Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2168
Peer-review started: February 27, 2021
First decision: May 13, 2021
Revised: August 18, 2021
Accepted: November 26, 2021
Article in press: November 26, 2021
Published online: December 27, 2021
Processing time: 303 Days and 1.4 Hours
Accurate detection of gastric antral vascular ectasia (GAVE) is critical for proper management of cirrhosis-related gastrointestinal bleeding. However, endoscopic diagnosis of GAVE can be challenging when GAVE overlaps with severe portal hypertensive gastropathy (PHG).
To determine the added diagnostic value of virtual chromoendoscopy to high definition white light for real-time endoscopic diagnosis of GAVE and PHG.
We developed an I-scan virtual chromoendoscopy criteria for diagnosis of GAVE and PHG. We tested our criteria in a cross-sectional cohort of cirrhotic adults with GAVE and PHG when high-definition white light endoscopy (HDWLE) diagnosis was in doubt. We then compared the accuracy of I-scan vs HDWLE alone to histology.
Twenty-three patients were included in this study (65.2% Caucasians and 60.9% males). Chronic hepatitis C was the predominant cause of cirrhosis (43.5%) and seven adults (30.4%) had confirmed GAVE on histology. I-scan had higher sensitivity (100% vs 85.7%) and specificity (75% vs 62.5%) in diagnosing GAVE compared to HDWLE. This translates into a higher, albeit not statistically significant, accuracy of I-scan in detecting GAVE compared to HDWLE alone (82% vs 70%). I-scan was less likely to lead to an accurate diagnosis of GAVE in patients on dialysis (P < 0.05) and in patients with elevated creatinine (P < 0.05). I-scan had similar accuracy to HDWLE in detecting PHG.
This pilot work supports that virtual chromoendoscopy may obviate the need for biopsies when the presence of GAVE is in doubt. Larger studies are needed to assess the impact of virtual chromoendoscopy on success of endoscopic therapy for GAVE.
Core Tip: Gastric antral vascular ectasia (GAVE) and portal hypertensive gastropathy (PHG) are two causes of GI bleeding in cirrhosis. Gastric biopsies, which are the gold standard to differentiate the two conditions, may be contraindicated given coagulopathy or thrombocytopenia in cirrhosis. We developed virtual chromoendoscopy (I-scan) criteria for diagnosis of GAVE and PHG. We tested our criteria in a prospective cohort of cirrhotic adults with GAVE and PHG when high-definition white light endoscopy (HDWLE) diagnosis was doubtful. We compared accuracy of I-scan vs HDWLE to histology. Compared to HDWLE, I-scan demonstrated superior performance for real-time diagnosis of PHG and GAVE in cirrhosis.
- Citation: Al-Taee AM, Cubillan MP, Hinton A, Sobotka LA, Befeler AS, Hachem CY, Hussan H. Accuracy of virtual chromoendoscopy in differentiating gastric antral vascular ectasia from portal hypertensive gastropathy: A proof of concept study. World J Hepatol 2021; 13(12): 2168-2178
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2168.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2168
Gastric antral vascular ectasia (GAVE) and portal hypertensive gastropathy (PHG) account for up to 10% of causes of gastrointestinal bleeding in patients with cirrhosis[1-3]. Management of GAVE is aimed at temporizing bleeding with endoscopic therapy. In contrast, management of PHG is targeted at reducing portal pressure with pharmacologic agents and portosystemic shunting[1-3]. As a result, accurate diagnosis is critical for optimal treatment of GAVE- and PHG-related bleeding[4,5]. Endoscopically, GAVE often manifests as red stripes radiating away from the pylorus commonly referred to as “watermelon stomach” but can also present in a more diffuse, ‘honeycomb’ pattern[6-8]. Alternatively, PHG usually involves the mucosa in the gastric fundus and body and is characterized by four main features: A mosaic-like pattern, presence of red point lesions, cherry red spots and black brown spots[9]. Despite their typical appearance, distinguishing between GAVE and PHG can be challenging with endoscopy alone as advanced PHG can have similar endoscopic features to GAVE.
