Published online Mar 27, 2021. doi: 10.4254/wjh.v13.i3.375
Peer-review started: November 5, 2020
First decision: November 30, 2020
Revised: January 4, 2021
Accepted: February 18, 2021
Article in press: February 18, 2021
Published online: March 27, 2021
Processing time: 134 Days and 13.6 Hours
Once daily tacrolimus regimen was found to exhibits similar bioavailability, safety and efficacy properties compared to twice-daily tacrolimus in kidney transplantation patients.
To compare the efficacy and safety of once-daily prolonged release tacrolimus compared to twice-daily tacrolimus in liver transplantation patients.
MEDLINE, EMBASE, CENTRAL databases were searched for clinical trials until December 2020. Efficacy outcome measured as the rate of treatment failure indicated by biopsy-proven acute rejection, Serum creatinine, graft loss, or death. Two reviewers independently selected studies, collected data and assessed risk of bias. The results are reported as risk ratio with 95% confidence interval (CI) for dichotomous data.
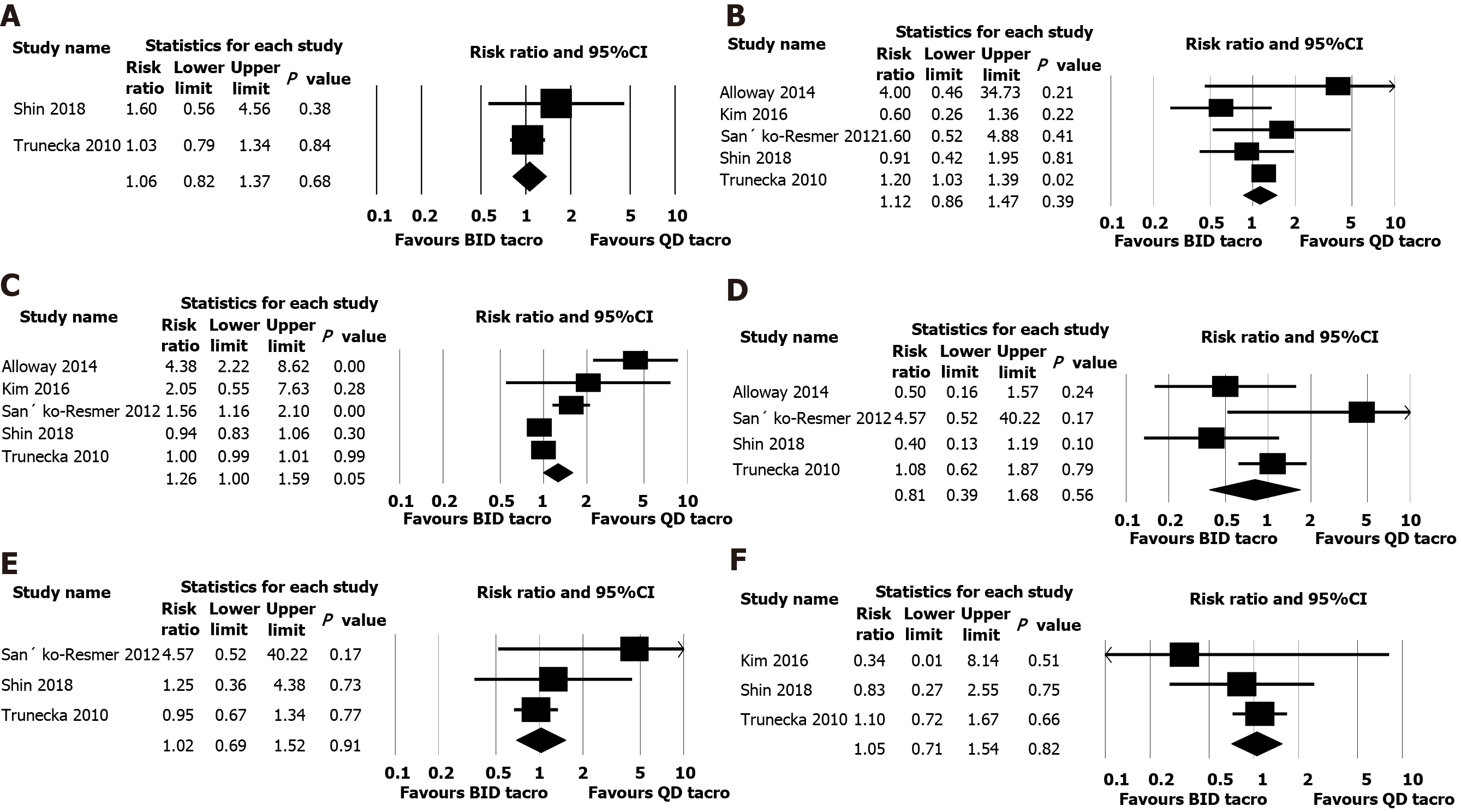
Seven studies included with 965 patients. All the included studies were of moderate quality according to the risk of bias assessment using Cochrane Risk of Bias tool. Biopsy-proven acute rejection was reported in four studies, and pooled analysis of those studies indicated similar rejections in both twice daily and once daily tacrolimus groups (risk ratio: 1.06, 95%CI: 0.84-1.34, n = 758, I2 = 0%) and also we found no significant difference between both groups for renal outcome (serum creatinine; mean difference, 0.001 mg/dL, 95%CI: -0.042 to 0.043, n = 846,
The analysis findings confirm that both once daily and twice daily tacrolimus formulations are comparable in terms of efficacy and safety outcomes.
Core Tip: Tacrolimus, a calcineurin inhibitor is an important component of the immunosuppressive regimens post liver transplantation. Compliance to immuno-suppression treatment generally is important and non-adherence is a major risk factor of graft rejection and loss. Compliance to medication declines over the course of time in patients after liver transplantation due to several factors and this contributes to about 20% of late acute rejection. The efficacy of once daily tacrolimus regimens has been reported in many studies and this systematic review/meta-analysis confirmed the evidence of comparable efficacy and safety of prolonged release tacrolimus to the twice daily immediate release formulation.
