliary stone disease had similar mortality compared to patients < 80 years old. With an increase in life expectancy globally, the elderly population is also increasing. In the elderly population where there is an increased prevalence of gallstones in the elderly population, biliary events including AC are also more common. The elderly poses a unique challenge due to underlying comorbidity, frailty, sarcopenia, functional decline, cognitive decline, and diminished reserves to withstand stress[4]. With diminished physiological reserves, sepsis resulting from AC poses a mortality risk, and our mortality outcomes are acceptable, considering mortality risk of up to 20% in patients with AC[1]. Our reported mortality is comparable to mortality of less than 11% cited in more recent studies[28,29]. The higher mortality compared to some reports may be due to advanced age or co-morbidity associated with ageing[30]. With regards to the exact cause of mortality, we did not collect separate data, and this remains a limitation of our study. However, locally, our institution tracks procedure-related mortality separately; ERCP-related mortality is < 1% locally. Further, it is difficult to distinguish ERCP-related complications such as post-ERCP cholangitis from the index-admission sepsis. Due to the retrospective nature of our study, it is difficult to establish a cause-effect relationship.
The principles of management of AC are early diagnosis, resuscitation, risk stratification, compliance to sepsis bundle, and source control[21]. Risk stratification is essential for resource allocation, patient and caregiver counselling, and timely proactive interventions. Source control is best achieved with endoscopic biliary decompression, i.e., ERCP. The traditional systemic inflammatory response criteria lack specificity in hepatobiliary sepsis, and thus alternative indices are for risk stratification and prognostication of outcomes[15]. The SI (heart rate/systolic blood pressure) is a validated tool[19,31]. Yussof et al[31] demonstrated abnormal SI predicted mortality of severe sepsis in the emergency department. Our study however demonstrated that patients who had abnormal SI were equally likely to undergo ERCP, and outcomes were comparable between elderly and non-elderly patients. SI is not reflective of the severity of sepsis as it does not take into account tissue perfusion indices and altered mental state. The decision for ERCP at the time of admission was based on the severity of AC and resources. Thus, SI does not predict the need for ERCP. Also, ERCP may occasionally be delayed in patients with abnormal SI in an attempt to resuscitate first. ERCP is an invasive procedure with approximately 10% risk of complications. Elderly patients undergoing ERCP are at higher risk of complications such as pancreatitis, hemorrhage, perforation, cardiorespiratory complications, and mortality[32]. This increased morbidity and mortality are attributed to underlying comorbidity and lower physiological reserves of the elderly[33].
However, several studies have shown no relationship between comorbidities and ERCP-related complications, except liver cirrhosis[34]. Many authors have demonstrated the safety and efficacy of ERCP in elderly patients[35,36]. In a single-center retrospective study reporting on efficacy and safety of ERCP in elderly patients with AC, Tohda et al[37] reported that patients ≥ 80 years old were more likely to have periampullary diverticulum (24.5% vs 13.3%), but equal technical success rates (95.1% vs 95.2%) and frequency of ERCP-related complications (6.9% vs 6.7%) as compared to patients < 80 years age. The authors reported a lower rate of post-ERCP pancreatitis in the elderly than non-elderly (1.0% vs 3.8%). We used PSM analysis to reduce the confounding effect of comorbidities on mortality outcomes, thus reducing the selection bias. We did not specifically compare procedure-related morbidity between elderly vs non-elderly and showed comparable LOS and mortality in both the unmatched and matched cohorts between elderly and non-elderly patients. Our experience shows that both stent insertion for biliary decompression and definitive stone removal can be safely performed. In particular, patient physiology, coagulopathy, and endoscopist experience are determinants of ERCP outcomes. Regarding the timing of ERCP, most authors agree that urgent ERCP should be done at the next available opportunity, and in clinical practice, timing is determined by local resources as well as clinical status. The majority of authors recommend ERCP within 24-72 h of admission[38]. Delay in ERCP in AC could influence patients’ outcomes, and many authors define delay variably as the time to ERCP of more than 48-72 h since admission. Khashab et al[39] defined delay in ERCP as > 72 h after admission and reported that it was associated with prolonged LOS (OR 19.8, 95%CI: 2.18-178, P = 0.008). Navaneethan et al[40] defined delay in ERCP as > 48 h after admission and reported that it was associated with an increased risk of 30-d readmission. We defined delay as > 72 h after admission and did not find any difference in clinical outcomes between elderly and non-elderly patients in both the unmatched and matched cohort. Khashab et al[39] demonstrated that delayed ERCP and age are associated with worse composite clinical outcomes (death, persistent organ failure and admission to ICU). However, as our 90-d mortality only had thirteen patients with delayed ERCP, it was not possible to perform subgroup analysis of age on clinical outcomes. It is possible that worse outcomes are associated with delay in ERCP but independent of age.