While endoscopic appearance can suggest the diagnosis, gastric biopsies are the current gold standard for differentiating PHG from GAVE. Biopsies may be contraindicated given coagulopathy or thrombocytopenia that are commonly seen with cirrhosis[10,11]. Recently, there has been an increasing interest in the use of digital chromoendoscopy for real-time optical diagnosis of various gastrointestinal pathologies[12]. Utilizing narrow band imaging (Olympus, Tokyo, Japan), Hayashi and Saeki[13], demonstrated that PHG had obscured collecting venules (CVs) and intramucosal hemorrhage as opposed to partial and marked dilation of the capillaries surrounding the gastric pits in patients with GAVE[13]. Achim et al[12] demonstrated that the I-scan virtual chromoendoscopy (Pentax, Tokyo, Japan) has an increased sensitivity in the diagnosis of PHG when compared with white light endoscopy[12]. Building on these studies, we aimed to compare the sensitivity, specificity and accuracy of I-scan to high-definition white light endoscopy (HDWLE) in distinguishing between GAVE and PHG. Our main hypothesis is that I-scan virtual chromoendoscopy is more sensitive and specific than HDWLE at diagnosing GAVE when compared to gastric biopsy.
A cross-sectional cohort study was conducted at Saint Louis University-affiliated hospitals in St. Louis Missouri between July 17, 2012 and July 8, 2013. Inclusion and exclusion criteria are highlighted in Figure 1. All adult patients with cirrhosis undergoing an upper endoscopy were considered candidates for this study. Cirrhosis was confirmed on liver biopsy or clinically coupled with laboratory tests (e.g. serum albumin less than 3.0 g/dL or blood platelet counts less than 150000 mm3) and radiologic evidence of cirrhosis. Patients were excluded from the study if GAVE or PHG