Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1753
Peer-review started: April 16, 2021
First decision: June 23, 2021
Revised: July 2, 2021
Accepted: September 1, 2021
Article in press: September 1, 2021
Published online: November 27, 2021
Processing time: 221 Days and 12.6 Hours
The high mortality rate of hepatocellular carcinoma (HCC) in Egypt is due mainly to the increasing prevalence of hepatitis C virus infection (HCV) and late diagnosis of the carcinoma. MicroRNAs (miRNA), which regulate tumor proliferation and metastasis in HCC, may serve as a useful diagnostic approach for the early detection of HCC, thus decreasing its mortality. Meanwhile, endocan is a protein with angiogenic and inflammatory properties that are associated with tumor progression and poor outcomes.
To analyze the levels of miRNA 9-3p and endocan in HCV-infected HCC patients and correlate them with clinicopathological parameters.
We compared levels of endocan and circulating miRNA 9-3p from 35 HCV-related HCC patients to 33 patients with HCV-induced chronic liver disease and 32 age and gender matched healthy controls recruited from inpatient and outpatient clinics of the National Liver Institute, Menoufia University, Egypt in the period from January to March 2021 in a case-control study. Serum samples from all groups were analyzed for HCV. Endocan was measured by enzyme-linked immunosorbent assays, and the expression levels of circulating miRNA 9-3p were measured by real-time quantitative reverse transcriptase PCR.
The levels of circulating miRNA 9-3p were significantly lower in the HCC group compared to the chronic liver disease (P < 0.001) and control (P < 0.001) groups, while levels in the chronic liver disease were significantly lower than those in the control group (P < 0.001). The levels of serum endocan were significantly higher in the HCC group compared to the chronic liver disease (P < 0.001) and control (P < 0.001) groups. Moreover miRNA 9-3p and endocan performed better than α-fetoprotein in discriminating HCC patients from cirrhosis and healthy patients. The levels of miRNA 9-3p were significantly inversely correlated to vascular invasion (P = 0.002), stage of advancement of Barcelona Clinical Liver Cancer (P < 0.001) and the metastatic site (P < 0.001) of the HCC group.
Circulating miRNA 9-3p and endocan can be used as novel biomarkers for the early diagnosis of HCV-related HCC.
Core Tip: The level of circulating microRNA 9-3p was significantly decreased in hepat
- Citation: Wahb AMSE, El Kassas M, Khamis AK, Elhelbawy M, Elhelbawy N, Habieb MSE. Circulating microRNA 9-3p and serum endocan as potential biomarkers for hepatitis C virus-related hepatocellular carcinoma. World J Hepatol 2021; 13(11): 1753-1765
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1753.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1753
Worldwide, hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third leading cause of cancer deaths[1]. In Egypt, HCC is a significant health problem, as it is the most prevalent and second most prevalent cancer in males and females, respectively. It is the most prevalent malignancy in general, accounting for 32.35% of the total cancer deaths[2,3]. One reason for these high prevalence rates is the high prevalence of hepatitis C in Egypt[4].
Moreover, HCC has been attributed to molecular aberrations, such as errors in regulation of gene expression, which may result in translational repression and/or degradation[5,6]. To improve the overall survival from HCC, extensive research is needed, focusing particularly on more accurate and monitored management of the disease[7].
MicroRNAs (miRNAs) are small (approximately 22 nucleotides long), endogenous, non-protein coding RNAs that are key post-transcriptional regulators of gene expression[8]. miRNAs regulate different cellular pathways, including the cell cycle, cell proliferation and apoptosis. Dysregulation of miRNAs can therefore impact cellular processes involved in tumorigenesis and cancer. Thus, serum miRNAs may serve as non-invasive biomarkers for the diagnosis of cancer[9].
Three genes encode miRNA 9-3p: MIR9-1, MIR9-2 and MIR9-3, located on chromo
Endocan is a 50 kDa soluble proteoglycan that circulates freely in the bloodstream of healthy individuals and is expressed by the vascular endothelium. It has angiogenic and inflammatory properties that may affect vascular permeability, thus it plays crucial roles in regulating major physiological and pathophysiological processes, such as cell adhesion, inflammation and tumor progression[13]. Endocan expression is upregulated in cancer cells derived from the lung, kidney, brain, astrocytes and liver[14].