- Citation: Bzeizi KI, Albenmousa A, Shawkat M, Ahmed Z, Alabbad S, Al-Hamoudi W, Troisi R, Broering D. Efficacy and safety of once daily tacrolimus compared to twice daily tacrolimus after liver transplantation. World J Hepatol 2021; 13(3): 375-383
- URL: https://www.wjgnet.com/1948-5182/full/v13/i3/375.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i3.375
Advances in immunosuppression regimens after solid-organ transplantation have significantly improved patient and graft survival. Tacrolimus, a calcineurin inhibitor is an important component of the immunosuppressive regimens widely used following liver transplantation (LT). Compliance to immunosuppression treatment however is important and non-adherence is a recognized contributing factor in rejection and graft loss[1,2].
Compliance to medication declines over the course of time in patients after LT due to several factors including the number of drugs to consume and the rate of rejection/infections increases. Previous reviews directed at recipients transplanted between the late 1980s and mid-2000s showed that the prevalence of non-adherence to immunosuppressive medications averaged about 25%. This non-adherence to medications was felt to contribute to about 20% of late acute rejection episodes and 16%-36% of graft losses[3]. To maintain good adherence, less frequently administering regimen were proved to be effective[4].
Recently, tacrolimus once-daily prolonged-release (PR) formulation was developed. Based on the previous literature, it was evident that conversion from the twice-daily, immediate release (IR) to PR tacrolimus was well tolerated, safe and conveniently used in stable patients after LT[5,6]. However, there is no systematic review that has been conducted till date to confirm the efficacy and safety of PR tacrolimus compared to IR tacrolimus.
This systematic review and meta-analysis was performed according to Cochrane Collaboration[7] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement[8].
We searched MEDLINE, EMBASE, CENTRAL databases since inception to December 2020 using an extensive search strategy to identify relevant literature. We used the following terms: Tacrolimus, liver transplantation and dosage forms (Supplementary file) while searching databases with human and English language restrictions. In addition, we also searched clinicaltrials.gov.in and Google Scholar and references of previously published relevant papers to find more relevant trials.