were absent or had a characteristic endoscopic appearance that could be clearly diagnosed without biopsy. We also excluded pregnant women or if a gastric biopsy did not confirm the diagnosis of GAVE or PHG. The study protocol was approved by the Saint Louis University Institutional Review Board. The study protocol, patient’s rights and obligations were reviewed with eligible patients and informed consent was obtained from all participants.
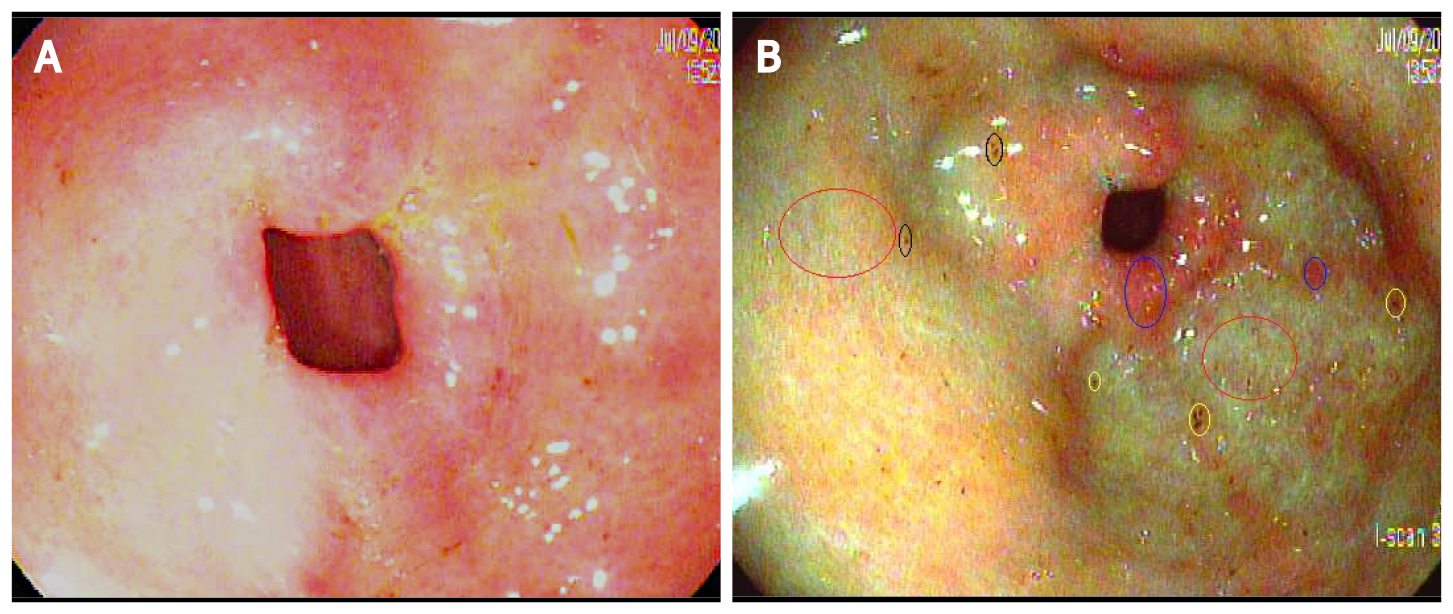
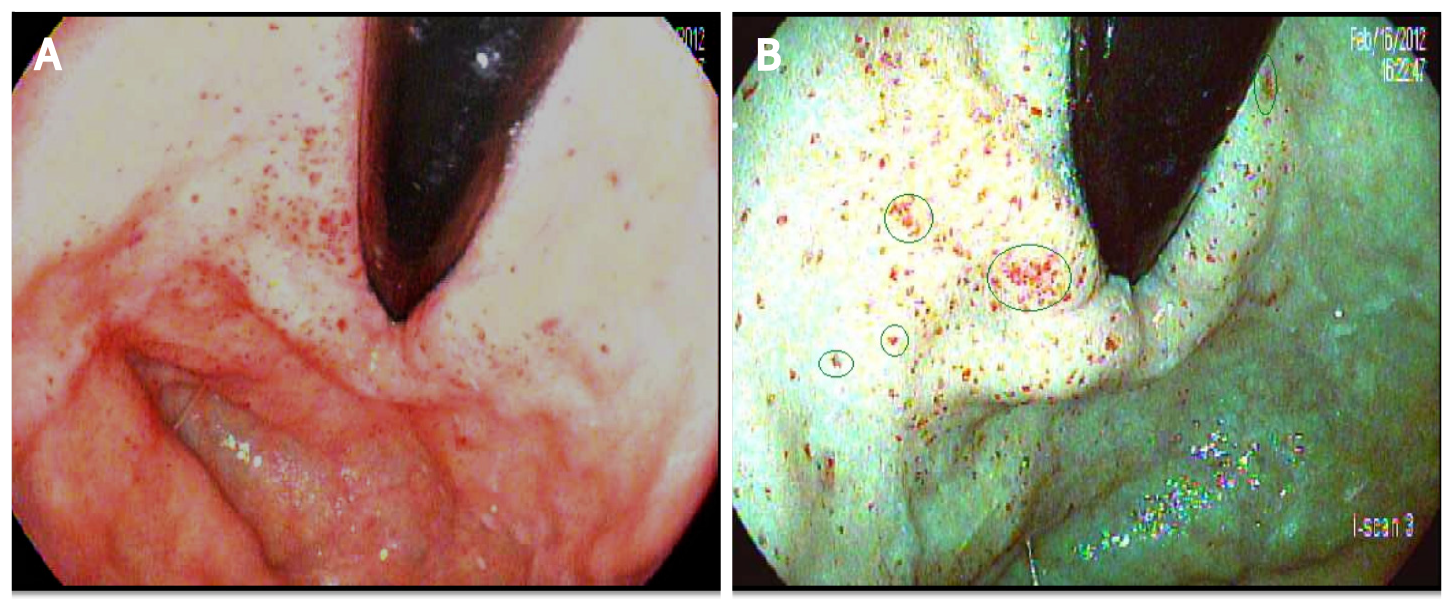
To create our diagnostic criteria, the author HH prospectively obtained I-scan pictures of the gastric mucosa when endoscopically evaluating classic PHG and GAVE in consenting adults with cirrhosis who underwent esophagogastroduodenoscopy (EGD). Upon review of the images and building on prior studies by Hayashi and Saeki[13] and Achim et al[12], the author HH created an I-scan criteria for diagnosis of GAVE and PHG. Gastric pits are usually round, pink, and surrounded by the subepithelial capillary network that drain into CVs. When PHG is present, there is pit edema and capillary engorgement on I-scan which manifests as the snake-skin appearance on HDWLE (Figure 2A). Similarly, CVs appear as dilated star-like dark-red spots with defined borders while intramucosal hemorrhage are typically lighter in color and have a hazier border compared to venules on I-scan (Figure 2B and C). In contrast, the classic appearance of GAVE on I-scan was defined as presence of capillary ectasia characterized by bright red spots with defined borders (Figure 2D)[12,13]. Additional examples of our PHG and GAVE under HDWLE and I-scan are in Figures 3 and 4. Participating endoscopists were then provided with a PowerPoint presentation explaining the visual appearance of GAVE and PHG with I-scan.