A single study has reported that miRNA 9-3p expression decreases in bladder cancer patients, resulting in reduced inhibition of endocan, thus increasing its expression, which promotes cell proliferation[15]. These results indicate that the gene encoding endocan is a target of miRNA 9-3p. To the best of our knowledge, simul
This case-control study included a total of 100 subjects recruited from inpatient and outpatient clinics of the National Liver Institute, Menoufia University, Egypt in the period from January to March 2021. Participants were categorized into three groups: Group I: 35 patients with HCV-related HCC; Group II: 33 patients with chronic liver disease due to chronic HCV; and Group III: 32 healthy and free of viral infection volunteers of matched age and gender. Patients were selected based on restrictive inclusion criteria including patients whose age was more than 18 years with confirmed HCV infection by both HCV antibody (anti-HCV) detection and positive HCV RNA. In Group I, HCC was diagnosed (triphasic spiral computed tomography or dynamic magnetic resonance imaging together with elevated α-fetoprotein and/or liver biopsy), and its stage was identified according to the Barcelona Clinical Liver Cancer (BCLC) system[16]. In Group II, chronic liver disease was diagnosed based on history, clinical examination, laboratory results and imaging that included abdominal ultrasonography and computed tomography. Liver disease severity was assessed by the Child-Pugh score. Patients with positive hepatitis B surface antigen and/or hepatitis B c antibody, secondary liver cancer, other malignancies, chronic hepatitis or cirrhosis due to any cause other than HCV infection, significant associated comorbidities (such as renal failure or heart failure) and those receiving chemotherapy, radiotherapy or on immunosuppression medication were excluded. Detailed histories of all participants were taken, and they all underwent physical examination, liver imaging (abdominal ultrasound) and routine laboratory tests that included complete blood counts, kidney and liver function tests [albumin, total bilirubin, direct bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], serum levels of α-fetoprotein, and serological tests for hepatitis B virus and HCV. Serum samples from Groups I and II were analyzed for HCV by reverse transcriptase PCR (RT-PCR).
Seven milliliters of venous blood were withdrawn by venipuncture; of this, 5 mL were transferred into a plain tube, left to clot and centrifuged for 10 min at 4000 rpm. The serum obtained was stored at -80 ºC until subsequent analyses for serum α-fetoprotein levels, liver function tests, hepatitis viral markers and endocan levels. The remaining 2 mL of blood were placed into an EDTA containing tube for HCV RT-PCR and miRNA 9-3p expression analysis. Anti-HCV levels were determined by electrochemiluminescence immunoassay using the Cobas immunoassay analyzer.
The hepatitis B surface antigen in serum was determined using (Sorin Biomedica Co. kits, Italy). Serum levels of ALT and AST were determined by the kinetic UV optimized method of the IFCC (ELTEC Kit, England). Serum levels of total bilirubin were measured using the DIAMOND diagnostics Kit, Germany. Serum albumin levels were quantified using a colorimetric method of enhanced specificity of bromocresol green (DIAMOND diagnostics Kit, Germany). Prothrombin time was determined by the STA-Stago Compact computed tomography autoanalyzer. Serum α-fetoprotein levels were measured by enzyme-linked immunosorbent assays using the IMMULITE 1000 system (Siemens Medical Solutions Diagnostics, United States).
Serum endocan levels were measured by enzyme-linked immunosorbent assay using the PicokineTM ELISA Kit for human ESMI/Endocan (Boster Biological Technology Co., Ltd., CA, United States, cat# EK0752).
Nucleic acids were extracted using the Qiagen viral RNA Mini Extraction Kit.
miRNA was isolated from plasma using the QiagenTM RNA extraction Kit MiRNeasy Kit (QIAGEN). miRNA was purified and then its concentration and purity were quantified using a NanoDrop® N50 nanophotometer (Implant GmbH and Implen, Inc. Schatzbogen 52 81829 München, Germany). Purified miRNA was stored at -80 °C until reverse transcription, which was accomplished using the Qiagen®miScript II RT Kit (QIAGEN) following the manufacturer’s instructions. Each 20-µl reaction tube contained 4 μL 5 × miScript HiSpec Buffer, 2 μL 10 × miScript Nuclease Mix, 2 μL RNase-free water, 2 μL miScript Reverse Transcriptase Mix, and 10 μL template RNA. Reverse transcription was carried out at 37 °C for 60 min and 95 °C for 5 min on an Applied Biosystems 2720 thermal cycler (Bioline, Singapore, United States). The cDNA product was diluted to 5 ng/ul before determining the transcript levels by real-time quantitative PCR. Real-time quantitative PCR was performed using the miScript SYBR Green PCR Kit (QIAGEN) according to the manufacturer's instructions. The reaction mixture contained 12.5 μL 2x QuantiTect SYBR Green PCR Master Mix, 2.5 μL 10x miScript Universal Primer based on mRNA sequences obtained from the miRBase database for miRNA 9-3p, 2.5 μL template cDNA and 3.5 μL RNase-free water. The Applied Biosystems®7500 real-time thermal cycler (Applied Biosystems, Foster City, CA, United States) was programmed to run 40 cycles of the following steps: 95 °C for 15 min (initial denaturation step), denaturation at 94 °C for 15 s, annealing for 30 s at 55 °C and extension for 30 s at 70 °C. U6 snRNA was used as an endogenous control. Relative quantification expression levels were calculated using the comparative 2−ΔΔCt method with Applied Biosystems 7500 software version 2.0.1. Each run was completed using melting curve analysis to confirm the specificity of the amplification and absence of primer dimers.