Clinical trials conducted on adult (> 18 years) patients who received a primary LT from a deceased or living donor, having an average serum tacrolimus level of 1-10 ng/mL for more than 6 wk, that compared once daily tacrolimus to twice daily tacrolimus in LT patients were included.
Studies were excluded if they had patients with a previous organ transplant other than liver and multiple organ transplantations. Studies also conducted on paediatric population and lack of a control group (the study had only included patients who received once daily tacrolimus. We also excluded studies only assessed pharmacokinetics of tacrolimus. Finally, studies without full-text such as conference proceedings, editorials, reviews, secondary analyses and letters excluded.
Outcomes: Efficacy was measured as the rate of treatment failure indicated by biopsy-proven acute rejection (BPAR), liver graft loss, or death while safety was assessed by the incidence of adverse events.
Two reviewers independently (KB and RT) screened the identified studies according to the aforementioned criteria and excluded studies that were found to be clearly irrelevant. We obtained the full text of the remaining studies and the same two reviewers screened full texts and selected trials for inclusion. The same two reviewers independently extracted data from included trials into the predesigned and validated data collection form. Disagreements were resolved by arbitration, and consensus was reached after discussion. We collected study characteristics (type of design with duration of intervention and methods), baseline demographics, and efficacy and safety outcome data from each included trial.
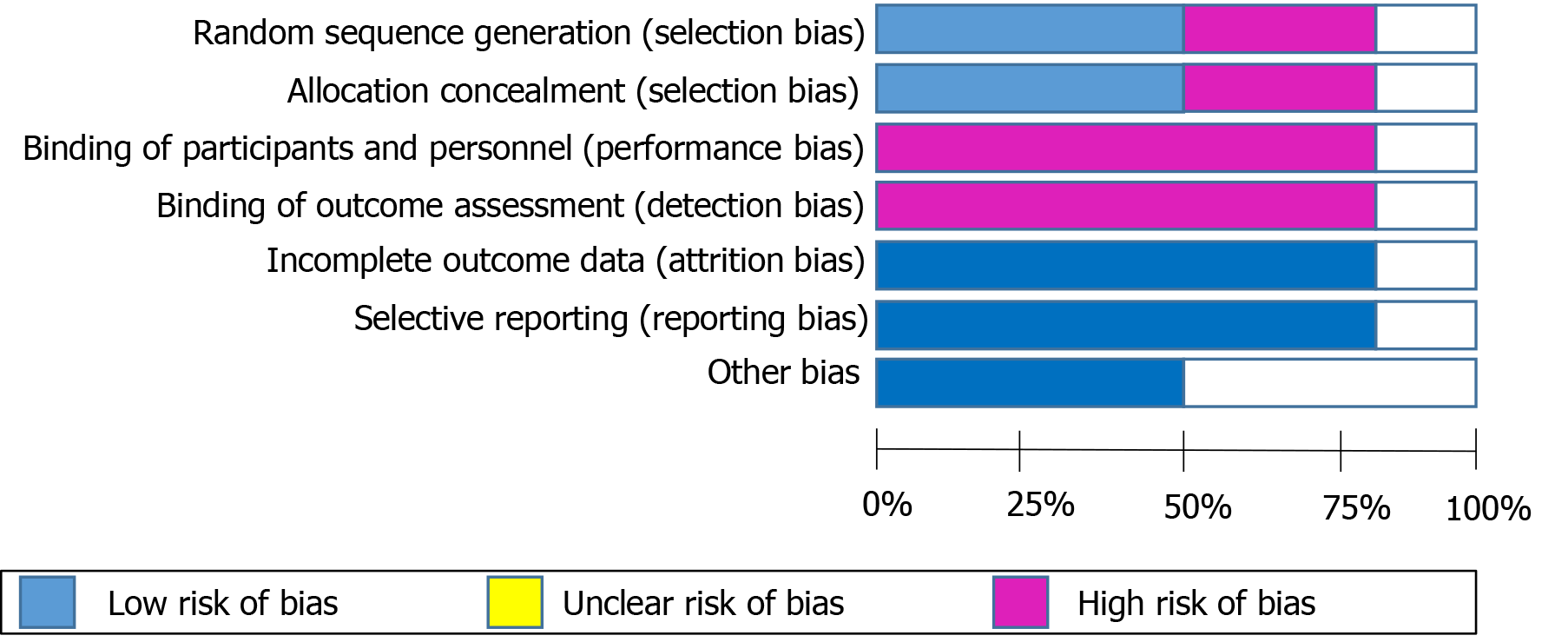