Prior to endoscopy, the following data were obtained from the patient once deemed to be eligible for this study: Age, gender, race, history of gastrointestinal bleeding in the past 3 mo, use of certain medications (non-steroidal anti-inflammatory drugs, aspirin, anticoagulants, iron tablets, or beta blockers), alcohol use, and the presence of ascites or lower extremity edema on exam.
All patients underwent an EGD similar to endoscopic evaluation performed in most clinical settings. Upper endoscope (models EG-3470K, EG-2990I, EG-3490K, and EG-2790K) developed by Pentax (Tokyo, Japan) were utilized in this study. Under direct visualization, the esophagus was intubated and the endoscope was advanced to the stomach. The gastric mucosa was first inspected using HDWLE for mucosal findings suggestive of GAVE and/or PHG. Patients who had abnormal gastric mucosal findings concerning for GAVE and/or PHG in whom the diagnosis was not certain utilizing HDWLE given lack of classic features underwent further evaluation with I-scan. Areas of abnormal gastric mucosa were carefully examined for 30 to 60 s utilizing HDWLE and the endoscopist determined the following: Visual diagnosis (PHG or GAVE), confidence level about diagnosis (high or low), location (antrum, antrum/ body, antrum/body/fundus, antrum/fundus, fundus, or body), PHG severity (mild, moderate, or severe), GAVE appearance (stripped, diffuse, punctate, past previous treatment), stigmata of recent bleeding, and presence of varices. High quality photos were taken. After HDWLE exam was completed, I-scan mode and electronic magnification (× 2) were activated. The tip of the scope was positioned about 2 cm away from the mucosa for careful examination. The endoscopist determined the following: Visual diagnosis (PHG or GAVE), confidence level about diagnosis (high or low), and presence of certain features on I-scan (pit edema, dilated capillaries, dilated venules, or intramucosal hemorrhage). High quality photos were taken. At completion of the visual inspection, biopsies of the abnormal gastric mucosa for histologic confirmation were taken using a standard biopsy forceps (Boston Scientific, Marlborough, MA).
Biopsy specimens were examined by a gastrointestinal pathologist using hematoxylin and eosin as well as special stains to establish the diagnosis. Pathologist commented on the presence of edema, vascular ectasia, acute and/or chronic inflammation, reactive epithelial cells, smooth fibers, microthrombi, hyalinosis, metaplasia, CD31 and CD61 positivity, and pathologic diagnosis. According to Westerhoff et al[14], staining for CD61 and CD31 has improved diagnostic accuracy of GAVE and PHG compared to H&E staining[14].
Analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC). To characterize the ability of HDWLE and I-scan to diagnose GAVE and PHG, sensitivities and specificities were calculated. Further, the percent accuracy of HDWLE and I-scan in diagnosing GAVE and PHG was compared with Fisher exact tests. Categorical data was summarized with frequencies and percentages while continuous data was summarized with medians and interquartile ranges (IQR). Differences between patients with correct and incorrect I-scan diagnoses of PHG were assessed through the use of Fisher exact tests or Wilcoxon rank-sum tests, as appropriate. Differences between patients with a correct and incorrect I-scan diagnosis of GAVE were analyzed similarly. All statistical tests were evaluated at the α = 0.05 significance level.