All procedures involving human participants were performed according to the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Menoufia University Faculty of Medicine Research Ethics Committee. Every patient in this research provided their written consent to participate in the research, provided that they were not identified in the paper.
Data were analyzed using IBM SPSS statistics software version 20 (SPSS Inc. Released 2011. IBM SPSS statistics for windows, version 20.0, Armonk, NY, United States: IBM Corp). Quantitative data are presented as means, standard deviations, medians and interquartile ranges, while qualitative data are presented as frequencies and percentages. The relationship between qualitative variables was evaluated by the χ2 test. Pairs of groups of non-normally distributed quantitative data were compared by the Mann–Whitney test, while three groups were compared by the Kruskal–Wallis test (non-parametric analysis of variance). Based on Kruskal–Wallis distribution, a post-hoc test was performed for pairwise comparisons. Correlations were assessed using the Spearman correlation test. The diagnostic values of serum miRNA 9-3p and endocan in HCC patients were evaluated by the receiver operating characteristic (ROC) curve analysis. The most independent factor associated with metastasis was identified by logistic regression analysis. P values of < 0.05 were considered statistically significant.
The patients in all three groups did not differ statistically in terms of age and gender. Biochemical analyses results (Table 1) showed that the HCC group and the chronic liver disease group differed in the following: ALT, direct bilirubin, international normalized ratio, endocan and miRNA 9-3p (P = 0.005, P = 0.003, P = 0.002, P < 0.001 and P < 0.001, respectively).
| Variables | Group I | Group II | Group III | P value | ||
| HCC | Chronic liver disease | Control | ||||
| n = 35 | n = 33 | n = 32 | ||||
| Gender | ||||||
| Male, n (%) | 30 (85.7) | 23 (69.7) | 27 (84.4) | NS | ||
| Female, n (%) | 5 (14.3) | 10 (30.3) | 5 (15.6) | |||
| Age (yr) | ||||||
| mean ± SD | 55.2 ± 5.2 | 52.7 ± 5.3 | 52.8 ± 5.6 | NS | ||
| ALT (IU/L), median (IQR) | 50.0 (35.0-55.0) | 34.0 (28.0-50.0) | 29.7 (23.5-31.7) | P < 0.001 | aP = 0.005; bP < 0.001; cP = 0.003 | |
| AST (IU/L), median (IQR) | 52.0 (39.0-70.0) | 42.0 (32.0-57.0) | 32.8 (30.0-36.0) | < 0.001 | aP = 0.060; bP < 0.001; cP < 0.001 | |
| Hb (mg/dL), mean ± SD | 13.1 ± 1.7 | 12.5 ± 1.6 | 13.5 ± 1.0 | 0.025 | aP = 0.260; bP = 0.438; cP = 0.019 | |
| Platelets, (× 10³/μL), median (IQR) | 141.0 (104.5-193.5) | 162.0 (134.0-213.0) | 197.5 (180.5-246.0) | 0.002 | aP = 0.225; bP < 0.001; cP = 0.022 | |
| Serum ALB (g/dL), mean ± SD | 3.6 ± 0.7 | 3.8 ± 0.6 | 4.1 ± 0.4 | 0.001 | aP = 0.382; bP = 0.001; cP = 0.050 | |
| INR, mean ± SD | 1.3 ± 0.2 | 1.1 ± 0.4 | 0.8 ± 0.2 | < 0.001 | aP = 0.002; bP = 0.001; cP = 0.001 | |
| α-fetoprotein (ng/mL), median (IQR) | 240.0 (28.2-635.0) | 124.0 (108.9-166.0) | 17.4 (14.0-24.0) | < 0.001 | aP = 0.895; bP < 0.001; cP < 0.001 | |
| Endocan (pg/mL), median (IQR) | 3450.0 (3188.5-4135.0) | 1934.0 (1450.0-2257.0) | 878.5 (850.0-1188.0) | < 0.001 | aP < 0.001; bP < 0.001; cP = 0.001 | |
| microRNA 9-3p, median (IQR) | 0.03 (0.02-0.05) | 0.42 (0.29-1.35) | 1.70 (1.40-2.15) | < 0.001 | aP < 0.001; bP < 0.001; cP < 0.001 | |
The HCC group differed significantly from the control group in terms of ALT, AST, platelet count, serum albumin, direct bilirubin, international normalized ratio, α-fetoprotein level, endocan and miRNA 9-3p (P < 0.001). The chronic liver disease group differed significantly from the control group in terms of ALT, AST, hemoglobin level, platelet count, international normalized ratio, α-fetoprotein level, endocan and miRNA 9-3p (P = 0.003, P < 0.001, P = 0.019, P = 0.022, P = 0.001, P < 0.001, P < 0.001 and P < 0.001 respectively).