Two reviewers (KB and RT) independently assessed quality of included studies using Cochrane Risk of Bias tool[9], and disagreements were resolved by discussion. If a consensus could not be reached, any discrepancy was resolved by a senior author. Seven domains of quality assessment included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
We performed statistical analysis using Comprehensive Meta-analysis Version 3.0[10]. We reported the results as risk ratio with 95% confidence interval (CI) for dichotomous data and continuous data as mean difference. We used a random-effects model to combine individual results regardless whether there was significant heterogeneity or not. We tested heterogeneity among trial results using the I2 statistic[7]. We considered a value greater than 50% as substantial heterogeneity. Publication bias was not assessed due to limited number of included studies in this review.
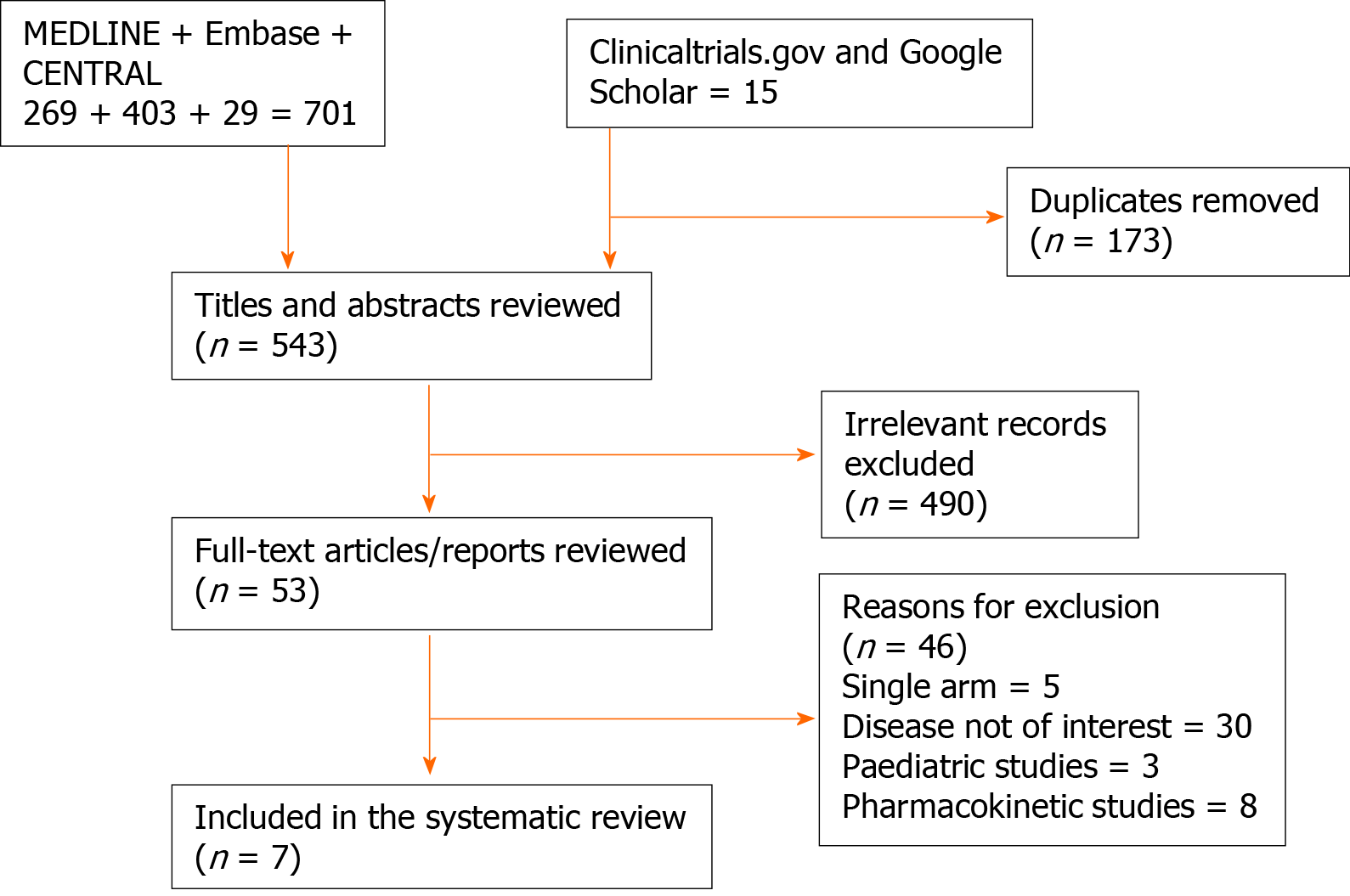
A total of 701 articles from databases search and 15 from additional searches identified. After removing duplicates 543 studies remained for screening. Upon screening titles and abstracts, 490 clearly irrelevant articles removed. The remaining 48 articles subjected to full text screening. Finally, seven clinical trials met the inclusion criteria[11-17]. The flow of the randomised controlled trial included in our analysis is shown in Figure 1.
A total of seven clinical trials were included with 965 patients. Study characteristics were summarized in Table 1. Studies included are conducted in various countries including United States, Japan, United Kingdom, and one study in another 16 countries.
| Ref. | Year | Country | Study design | Follow-up period | Sample size | Donor type | Mean age | Concurrent therapy |
| Alloway et al[11] | 2014 | United States | Phase-II, 3-sequence, open-label, multicenter, prospective study | 1 yr | 59 | NR | 49.8 | Mycophenolate mofetil |