The study protocol was approved by the Saint Louis University Institutional Review Board. The study protocol, patient’s rights and obligations were reviewed with eligible patients and informed consent was obtained from all participants.
A total of 25 patients met the initial inclusion criteria and were eligible to participate. Two patients were subsequently excluded given biopsies did not show GAVE or PHG. Baseline characteristics of the study cohort including medications and laboratory analysis are summarized in Table 1. The majority of the patients included in this study were Caucasian (65.2%), male (60.9%) and had chronic hepatitis C causing cirrhosis (43.5%). None of the patients were prescribed anticoagulation or antiplatelet agents other than aspirin (31.8%). Median blood work for included patients included hemoglobin 10.6 g/dL, platelets 125000 per mm3, INR 1.1 and creatinine 1.0 mg/dL. The majority of patients underwent an upper endoscopy for management of esophagogastric varices (73.9%). Some patients already had some form of therapy for portal HTN including TIPS (8.7%), history of liver transplantation (13%) or use of beta blockers (45.5%).
| Overall (n = 23) | ||
| Age (median, IQR), n (%) | 60 | |
| Male | 14 | 60.9 |
| Caucasian | 15 | 65% |
| Etiology of cirrhosis | ||
| Alcohol (EtOH) | 3 | 13.0 |
| Granulomatous hepatitis | 1 | 4.4 |
| HBV | 1 | 4.4 |
| HCV | 10 | 43.5 |
| HCV, EtOH | 1 | 4.4 |
| Nonalcoholic steatohepatitis | 6 | 26.1 |
| Primary sclerosing cholangitis | 1 | 4.4 |
| Liver biopsy | 10 | 43.5 |
| Liver transplantation | 3 | 13.0 |
| Portal hypertension on imaging | 17 | 73.9 |
| TIPS | 2 | 8.7 |
| Cirrhosis on CT/US | 23 | 100.0 |
| History of connective tissue disease | 1 | 4.4 |
| Dialysis | 2 | 8.7 |
| Endoscopy suite, n (%) | ||
| Reason for EGD | ||
| Anemia | 1 | 4.4 |
| GI Bleed | 4 | 17.4 |
| Varices | 18 | 78.2 |
| Anticoagulation | 0 | 0.0 |
| Alcohol use in the past 15 d | 5 | 21.7 |
| ASA in the past 15 d | 7 | 31.8 |
| NSAIDS use in the past 15 d | 0 | 0.0 |
| Plavix | 0 | 0.0 |
| Beta blockers | 10 | 45.5 |
| Labs within 3 mo Pre EGD1 | median | IQR |
| Hemoglobin | 10.6 | 9.5–13.3 |
| Mean corpuscular volume | 89.2 | 87.0–90.5 |
| Platelet count | 126.5 | 68.0–152.0 |
| INR | 1.1 | 1.1–1.2 |
| Serum sodium | 139.0 | 137.0–142.0 |
| Alanine aminotransferase | 30.0 | 25.0–54.0 |
| Aspartate aminotransferase | 50.0 | 32.0–79.0 |
| Total bilirubin | 1.6 | 1.2–2.6 |
| Alkaline phosphatase | 108.0 | 85.0–134.0 |
| Serum albumin | 3.2 | 2.4–3.4 |
| Ferritin | 74.3 | 5.0–2458.0 |
| Creatinine | 1.0 | 0.70–1.47 |
| Labs within 4-8 wk after EGD1 | median | IQR |
| Hemoglobin | 11.4 | 8.9–12.8 |
| Mean corpuscular volume | 87.9 | 84.8–91.6 |
| Platelet count | 117.0 | 63.0–166.0 |
| INR | 1.2 | 1.1–1.3 |
| Serum sodium | 140.0 | 137.0–142.0 |
| Alanine aminotransferase | 31.0 | 21.0–42.0 |
| Aspartate aminotransferase | 44.0 | 29.0–68.0 |
| Total bilirubin | 1.2 | 0.9–1.9 |
| Alkaline phosphatase | 132.0 | 79.0–185.0 |
| Serum albumin | 3.0 | 2.6–3.3 |
| Ferritin | 197.4 | 63.0–199.0 |
| Creatinine | 1.0 | 0.70–1.50 |