Serum endocan levels in the HCC group were significantly higher than those in the chronic liver disease and control groups (P < 0.001). Furthermore, serum miRNA 9-3p expression levels in the HCC group were significantly lower than those in the chronic liver disease and control groups (P < 0.001), while levels in the chronic liver disease group were significantly lower than those in the control group (P < 0.001).
In the HCC group, 62.9% of patients (22 patients) were classified as grade A and (37.1%) of patients (13 patients) were classified as grade B according to Child-Pugh classifications; 4 (11.4%) patients were classified as grade A in BCLC stage, 18 (51.4 %) patients in stage B and 13 (37.1%) patients were in stage C. Detailed tumor characteristics of the HCC group are shown in Table 2.
| Number of the focal lesions | n (%) |
| Single | 16 (45.7) |
| Multiple | 19 (54.3) |
| Tumor size in cm | |
| Small < 3 | 7 (20.0) |
| Medium 3-5 | 15 (42.9) |
| Large > 5 | 13 (37.1) |
| Location of the focal lesions | |
| Rt. Lobe | 19 (54.3) |
| Lt. Lobe | 8 (22.9) |
| Both | 7 (20.0) |
| Caudate lobe | 1 (2.9) |
| BCLC stage | |
| A | 4 (11.4) |
| B | 18 (51.4) |
| C | 13 (37.1) |
| Vascular invasion | |
| Negative | 25 (71.4) |
| Positive | 10 (28.6) |
| LN metastasis | |
| Negative | 28 (80.0) |
| Positive | 7 (20.0) |
| Ascites | |
| No | 25 (73.5) |
| Mild | 8 (23.5) |
| Moderate | 1 (2.9) |
| Child Pugh classA | 22 (62.9) |
| B | 13 (37.1) |
| C | 0 (0) |
| Distant metastasis | |
| No | 23 (65.7) |
| Yes | 12 (34.3) |
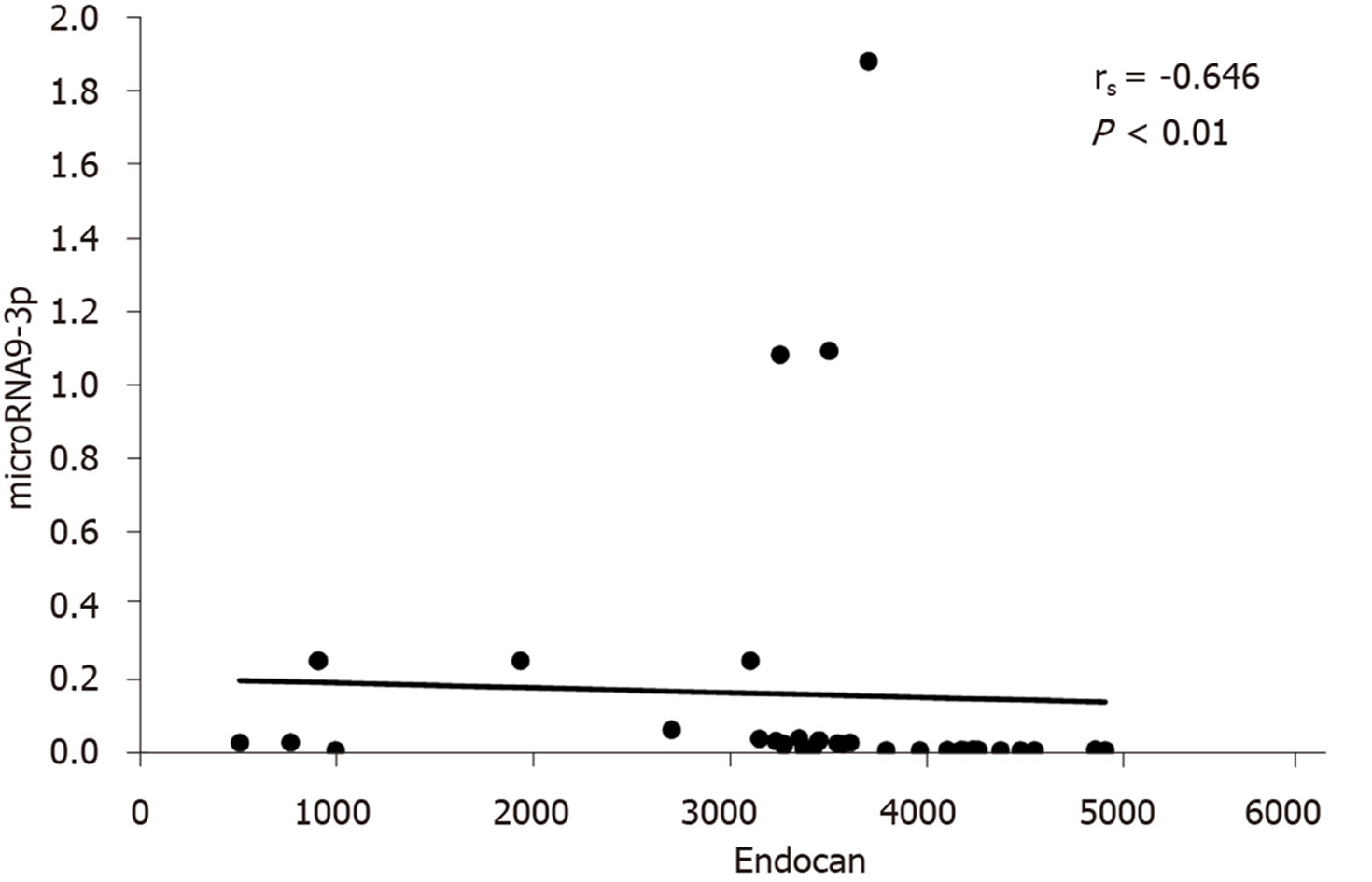