| Kim et al[13] | 2016 | South Korea | 2-armed, parallel group, prospective, randomized, open-label, phase IV | 1 yr | 79 | Deceased | 54 | NR |
| Sańko-Resmer et al[14] | 2012 | United Kingdom | Multicentre, open-label, single-sequence, crossover, phase IIIb | 3 mo | 98 | NR | 55 | None |
| Shin et al[17] | 2018 | South Korea | Phase IV, randomized, open-label, comparative, single-center study | 6 mo | 100 | NR | 52 | Corticosteroid, mycophenolate mofetil, and basiliximab. |
| Trunečka et al[15] | 2010 | 16 countries | 1:1-randomized, double-blind, double-dummy, two-arm, parallel-group phase III, comparative study | 1 yr | 471 | NR | 52 | Mycophenolate sodium |
| DuBay et al[12] | 2019 | United States | Phase II, open label, multicenter, randomized trial | 1 yr | 29 | Deceased | 54.4 | Mycophenolate mofetil, mycophenolic acid sodium, prednisone, or azathioprine |
| Fischer et al[16] | 2010 | Germany | Randomized, phase II, multicenter, open-label, prospective trial | 6 wk | 129 | NR | 47 | Anti-fungal, antibiotics, anti-hypertensives, antiepileptics and rifampicin) |
The mean age of included patients was 52.8 years and majority (71%) of them were males. Four studies had follow-up for one year while the other two had follow up for 3 and 6 mo. In four studies, concomitant treatment with mycophenolate mofetil and steroids was allowed. All the included studies were of moderate quality according to the risk of bias assessment using Cochrane Risk of Bias tool (Figure 2).
Acute rejection confirmed by biopsy was reported in four studies[12,15-17], and pooled analysis of those studies indicated similar rejection rate in both twice daily and once daily tacrolimus groups (risk ratio 1.06, 95%CI: 0.84-1.34, n = 758, I2 = 0%; Figure 3).