Seven adults (30.4%) had confirmed GAVE on histology. HDWLE had a sensitivity of 85.7% and specificity of 62.5% in diagnosing GAVE compared to a sensitivity of 100% and 75% specificity utilizing our I-scan criteria (examples of GAVE and PHG under I-scan are in Supplementary Figures 1 and 2). As a result, utilizing HDWLE alone, the diagnosis of GAVE was accurately made in 69.57% (n = 16) of cases compared to 82.61% (n = 19) when utilizing I-scan technology (P = 0.491; Fisher exact test Table 2). In contrast, HDWLE has a sensitivity of 93.8% and a 75% specificity in diagnosing PHG compared to a sensitivity of 87.5% and specificity of 71.4% utilizing I-scan (accuracy of 82.61% with or without I-scan, P = 1.000 as in Table 3). I-scan was more likely to make an incorrect diagnosis of PHG in patients with alcoholic cirrhosis, alcohol use, or in patients with lower bilirubin levels while a better diagnosis of PHG was made antrum using I-scan when the antrum is involved (P < 0.05) (SupplementaryTable 1). I-scan was more likely to make an incorrect diagnosis of GAVE if the patient was on dialysis or an elevated creatinine (P < 0.05) (Supplementary Table 2). Other factors including age, gender, race, ascites, presence of varices, or laboratory findings were no significant.
| Biopsy | ||||
| No GAVE | GAVE | |||
| White Light | No GAVE | 10 | 1 | Sensitivity: 85.7% |
| GAVE | 6 | 6 | Specificity: 62.5% | |
| I-Scan | No GAVE | 12 | 0 | Sensitivity: 100% |
| GAVE | 4 | 7 | Specificity: 75.0% | |
| Biopsy | ||||
| No PHG | PHG | |||
| White Light | No PHG | 4 | 1 | Sensitivity: 93.8% |
| PHG | 3 | 15 | Specificity: 57.1% | |
| I-Scan | No PHG | 5 | 2 | Sensitivity: 87.5% |
| PHG | 2 | 14 | Specificity: 71.4% | |
In this pilot study, I-scan with magnification demonstrated a trend towards superior overall performance characteristics for real-time visual diagnosis of PHG and GAVE compared to HDWLE in patients with cirrhosis and ambiguous findings on endoscopic evaluation. This novel method may allow for an accurate, real time diagnosis in multiple critical clinical situations, such as when biopsy is contraindicated or when more urgent decisions regarding endoscopic management of gastrointestinal bleeding is needed. Therefore, I-scan should be considered a valuable diagnostic tool in such challenging clinical scenarios, although further prospective evaluation is needed.
The superiority of I-scan compared to HDWLE can be contributed to I-scan’s ability to provide real-time structural and vascular enhancement of HDWLE images. I-scan image processing involves three algorithms: Surface enhancement (SE), contrast enhancement (CE), and tone enhancement (TE). SE improves the delineation of the examined mucosa by accentuating blood vessels. CE can sharpen the appearance of surface vessels and enhance the visualized details of mucosa surface texture. TE accentuates mucosal patterns and vascular structures to aid in lesion characterization. These enhancements significantly contribute to the endoscopist ability to perform an accurate diagnosis based on the endoscopic appearance which is noted in this study when comparing the ability for the endoscopist to accurately diagnose GAVE based on visual appearance of the gastric mucosa. The utilization of I-scan technology allowed for increased sensitivity and specificity when diagnosing GAVE compared to standard HDWLE. This translated into an accuracy of 82% for I-scan and 70% for HDLWE. While this finding was not statistically significant likely due to small sample size, it does show a trend towards statistical significance. A more recent study using Narrow Band Imaging showed an increased accuracy of virtual chromoendoscopy at diagnosing GAVE. However, our study relied on more extensive advanced imaging diagnosis criteria and used special stains to confirm GAVE[15].