The correlations between serum miRNA 9-3p levels and clinical data in HCC patients are shown in Table 3. miRNA 9-3p expression levels were significantly inversely correlated to vascular invasion, BCLC classification and metastatic site. Moreover, miRNA 9-3p expression levels were also significantly inversely correlated to serum endocan levels (Figure 1).
| n | microRNA 9-3p | |||
| Median (IQR) | P value | |||
| Vascular invasion | ||||
| Negative | 25 | 0.04 (0.02-0.26) | 0.002 | |
| Positive | 10 | 0.02 (0.02-0.02) | ||
| LN metastasis | ||||
| Negative | 28 | 0.04 (0.02-0.17) | 0.072 | |
| Positive | 7 | 0.02 (0.02-0.03) | ||
| Distant metastasis | ||||
| No | 23 | 0.04 (0.03-0.26) | < 0.001 | |
| Yes | 12 | 0.02 (0.02-0.02) | ||
| Child Pugh class | ||||
| A | 22 | 0.03 (0.02-0.04) | 0.389 | |
| B | 13 | 0.04 (0.02-0.26) | ||
| Tumor number | ||||
| Single | 16 | 0.03 (0.02-0.17) | 0.935 | |
| Multiple | 19 | 0.03 (0.02-0.05) | ||
| Tumor size in cm | ||||
| Small < 3 | 7 | 0.03 (0.02-0.15) | 0.852 | |
| Medium 3-5 | 15 | 0.03 (0.02-0.06) | ||
| Large > 5 | 13 | 0.04 (0.02-0.04) | ||
| Tumor site | ||||
| Rt lobe | 19 | 0.04 (0.02-0.06) | 0.432 | |
| Lt lobe | 8 | 0.04 (0.03-0.15) | ||
| Both | 7 | 0.02 (0.02-0.04) | ||
| Caudate lobe | 1 | |||
| BCLC stage | ||||
| A | 4 | 0.26 (0.17-0.26) | < 0.001 | |
| B | 18 | 0.04 (0.03-0.05) | ||
| C | 13 | 0.02 (0.02-0.02) | ||
Univariate and multivariate logistic regression analyses on the HCC group indicated that miRNA 9-3p is an independent predictor factor of metastasis (P = 0.041; 95% confidence interval: 0.089-0.951) (Table 4).