In addition, it is essential for patients with haemodynamic instability to be adequately resuscitated with airway management, prompt administration of vasopressor after volume replacement, and early engagement of critical care specialist or anesthetist, followed by prompt and early biliary decompression[41]. A recent study by Novy et al[14] in 2020, which analyzed the outcomes of 85 patients ≥ 75 years old with severe AC and admitted to ICU, showed that the majority (76%) of the ICU patients had ERCP within 24 h, which was attributed to the ease of access to facilities. Institutions with availability of ERCP services should consider early ERCP synchronized with resuscitation measures as delaying ERCP is associated with poor clinical outcomes[39]. Despite a policy for early ERCP, Novy et al[14] reported ICU mortality of 18%. This highlights that there are other determinants of mortality in critically ill patients. It is important to note that there is an inherent selection bias for elderly patients included in the study; patients not eligible for ICU admission may have more inferior pre-morbid status and deemed not suitable based on medical futility, or may have had advanced care planning performed and decided that ICU admission is unlikely to provide benefit for the patient[42]. Moreover, ICU admission implies the need for vasopressor therapy or intubation, which reflects the severity of the disease. We did not differentiate our patients based on their need for ICU admission or otherwise; or the use of vasopressor therapy. There is a paucity of data related to causative organisms and their impact on AC's clinical outcomes compared to other hepatobiliary diseases, such as acute cholecystitis or pyogenic liver abscesses[43]. Microbiology of patients with AC was also consistent with existing studies, where Escherichia coli and Klebsiella pneumoniae were the most typical organisms[44].
An alternative to biliary decompression is the use of PTBD. Our study demonstrated a significantly higher number of elderly patients who underwent PTBD compared to non-elderly patients [n = 13 (11.6%) vs n = 5 (4.5%), OR 2.81, P = 0.049] in the matched cohort. ERCP is traditionally the gold standard management for AC and has been proven to be safe and effective in the elderly population[36,37]. PTBD is regarded as a second-line treatment for patients who failed ERCP, with altered biliary anatomy, or were contra-indicated for ERCP. However, unlike ERCP which requires the use of moderate sedation or general anaesthesia, PTBD only requires the use of local anaesthesia. Despite the safety of ERCP in elderly patients, elderly patients are at higher risk of complications from the use of sedation[45]. Weighing the risks and benefits of endoscopic biliary decompression vs the use of sedation is also essential in the management of AC. Patient and/or family members may opt for PTBD which is deemed to be “less invasive” without the need for moderate sedation/general anaesthesia.