This systematic review and meta-analysis compared PR tacrolimus to IR tacrolimus in LT recipients. The efficacy and safety outcomes were found to be similar for both regimens.
Adherence to the immunosuppressant regimen post-LT is important for preventing rejection and graft loss. The reported rate of non-adherence to immunosuppressant regimens is 15%-40%, which could lead to significantly higher rate of graft rejection, graft loss and severe impact on long-term survival[18]. It was observed that once daily tacrolimus is safe and is associated with better adherence and low variability of liver function tests[18,19].
A study by Muduma et al[20] looked at the cost effectiveness of PR tacrolimus in LT recipients. Based on a United Kingdom specific analysis of the projected cost-utility of PR tacrolimus relative to IR tacrolimus and cyclosporin, once daily tacrolimus was cost-effective, improved life expectancy and quality adjusted life year and incremental cost effectiveness ratio below £20000 per a quality adjusted life year gained. Over a 3-year time horizon, one graft would be saved for every 14 patients treated with PR tacrolimus with minimal impact on cost when compared to IR tacrolimus.
The results of recently published systematic review showed that PR tacrolimus when compared to the IR tacrolimus resulted in no significant difference in the glomerular filtration rate, BPAR and the safety outcomes among the kidney transplant recipients[21]. The findings of our review are also in congruent with the previous review. In contrast, another meta-analysis based on combination of two clinical trials and four observational studies found that once daily tacrolimus is effective for the first year after liver transplantation, however, there was no significant difference in 1-year mortality and adverse events between once daily and twice daily tacrolimus groups[22].
PR tacrolimus has been introduced as helpful therapeutic option to increase the patient adherence to immunosuppressive treatment. Studies with short follow-up and pharmacokinetic evaluation were not included in this review, however one study which evaluated pharmacokinetic outcomes along with efficacy outcomes showed similar BPAR, graft losses and safety outcomes such as hypertension, infections and blood related disorders[16] between groups. Of the included studies in our systematic review, four reported concomitant immunosuppressant therapies administration such as corticosteroids, and mycophenolate mofetil. It was evident that those concomitant drugs have negative association with occurred adverse events with tacrolimus[23]. An eight years long-term follow up study based on European Liver Transplant Registry has recently been published study and the findings were in favour of PR in terms of graft losses and acute rejections. This very large population study also reported better outcome in those converted from IR to PR tacrolimus after 1 mo compared to those maintained on IR tacrolimus-based immunosuppression. They concluded that patients on PR tacrolimus continues to provide ongoing benefits for graft and patient survival beyond 3 years post transplantation[24]. The major limitation of the study, were the lack of data on the dosages and the trough levels of tacrolimus were not captured. In addition, the lack of clarity on the IR tacrolimus preparations the cohort received. The retrospective design of the study was the main reason for its exclusion in our analyses as it did not meet the eligibility criteria.
The major strength of our study is that, we have only included clinical trials of long-term follow-up to address efficacy and safety of PR tacrolimus. There was no heterogeneity found for all the outcomes assessed, except for any adverse events. One of the limitations of our review is that, we have only included studies published in English language, which means some of the studies published in other language might have been missed. Publication bias assessment was also not assessed due to less than ten studies included in the analysis, however due to our intense search effort it was evident that we did not miss any study meeting this review’s eligibility criteria. Majority of the studies were of open-label design, that could have introduced bias, however this could not be avoided due to the nature of administration. In addition, the paucity of studies of PR tacrolimus in Asian patients renders data from this review of high interest to the transplant community.
Our systematic review and meta-analysis indicate that both PR and IR tacrolimus formulations are comparable in terms of efficacy and safety outcomes. However, to confirm these findings, long-term follow-up randomized controlled trials with large sample sizes are required. Also, to assess acceptability by patients, quality of life and economic evaluations should be conducted.
Tacrolimus, a calcineurin inhibitor is an important immunosuppressive medication post liver transplantation. Compliance to immunosuppression is important and non-adherence can lead to rejection and graft loss. To maintain good adherence, less frequently administering regimen were proved to be effective.