| Univariate | Multivariate | |||
| P value | OR (95%CI) | P value | OR (95%CI) | |
| microRNA 9-3p | 0.008 | 0.193 (0.057-0.653) | 0.041 | 0.291 (0.089-0.951) |
| Endocan | 0.023 | 1.002 (1.000-1.003) | 0.358 | 1.001 (0.999-1.002) |
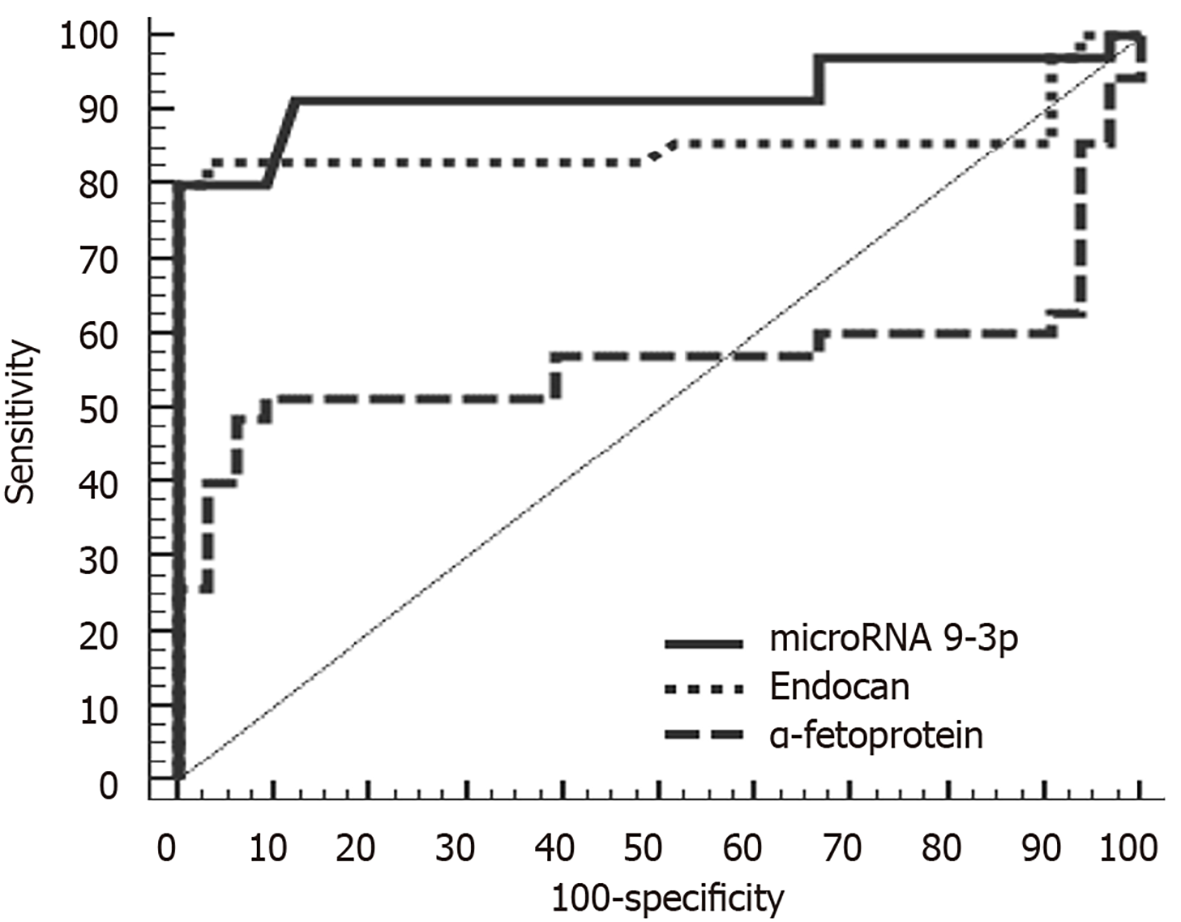
ROC analysis of miRNA 9-3p and endocan levels indicated that at a cutoff point of 0.26, miRNA 9-3p can discriminate between patients with HCC and those with chronic liver disease with a sensitivity of 91.43%, a specificity of 87.88%, a positive predictive value of 88.90% and a negative predictive value of 90.60%. Meanwhile, at a cutoff point of 2370 pg/mL, endocan can discriminate between HCC and chronic liver disease patients with a sensitivity of 82.86%, a specificity of 84.85%, a positive predictive value of 85.30% and a negative predictive value of 82.40%. In comparison, α-fetoprotein was less sensitive and specific (60.00% and 33.30%, respectively).