Following the acute management of AC, cholecystectomy should be offered to patients to prevent future recurrences. In our experience, non-elderly patients are more likely to undergo index admission LC (Matched cohort: P = 0.006). Five out of 12 patients in the non-elderly group who underwent index admission LC in the matched cohort did not receive ERCP. It is likely that in addition to age, underlying comorbidity and personal choices impact the decision for surgery. These findings are similar to a single-center retrospective study of Discolo et al[46]. In an eight-year study including 151 cholecystectomies for AC, Discolo et al[46] reported a more than 61% rate of index admission cholecystectomy, and patients with age > 75 years were more likely to receive delayed cholecystectomy (41.4% vs 21.5%, P = 0.01). The authors also showed that TG severity grading did not impact the decision for index admission cholecystectomy (P = 0.46). Furthermore, there was no difference in average operative time (P = 0.36), open conversion (P = 0.34), and intra-operative complications (P = 0.28) based on the timing of cholecystectomy. We did not perform subgroup analysis on postoperative outcomes in patients who underwent index admission cholecystectomy given the small sample size. In general, index admission cholecystectomy could reduce the risk of recurrent biliary events; however, more evidence is needed in patients with AC. We have previously reported our views on a policy of ‘universal cholecystectomy’, i.e., patients with a diagnosis that requires cholecystectomy (e.g., acute cholecystitis, AC, or acute biliary pancreatitis) procedure should receive index admission surgery unless contraindicated for general anesthesia or patient refusal[47].
The important issue that surfaces from our study is, if age should be considered as part of a risk stratification tool for the severity of AC. Age is usually included in severity classifications as a surrogate marker for functional capacity and extent of comorbidities. The use of other surrogate markers such as the clinical frailty scale or Charlson co-morbidity index may be a better predictor of disease severity in AC[48]. In reality however, age serves as a useful tool in view of its ease of use as well as age-associated reduced functional reserves that are not associated with any co-morbidity. While clinical outcomes are not determined by age in patients with AC in our study; based on available literature, we advocate that age should continue to remain as one of the component variables that determines disease severity in patients with AC.
There are several limitations of our study. A retrospective study is inherently prone to selection bias, and thus cause-effect cannot be established. PSM helps to reduce this bias, and such analysis ranks higher than traditional observational studies[24]. To the best of our knowledge, this is the first study using PSM to compare outcomes of AC secondary to biliary stones between elderly and non-elderly patients. PSM analysis cannot account for unknown confounding variables, and only a randomized controlled trial can overcome this bias. Our study included patients treated in 2012, i.e., before the TG13 guidelines, and we retrospectively assigned TG13 criteria with possible reporting bias. We did not study the effect of polypharmacy, frailty, and Charlson’s comorbidity index on AC outcomes. In a large population study over a decade in the Korean general population, Min et al[49] have reported that the use of proton pump inhibitor is associated with increased AC risk (hazard ratio 5.75, 95%CI: 4.39-7.54). We also did not evaluate comorbidities like cerebrovascular accident and liver cirrhosis, as data was not available for all the patients. Our study used the age of 80 years old as a cut-off compared to 75 years, used in TG13/18 guidelines. Existing studies evaluating the safety of ERCP in elderly patients have used a variety of cut-offs for age, ranging from 80 years old to 90 years old[35-37]. In addition, use of 75 years as a cut-off will reduce our sample size and impact the statistical power of study (96 patients < 75 years and 361 patients ≥ 75 years compared to 139 patients < 80 years and 318 patients ≥ 80 years respectively). Nevertheless, this difference in age cut-off reduces our study's generalizability from being considered an accurate validation study of TG13/18 guidelines. We also did not categorize which patients with history of biliary disease had prior ERCP and papillotomy. It is possible that elderly patients were more likely to have prior ERCP and papillotomy, and this could impact results of our study. We also did not collect data on disease or procedure-related morbidity and causes of mortality.
Elderly patients (≥ 80 years old) with AC have similar outcomes as compared to non-elderly patients (< 80 years old). In a subgroup of patients who underwent ERCP or with delayed ERCP, clinical outcomes are comparable between the elderly and non-elderly. Age alone may not predict the outcomes of AC and its use in the Tokyo Guidelines should be re-evaluated.
Acute cholangitis (AC) is a disease spectrum with varying extent of severity. Age ≥ 75 years forms part of the criteria for moderate (Grade II) severity in the Tokyo Guidelines (TG13 and TG18). Aging is associated with reduced physiological reserves, frailty, and sarcopenia. However, there is evidence that age itself is not the determinant of inferior outcomes in elective and emergency biliary diseases.