Recently, tacrolimus once-daily prolonged-release (PR) formulation was developed. Several studies have shown evidence that conversion from the twice-daily, immediate release to PR tacrolimus was well tolerated, safe and conveniently used in stable patients after liver transplantation.
Our objective was to conduct a metanalysis and systematic review of the published clinical trials that studied the safety and efficacy of PR tacrolimus compared to immediate release tacrolimus.
MEDLINE, EMBASE, CENTRAL databases were searched for clinical trials until December 2020. Efficacy outcome measured as the rate of treatment failure indicated by biopsy-proven acute rejection, Serum creatinine, graft loss, or death. Two reviewers independently selected studies, collected data and assessed risk of bias. The results are reported as risk ratio with 95%CI for dichotomous data.
Seven studies included with 965 patients. All the included studies were of moderate quality according to the risk of bias assessment using Cochrane Risk of Bias tool. Biopsy-proven acute rejection was reported in four studies, and pooled analysis of those studies indicated similar rejections in both twice daily and once daily tacrolimus groups. We also found no significant difference between both groups for renal outcome (serum creatinine; mean difference, 0.001 mg/dL, 95%CI: -0.042 to 0.043, n = 846, I2 = 18.6%). Similarly, there was similar number of adverse events such as hypertension, headache, back pain, blood related disorders, infections and nausea observed in both groups.
The analysis findings confirm that both once daily and twice daily tacrolimus formulations are comparable in terms of efficacy and safety outcomes.
Long-term follow-up randomized controlled trials with large sample sizes are required. Also, to assess acceptability by patients, quality of life and economic evaluations should be conducted.
| 1. | Busuttil RW, Lake JR. Role of tacrolimus in the evolution of liver transplantation. Transplantation. 2004;77:S44-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 763] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58:824-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Atreja A, Bellam N, Levy SR. Strategies to enhance patient adherence: making it simple. MedGenMed. 2005;7:4. [PubMed] |
| 5. | Comuzzi C, Lorenzin D, Rossetto A, Faraci MG, Nicolini D, Garelli P, Bresadola V, Toniutto P, Soardo G, Baroni GS, Adani GL, Risaliti A, Baccarani U. Safety of conversion from twice-daily tacrolimus (Prograf) to once-daily prolonged-release tacrolimus (Advagraf) in stable liver transplant recipients. Transplant Proc. 2010;42:1320-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Wu YJ, Lin YH, Yong CC, Li WF, Wang SH, Wang CC, Lin TL, Chen CL, Lin CC. Safe One-to-One Dosage Conversion From Twice-Daily to Once-Daily Tacrolimus in Long-Term Stable Recipients After Liver Transplantation. Ann Transplant. 2016;21:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Higgins, Julian PT, James Thomas, Jacqueline Chandler, Miranda Cumpston, Tianjing Li, Matthew J. Page, and Vivian A. Welch, eds. Cochrane handbook for systematic reviews of interventions. John Wiley and Sons, 2019. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5433] [Cited by in RCA: 7959] [Article Influence: 1137.0] [Reference Citation Analysis (0)] |
| 8. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13792] [Article Influence: 811.3] [Reference Citation Analysis (3)] |
| 9. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26257] [Article Influence: 1750.5] [Reference Citation Analysis (4)] |
| 10. | Biostat Inc. Comprehensive meta-analysis version 3 [cited 3 Feb, 2021]. 2016. Available from: https://www.Meta-Analysis.com. |