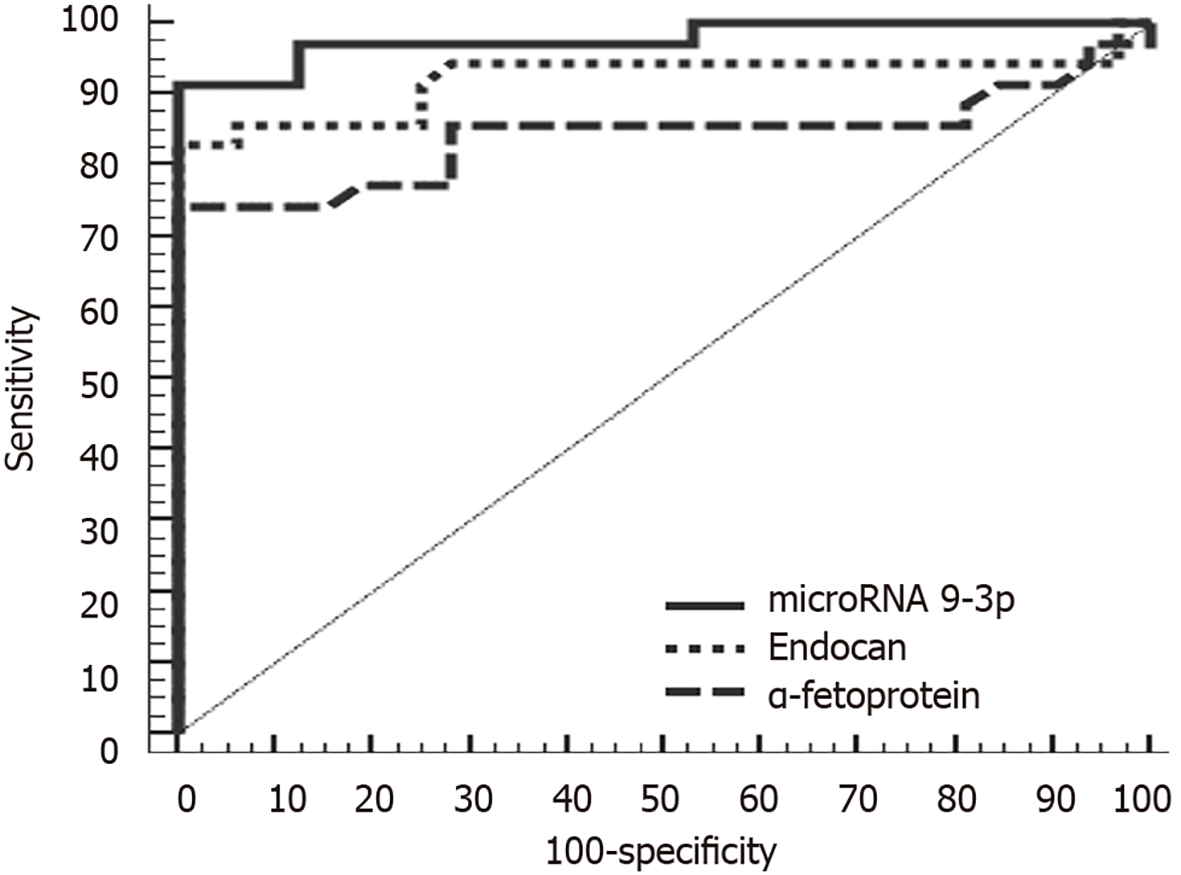
At a cutoff point of 1.01, miRNA 9-3p can discriminate between HCC and control group patients with a sensitivity of 91.43%, a specificity of 87.50%, a positive predictive value of 88.90% and a negative predictive value of 90.30%. Meanwhile, at a cutoff point of 1510 pg/mL, endocan can discriminate between HCC and chronic liver disease patients with a sensitivity of 85.71%, a specificity of 87.50%, a positive predictive value of 88.20% and a negative predictive value of 84.80%. In comparison, α-fetoprotein was less sensitive and specific (80.00% and 71.87%, respectively). Diagnostically, both miRNA 9-3p and endocan performed better than α-fetoprotein at discriminating HCC patients from both chronic liver disease and healthy patients (Figures 2 and 3).
ROC analysis of miRNA 9-3p levels in the HCC group indicated that at a cutoff point of 0.02, miRNA 9-3p can discriminate between metastatic and non-metastatic HCC patients with a sensitivity of 91.67%, a specificity of 82.61%, a positive predictive value of 73.30% and a negative predictive value of 95.00%.
The increasing prevalence of HCC worldwide and its associated poor prognosis make it a global health problem. Studies in Egypt shows the increasing role of HCV infection in liver cancer etiology, and among all cancer deaths in Egypt, HCC is the primary cause[17,18].
HCC is often detected late, when it is no longer operable, which limits curative surgical treatment to only a few cases involving small HCC malignancies. Moreover, as a diagnostic tool, α-fetoprotein is limited in its accuracy[19]. In contrast, circulating miRNAs may serve as biomarkers and a useful diagnostic approach for the early detection of HCC[20].
In the present study, clinical and laboratory data from the three different groups of patients revealed that serum α-fetoprotein levels of HCC and chronic liver disease patients was significantly different from those of control patients. α-fetoprotein is known to be overexpressed in HCC[21-23], and the severity of cirrhosis is a significant predictor of elevated serum α-fetoprotein levels; higher serum α-fetoprotein levels are significantly correlated with advanced cirrhosis in patients with chronic HCV[24].