Endoscopic retrograde cholangiopancreatography is deemed to be safe in elderly patients with AC. There is paucity of data on outcome determinants in elderly patients with AC. This era of ageing population prompted our interest to study the impact of age alone on outcomes of AC through the use of propensity score matching.
Our primary outcomes are in-hospital mortality, 30-d mortality and 90-d mortality. Secondary outcome is morbidity (length of hospital stay).
This is a single-center retrospective cohort study of all patients diagnosed with calculous AC (January 2016 to December 2016) and ≥ 80 years old (January 2012 to December 2016) at a tertiary university-affiliated teaching hospital. Elderly was defined as ≥ 80 years old while non-elderly was defined as < 80 years old.
Four hundred fifty-seven patients with AC were included in this study (318 elderly, 139 non-elderly). Propensity score matching analysis resulted in a total of 224 patients (112 elderly, 112 non-elderly). The overall in-hospital mortality, 30-d mortality and 90-d mortality were 4.6%, 7.4% and 8.5% respectively, with no statistically significant differences between the elderly and non-elderly in both the unmatched and matched cohorts. Length of hospital stay was longer in the unmatched cohort [elderly 8 d, interquartile range (IQR) 6-13 vs non-elderly 8 d, IQR 5-11, P = 0.040], but was comparable in the matched cohort (elderly 7.5 d, IQR 5-11 vs non-elderly 8 d, IQR 5-11, P = 0.982).
Mortality is indifferent in the elderly (≥ 80 years old) and non-elderly patients (< 80 years old) with AC.
Age alone may not predict the outcomes of AC and its use in the Tokyo Guidelines should be re-evaluated.
| 1. | Salek J, Livote E, Sideridis K, Bank S. Analysis of risk factors predictive of early mortality and urgent ERCP in acute cholangitis. J Clin Gastroenterol. 2009;43:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Miura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Gomi H, Solomkin JS, Schlossberg D, Han HS, Kim MH, Hwang TL, Chen MF, Huang WS, Kiriyama S, Itoi T, Garden OJ, Liau KH, Horiguchi A, Liu KH, Su CH, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Endo I, Suzuki K, Yoon YS, de Santibañes E, Giménez ME, Jonas E, Singh H, Honda G, Asai K, Mori Y, Wada K, Higuchi R, Watanabe M, Rikiyama T, Sata N, Kano N, Umezawa A, Mukai S, Tokumura H, Hata J, Kozaka K, Iwashita Y, Hibi T, Yokoe M, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 3. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 487] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 4. | Mohan R, Huey CWT, Junnarkar S, Low JK, Shelat VG. Prehabilitation in elderly patients scheduled for liver resection and protocol for Recovery of Surgery in Elderly. Hepatoma Res. 2020;6:13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 691] [Article Influence: 28.8] [Reference Citation Analysis (5)] |
| 6. | Li W, Ding C, Yin S. Severe pneumonia in the elderly: a multivariate analysis of risk factors. Int J Clin Exp Med. 2015;8:12463-12475. [PubMed] |
| 7. | Tal S, Guller V, Levi S, Bardenstein R, Berger D, Gurevich I, Gurevich A. Profile and prognosis of febrile elderly patients with bacteremic urinary tract infection. J Infect. 2005;50:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Ortega M, Marco F, Soriano A, Almela M, Martínez JA, López J, Pitart C, Mensa J. Epidemiology and prognostic determinants of bacteraemic biliary tract infection. J Antimicrob Chemother. 2012;67:1508-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Boey JH, Way LW. Acute cholangitis. Ann Surg. 1980;191:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Shelat VG, Chia VJ, Low J. Common bile duct exploration in an elderly Asian population. Int Surg. 2015;100:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Tabak F, Wang HS, Li QP, Ge XX, Wang F, Ji GZ, Miao L. Endoscopic retrograde cholangiopancreatography in elderly patients: Difficult cannulation and adverse events. World J Clin Cases. 