The clinical implications of improved visual diagnosis of GAVE are significant. Utilizing I-scan with magnification may potentially obviate the need for obtaining biopsies when visual diagnosis of GAVE can be made using I-scan. This can be especially helpful in situations where obtaining biopsies is discouraged given coagulopathy or active gastrointestinal bleeding which are relatively common scenarios in patients with cirrhosis. An accurate, real time diagnosis allows the endoscopist to initiate definitive management for gastrointestinal bleeding in a timely manner instead of delaying to confirm diagnosis via pathology evaluation. Ultimately, we suspect this will improve patient outcomes and utilization of hospital resources. In addition, an accurate visual diagnosis can obliviate the need to obtain biopsy which will results significant cost savings.
Patients with alcoholic cirrhosis or alcohol use were less likely to have an accurate diagnosis of PHG, suggesting that alcohol may alter the gastric pit and vascular patterns leading to a difficult PHG diagnosis. Indeed, alcohol use is known to alter the upper gastrointestinal mucosa and lead to atrophy and inflammation[16]. In contrast, I-scan had better ability to diagnosis PHG in the antrum and which is the stomach location where GAVE usually appears. These findings highlights the ability of I-scan in making accurate diagnosis of GAVE vs PHG in the antrum which is critical for management. We do note that patient with an elevated creatinine, and on dialysis were more likely to have an incorrect diagnosis of GAVE utilizing I-scan technology. At this time, the association between renal dysfunction on incorrect diagnosis using I-scan remain unclear and may have only been noted in this study due to the small sample size or could be due to underlying edema leading to obscured diagnosis. These findings are novel and have not been noted in other studies evaluating the accuracy of I-scan technology in diagnosing gastrointestinal pathology.
In light of the emerging technologies in endoscopic imaging, the preservation and incorporation of valuable endoscopic innovations (PIVI) initiative was developed by the American Society for Gastrointestinal Endoscopy to set thresholds that any new technology should meet before it can replace the current practice of random biopsies. These thresholds have been described for diminutive colonic polyps[17] and Barrett’s esophagus[18] but not for PHG or GAVE. This study shows promising results in utilizing I-scan technology to assist with accurate visual diagnosis. Despite the promising results notes in this study, there is limitation to this data. First, the small sample size may have affected the results and these results should be confirmed with a larger study prior to implementing into clinic practice. Given multiple endoscopist performed the procedures after a short PowerPoint presentation on the visual diagnosis of GAVE and PHG utilizing I-scan technology, there was likely some variability in endoscopist’s diagnosis. Finally, we could not account for the learning curve leading to more accurate diagnosis for GAVE and PHG with HDWLE later in the study.
We conclude that, utilizing I-scan with magnification may obviate the need for biopsies when visual diagnosis of either PHG or GAVE can be made with high confidence. This pilot work supports the further evaluation of I-scan in these challenging clinical situations using a larger sample size and a follow up of outcomes in a randomized fashion.
Gastric antral vascular ectasia (GAVE) and portal hypertensive gastropathy (PHG) are two not uncommon causes of upper gastrointestinal bleeding in patients with cirrhosis. While endoscopic appearance can suggest the diagnosis, gastric biopsies are the current gold standard for differentiating PHG from GAVE.
Distinguishing GAVE from PHG is important as the management is different for the two conditions. Obtaining gastric biopsies to diagnose GAVE and PHG may be contraindicated given coagulopathy or thrombocytopenia which are commonly seen with cirrhosis. Here we hypothesized that I-scan virtual chromoendoscopy is more sensitive and specific than high-definition white light endoscopy (HDWLE) at diagnosing GAVE when compared to gastric biopsy.
The main objective of this work was to determine the added diagnostic value of virtual chromoendoscopy to high definition white light for real-time endoscopic diagnosis of GAVE and PHG.
We developed an I-scan virtual chromoendoscopy criteria for diagnosis of GAVE and PHG. We then tested these criteria in a prospective cohort of cirrhotic adults with GAVE and PHG when HDWLE diagnosis was in doubt. We then compared the accuracy of I-scan vs HDWLE alone compared to histology.
I-scan with magnification demonstrated superior overall performance characteristics for real-time visual diagnosis of PHG and GAVE compared to HDWLE in patients with cirrhosis and ambiguous findings on endoscopic evaluation.