| 11. | Alloway RR, Eckhoff DE, Washburn WK, Teperman LW. Conversion from twice daily tacrolimus capsules to once daily extended-release tacrolimus (LCP-Tacro): phase 2 trial of stable liver transplant recipients. Liver Transpl. 2014;20:564-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | DuBay DA, Teperman L, Ueda K, Silverman A, Chapman W, Alsina AE, Tyler C, Stevens DR. Pharmacokinetics of Once-Daily Extended-Release Tacrolimus Tablets Versus Twice-Daily Capsules in De Novo Liver Transplant. Clin Pharmacol Drug Dev. 2019;8:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kim JM, Kwon CH, Joh JW, Sinn DH, Lee S, Choi GS, Lee SK. Conversion of once-daily extended-release tacrolimus is safe in stable liver transplant recipients: A randomized prospective study. Liver Transpl. 2016;22:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sańko-Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice- to once-daily tacrolimus in stable liver recipients: an open-label multicenter study. Transpl Int. 2012;25:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Trunečka P, Boillot O, Seehofer D, Pinna AD, Fischer L, Ericzon BG, Troisi RI, Baccarani U, Ortiz de Urbina J, Wall W; Tacrolimus Prolonged Release Liver Study Group. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010;10:2313-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Fischer L, Trunečka P, Gridelli B, Roy A, Vitale A, Valdivieso A, Varo E, Seehofer D, Lynch S, Samuel D, Ericzon BG, Boudjema K, Karpf C, Undre N. Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: a randomized, open-label trial. Liver Transpl. 2011;17:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Shin MH, Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Yun YI, Kim WJ, Kang WH, Kim SH, Jiang H, Lee S, Tak EY. Once-daily, prolonged-release tacrolimus vs twice-daily, immediate-release tacrolimus in de novo living-donor liver transplantation: A Phase 4, randomized, open-label, comparative, single-center study. Clin Transplant. 2018;32:e13376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Beckebaum S, Iacob S, Sweid D, Sotiropoulos GC, Saner F, Kaiser G, Radtke A, Klein CG, Erim Y, de Geest S, Paul A, Gerken G, Cicinnati VR. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011;24:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Kim SH, Lee SD, Kim YK, Park SJ. Conversion of twice-daily to once-daily tacrolimus is safe in stable adult living donor liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2015;14:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Muduma G, Odeyemi I, Pollock RF. Evaluating the Cost-Effectiveness of Prolonged-Release Tacrolimus Relative to Immediate-Release Tacrolimus in Liver Transplant Patients Based on Data from Routine Clinical Practice. Drugs Real World Outcomes. 2016;3:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Saengram W, Vadcharavivad S, Poolsup N, Chancharoenthana W. Extended release versus immediate release tacrolimus in kidney transplant recipients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Huang B, Liu J, Li J, Schroder PM, Chen M, Deng R, Deng S. Once-daily prolonged-release tacrolimus versus twice-daily tacrolimus in liver transplantation. J Am Pharm Assoc (2003) 2019; 59: 816-823. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Riva N, Dip M, Halac E, Cáceres Guido P, Woillard JB, Licciardone N, Chan D, Buendía J, Borgnia D, Bosaleh A, de Davila MT, Imventarza O, Schaiquevich P. Survival Time to Biopsy-Proven Acute Rejection and Tacrolimus Adverse Drug Reactions in Pediatric Liver Transplantation. Ther Drug Monit. 2018;40:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Adam R, Karam V, Cailliez V, Trunečka P, Samuel D, Tisone G, Němec P, Soubrane O, Schneeberger S, Gridelli B, Bechstein WO, Risaliti A, Line PD, Vivarelli M, Rossi M, Pirenne J, Klempnauer JL, Rummo A, Di Benedetto F, Zieniewicz K, Troisi R, Paul A, Vali T, Kollmar O, Boudjema K, Hoti E, Colledan M, Pratschke J, Lang H, Popescu I, Ericzon BG, Strupas K, De Simone P, Kochs E, Heyd B, Gugenheim J, Pinna AD, Bennet W, Kazimi M, Bachellier P, Wigmore SJ, Rasmussen A, Clavien PA, Hidalgo E, O'Grady JG, Zamboni F, Kilic M, Duvoux C; all contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Improved Survival in Liver Transplant Patients Receiving Prolonged-release Tacrolimus-based Immunosuppression in the European Liver Transplant Registry (ELTR): An Extension Study. Transplantation. 2019;103:1844-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khaliq S S-Editor: Zhang L L-Editor: A P-Editor: Wang LL