2020;8:2988-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 12. | Agarwal N, Sharma BC, Sarin SK. Endoscopic management of acute cholangitis in elderly patients. World J Gastroenterol. 2006;12:6551-6555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Amirthalingam V, Low JK, Woon W, Shelat V. Tokyo Guidelines 2013 may be too restrictive and patients with moderate and severe acute cholecystitis can be managed by early cholecystectomy too. Surg Endosc. 2017;31:2892-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Novy E, Carrara L, Remen T, Chevaux JB, Losser MR, Louis G, Guerci P. Prognostic factors associated with six month mortality of critically ill elderly patients admitted to the intensive care unit with severe acute cholangitis. HPB (Oxford). 2021;23:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mak MHW, Low JK, Junnarkar SP, Huey TCW, Shelat VG. A prospective validation of Sepsis-3 guidelines in acute hepatobiliary sepsis: qSOFA lacks sensitivity and SIRS criteria lacks specificity (Cohort Study). Int J Surg. 2019;72:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M, Kimura Y, Tsuyuguchi T, Itoi T, Yoshida M, Miura F, Yamashita Y, Okamoto K, Gabata T, Hata J, Higuchi R, Windsor JA, Bornman PC, Fan ST, Singh H, de Santibanes E, Gomi H, Kusachi S, Murata A, Chen XP, Jagannath P, Lee S, Padbury R, Chen MF, Dervenis C, Chan AC, Supe AN, Liau KH, Kim MH, Kim SW; Tokyo Guidelines Revision Committee. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Itoi T, Tsuyuguchi T, Takada T, Strasberg SM, Pitt HA, Kim MH, Belli G, Mayumi T, Yoshida M, Miura F, Büchler MW, Gouma DJ, Garden OJ, Jagannath P, Gomi H, Kimura Y, Higuchi R; Tokyo Guideline Revision Committee. TG13 indications and techniques for biliary drainage in acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 18. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 7450] [Article Influence: 620.8] [Reference Citation Analysis (1)] |
| 19. | Berger T, Green J, Horeczko T, Hagar Y, Garg N, Suarez A, Panacek E, Shapiro N. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14:168-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Birkhahn RH, Gaeta TJ, Terry D, Bove JJ, Tloczkowski J. Shock index in diagnosing early acute hypovolemia. Am J Emerg Med. 2005;23:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3206] [Article Influence: 246.6] [Reference Citation Analysis (0)] |
| 22. | Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, Ansaloni L, Bala M, Balogh ZJ, Beltrán MA, Ben-Ishay O, Biffl WL, Birindelli A, Cainzos MA, Catalini G, Ceresoli M, Che Jusoh A, Chiara O, Coccolini F, Coimbra R, Cortese F, Demetrashvili Z, Di Saverio S, Diaz JJ, Egiev VN, Ferrada P, Fraga GP, Ghnnam WM, Lee JG, Gomes CA, Hecker A, Herzog T, Kim JI, Inaba K, Isik A, Karamarkovic A, Kashuk J, Khokha V, Kirkpatrick AW, Kluger Y, Koike K, Kong VY, Leppaniemi A, Machain GM, Maier RV, Marwah S, McFarlane ME, Montori G, Moore EE, Negoi I, Olaoye I, Omari AH, Ordonez CA, Pereira BM, Pereira Júnior GA, Pupelis G, Reis T, Sakakhushev B, Sato N, Segovia Lohse HA, Shelat VG, Søreide K, Uhl W, Ulrych J, Van Goor H, Velmahos GC, Yuan KC, Wani I, Weber DG, Zachariah SK, Catena F. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (4)] |