This novel finding allows for an accurate, real time diagnosis in multiple critical clinical situations, such as when biopsy is contraindicated or when more urgent decisions regarding endoscopic management of gastrointestinal bleeding is needed.
Utilizing I-scan with magnification may obviate the need for biopsies when visual diagnosis of either PHG or GAVE can be made with high confidence. This pilot work supports the further evaluation of I-scan in these challenging clinical situations using a larger sample size and a follow up of outcomes in a randomized fashion.
The authors would like to thank Dr. Kiyoko Oshima who participated in the initial design of this study.
| 1. | Qureshi K, Al-Osaimi AM. Approach to the management of portal hypertensive gastropathy and gastric antral vascular ectasia. Gastroenterol Clin North Am. 2014;43:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Tekola BD, Caldwell S. Approach to the management of portal hypertensive gastropathy and gastric antral vascular ectasia. Clin Liver Dis (Hoboken). 2012;1:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Pérez-Ayuso RM, Piqué JM, Bosch J, Panés J, González A, Pérez R, Rigau J, Quintero E, Valderrama R, Viver J. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 167] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Gjeorgjievski M, Cappell MS. Portal hypertensive gastropathy: A systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J Hepatol. 2016;8:231-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 5. | Patwardhan VR, Cardenas A. Review article: the management of portal hypertensive gastropathy and gastric antral vascular ectasia in cirrhosis. Aliment Pharmacol Ther. 2014;40:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 6. | Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (8)] |
| 7. | Ito M, Uchida Y, Kamano S, Kawabata H, Nishioka M. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc. 2001;53:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165-1170. [PubMed] |
| 9. | Carpinelli L, Primignani M, Preatoni P, Angeli P, Battaglia G, Beretta L, Bortoli A, Capria A, Cestari R, Cosentino F, Crotta S, Gerunda G, Lorenzini I, Maiolo P, Merighi A, Rossi A, Sangiovanni A, de Franchis R. Portal hypertensive gastropathy: reproducibility of a classification, prevalence of elementary lesions, sensitivity and specificity in the diagnosis of cirrhosis of the liver. A NIEC multicentre study. New Italian Endoscopic Club. Ital J Gastroenterol Hepatol. 1997;29:533-540. [PubMed] |
| 10. | Sarin SK, Sreenivas DV, Lahoti D, Saraya A. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;102:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 111] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Achim AC, Vesa SC, Dumitru E. The Efficacy of Virtual Chromoendoscopy in the Diagnosis of Portal Hypertensive Gastropathy. J Gastrointestin Liver Dis. 2016;25:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Hayashi S, Saeki S. Endoscopic microvascular architecture of the portal hypertensive gastric muscosa on narrow band imaging. Diges Endosc. 2007;116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Westerhoff M, Tretiakova M, Hovan L, Miller J, Noffsinger A, Hart J. CD61, CD31, and CD34 improve diagnostic accuracy in gastric antral vascular ectasia and portal hypertensive gastropathy: An immunohistochemical and digital morphometric study. Am J Surg Pathol. 2010;34:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Chang CY, Chen PH, Hou MC, Chang WC, Yang TC, Hsin IF, Liao WC, Lee FY. Magnifying endoscopy with narrow-band image for diagnosing diffuse type of gastric antral vascular ectasia in cirrhotic patients. Eur J Gastroenterol Hepatol. 2021;33:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Bienia A, Sodolski W, Luchowska E. The effect of chronic alcohol abuse on gastric and duodenal mucosa. Ann Univ Mariae Curie Sklodowska Med. 2002;57:570-582. [PubMed] |
| 17. | Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, Lieberman DA. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 483] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 18. | Sharma P, Savides TJ, Canto MI, Corley DA, Falk GW, Goldblum JR, Wang KK, Wallace MB, Wolfsen HC; ASGE Technology and Standards of Practice Committee. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett's Esophagus. Gastrointest Endosc. 2012;76:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Kothari HG S-Editor: Fan JR L-Editor: A P-Editor: Fan JR