| 23. | Sartelli M, Weber DG, Ruppé E, Bassetti M, Wright BJ, Ansaloni L, Catena F, Coccolini F, Abu-Zidan FM, Coimbra R, Moore EE, Moore FA, Maier RV, De Waele JJ, Kirkpatrick AW, Griffiths EA, Eckmann C, Brink AJ, Mazuski JE, May AK, Sawyer RG, Mertz D, Montravers P, Kumar A, Roberts JA, Vincent JL, Watkins RR, Lowman W, Spellberg B, Abbott IJ, Adesunkanmi AK, Al-Dahir S, Al-Hasan MN, Agresta F, Althani AA, Ansari S, Ansumana R, Augustin G, Bala M, Balogh ZJ, Baraket O, Bhangu A, Beltrán MA, Bernhard M, Biffl WL, Boermeester MA, Brecher SM, Cherry-Bukowiec JR, Buyne OR, Cainzos MA, Cairns KA, Camacho-Ortiz A, Chandy SJ, Che Jusoh A, Chichom-Mefire A, Colijn C, Corcione F, Cui Y, Curcio D, Delibegovic S, Demetrashvili Z, De Simone B, Dhingra S, Diaz JJ, Di Carlo I, Dillip A, Di Saverio S, Doyle MP, Dorj G, Dogjani A, Dupont H, Eachempati SR, Enani MA, Egiev VN, Elmangory MM, Ferrada P, Fitchett JR, Fraga GP, Guessennd N, Giamarellou H, Ghnnam W, Gkiokas G, Goldberg SR, Gomes CA, Gomi H, Guzmán-Blanco M, Haque M, Hansen S, Hecker A, Heizmann WR, Herzog T, Hodonou AM, Hong SK, Kafka-Ritsch R, Kaplan LJ, Kapoor G, Karamarkovic A, Kees MG, Kenig J, Kiguba R, Kim PK, Kluger Y, Khokha V, Koike K, Kok KY, Kong V, Knox MC, Inaba K, Isik A, Iskandar K, Ivatury RR, Labbate M, Labricciosa FM, Laterre PF, Latifi R, Lee JG, Lee YR, Leone M, Leppaniemi A, Li Y, Liang SY, Loho T, Maegele M, Malama S, Marei HE, Martin-Loeches I, Marwah S, Massele A, McFarlane M, Melo RB, Negoi I, Nicolau DP, Nord CE, Ofori-Asenso R, Omari AH, Ordonez CA, Ouadii M, Pereira Júnior GA, Piazza D, Pupelis G, Rawson TM, Rems M, Rizoli S, Rocha C, Sakakushev B, Sanchez-Garcia M, Sato N, Segovia Lohse HA, Sganga G, Siribumrungwong B, Shelat VG, Soreide K, Soto R, Talving P, Tilsed JV, Timsit JF, Trueba G, Trung NT, Ulrych J, van Goor H, Vereczkei A, Vohra RS, Wani I, Uhl W, Xiao Y, Yuan KC, Zachariah SK, Zahar JR, Zakrison TL, Corcione A, Melotti RM, Viscoli C, Viale P. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J Emerg Surg. 2016;11:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (4)] |
| 24. | Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15213] [Cited by in RCA: 15340] [Article Influence: 356.7] [Reference Citation Analysis (0)] |
| 25. | Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1784] [Cited by in RCA: 2729] [Article Influence: 194.9] [Reference Citation Analysis (0)] |
| 26. | Tsuyuguchi T, Sugiyama H, Sakai Y, Nishikawa T, Yokosuka O, Mayumi T, Kiriyama S, Yokoe M, Takada T. Prognostic factors of acute cholangitis in cases managed using the Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083-3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3915] [Cited by in RCA: 4857] [Article Influence: 285.7] [Reference Citation Analysis (1)] |
| 28. | Csendes A, Diaz JC, Burdiles P, Maluenda F, Morales E. Risk factors and classification of acute suppurative cholangitis. Br J Surg. 1992;79:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Chijiiwa K, Kozaki N, Naito T, Kameoka N, Tanaka M. Treatment of choice for choledocholithiasis in patients with acute obstructive suppurative cholangitis and liver cirrhosis. Am J Surg. 1995;170:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Kiriyama S, Takada T, Hwang TL, Akazawa K, Miura F, Gomi H, Mori R, Endo I, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Clinical application and verification of the TG13 diagnostic and severity grading criteria for acute cholangitis: an international multicenter observational study. J Hepatobiliary Pancreat Sci. 2017;24:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH. Value of Shock Index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. 2012;67:406-411. [PubMed] |
| 32. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 801] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 33. | Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, Wong J. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 329] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Freeman ML. Complications of endoscopic biliary sphincterotomy: a review. Endoscopy. 1997;29:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 35. | Mitchell RM, O'Connor F, Dickey W. Endoscopic retrograde cholangiopancreatography is safe and effective in patients 90 years of age and older. J Clin Gastroenterol. 2003;36:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Katsinelos P, Paroutoglou G, Kountouras J, Zavos C, Beltsis A, Tzovaras G. Efficacy and safety of therapeutic ERCP in patients 90 years of age and older. Gastrointest Endosc. 2006;63:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Tohda G, Ohtani M, Dochin M. Efficacy and safety of emergency endoscopic retrograde cholangiopancreatography for acute cholangitis in the elderly. World J Gastroenterol. 2016;22:8382-8388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Boender J, Nix GA, de Ridder MA, Dees J, Schütte HE, van Buuren HR, van Blankenstein M. Endoscopic sphincterotomy and biliary drainage in patients with cholangitis due to common bile duct stones. Am J Gastroenterol. 1995;90:233-238. [PubMed] |
| 39. | Khashab MA, Tariq A, Tariq U, Kim K, Ponor L, Lennon AM, Canto MI, Gurakar A, Yu Q, Dunbar K, Hutfless S, Kalloo AN, Singh VK. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Butt M, Sanaka MR, Vargo JJ, Parsi MA. Delay in performing ERCP and adverse events increase the 30-day readmission risk in patients with acute cholangitis. Gastrointest Endosc. 2013;78:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Wang Z, Ahmed S, Shelat VG. Acute Cholangitis. In: Sartelli M, Bassetti M, Martin-Loeches I. Abdominal Sepsis. Hot Topics in Acute Care Surgery and Trauma. Cham: Springer, 2018: 65-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med. 2015;43:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 43. | Teng TZJ, Shelat VG. Biliary Candidiasis Caused by Candida dubliniensis Causing Perforated Cholecystitis. Surg Infect (Larchmt). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Gomi H, Takada T, Hwang TL, Akazawa K, Mori R, Endo I, Miura F, Kiriyama S, Matsunaga N, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Updated comprehensive epidemiology, microbiology, and outcomes among patients with acute cholangitis. J Hepatobiliary Pancreat Sci. 2017;24:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Finkelmeier F, Tal A, Ajouaou M, Filmann N, Zeuzem S, Waidmann O, Albert J. ERCP in elderly patients: increased risk of sedation adverse events but low frequency of post-ERCP pancreatitis. Gastrointest Endosc. 2015;82:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Discolo A, Reiter S, French B, Hayes D, Lucas G, Tan L, Scanlan J, Martinez R. Outcomes following early vs delayed cholecystectomy performed for acute cholangitis. Surg Endosc. 2020;34:3204-3210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Yu H, Chan EE, Lingam P, Lee J, Woon WWL, Low JK, Shelat VG. Index admission laparoscopic cholecystectomy for acute cholecystitis restores Gastrointestinal Quality of Life Index (GIQLI) score. Ann Hepatobiliary Pancreat Surg. 2018;22:58-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Lee F, Ohanian E, Rheem J, Laine L, Che K, Kim JJ. Delayed endoscopic retrograde cholangiopancreatography is associated with persistent organ failure in hospitalised patients with acute cholangitis. Aliment Pharmacol Ther. 2015;42:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Min YW, Kang D, Shin JY, Kang M, Park JK, Lee KH, Lee JK, Lee KT, Rhee PL, Kim JJ, Guallar E, Cho J, Lee H. Use of proton pump inhibitors and the risk of cholangitis: a nationwide cohort study. Aliment Pharmacol Ther. 2019;50:760-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gevers TJG, Kang KJ S-Editor: Gao CC L-Editor: A P-Editor: Wang LL