We found that serum α-fetoprotein levels in the HCC group did not differ significantly from those of the chronic liver disease group. This agrees with the results of Massironi et al[25], who reported similar findings in HCC and liver cirrhosis subjects. In our study, at a cutoff value of 23 ng/dL, α-fetoprotein discriminates between HCC and control patients at a sensitivity of 80.00% and a specificity of 71.87%. These results are similar to those of Massironi et al[25] and Metwaly et al[26], who reported a sensitivity of 75% and a specificity of 80% at a cutoff value of 16.9 ng/dL.
Our findings show higher serum endocan levels in HCC patients than in chronic liver disease patients, which agrees with previous studies by Nault et al[27] and Ozaki et al[28].
Recent studies on HCC show that elevated serum endocan levels and endocan expression by stromal endothelial cells in HCC tissues are correlated with poor survival[29]. Endocan expression in tumors undergoing angiogenesis reflects the processes of angiogenesis and tumor invasion. Structurally, the glycan form and phenylalanine-rich region of endocan are its key effective sections through the nuclear factor-κB/IκB pathway[30]. However, the involvement of endocan in HCC develo
We found that plasma miRNA 9-3p levels are significantly lower in HCC patients compared to chronic liver disease and control patients. Overall, the order of miRNA 9-3p expression among the different groups is as follows: HCC < chronic liver disease < control.
This supports the concept of the antitumor function of miRNA 9-3p as reported by Higashi et al[12], Yang et al[31] and Tang et al[32]. In contrast, Sun et al[33] showed that miR-9 increases the levels of migration and invasion of HCC cell lines. It is possible that miR-9 (i.e. miR-9-5p) and miR-9* (miR-9-3p) are two different miRNAs that originate from the same precursor, and they can play either synergistic or opposite roles within one malignancy[34].
Interestingly, we observed significantly lower levels of miRNA 9-3p expression and vascular invasion at the advanced stage of BCLC and at the metastatic site of the HCC group.
In cervical adenocarcinoma, miRNA 9-3p is downregulated and acts as a tumor suppressor. Ectopic expression of miR-9-3p inhibits the JAK/STAT3 pathway by targeting interleukin 6, leading to the upregulation of vascular endothelial growth factor and increased angiogenesis. This results in decreased proliferation and migration and reduced tumor growth in vivo[35]. Moreover, Tang et al[32] reported that exosomal miRNA 9-3p suppresses the development and progression of HCC.
Cai et al[15] reported that increased exosomal miR-9-3p counteracts bladder cancer growth and metastasis and decreases endocan protein expression in nude mice. We similarly observed that miR-9-3p expression is inversely correlated to serum endocan levels in the HCC group.
We performed ROC analysis to compare the diagnostic accuracies of miRNA 9-3p, endocan and the traditional HCC tumor marker, α-fetoprotein. Diagnostically, both miRNA 9-3p and endocan perform better than α-fetoprotein in discriminating patients with HCC from those with or without (i.e. healthy) chronic liver disease. Furthermore, ROC analysis revealed that miRNA 9-3p performed well at discriminating between metastatic and non-metastatic patients in the HCC group. Statistically, miRNA 9-3p is an independent predictor factor of metastasis. This study could be the nucleus of a larger study working on a larger number of patients that may include those with other causes of chronic liver disease like alcoholism as our study was limited to HCV-induced chronic liver disease as it is highly prevalent in Egypt.
Endocan and miRNA 9-3p could be biomarkers with potential use for the early diagnosis of HCV-related HCC. In this regard, they are more valuable than α-fetoprotein. Moreover, miRNA 9-3p is an independent predictor of metastasis in HCC patients.
The high mortality rate of hepatocellular carcinoma (HCC) in Egypt is due mainly to the increasing prevalence of hepatitis C virus infection (HCV) and late diagnosis of the carcinoma.
MicroRNAs (miRNA), which regulate tumor proliferation and metastasis in HCC, may serve as a useful diagnostic approach for the early detection of HCC, thus decreasing its mortality. Meanwhile, endocan is a protein with angiogenic and inflammatory properties that are associated with tumor progression and poor outcomes.
To analyze the levels of miRNA 9-3p and endocan in HCV-infected HCC patients and correlate them with clinicopathological parameters.
We compared levels of endocan and circulating miRNA 9-3p from 35 HCV-related HCC patients to 33 patients with HCV-induced chronic liver disease and 32 age and gender matched healthy controls.
The levels of circulating miRNA 9-3p were significantly lower in the HCC group compared to the chronic liver disease (P < 0.001) and control (P < 0.001) groups, while levels in the chronic liver disease were significantly lower than those in the control group (P < 0.001). While the levels of serum endocan were significantly higher in the HCC group compared to the chronic liver disease (P < 0.001) and control (P < 0.001) groups. Moreover, miRNA 9-3p and endocan performed better than α-fetoprotein in discriminating HCC patients from cirrhosis and healthy patients. The levels of miRNA 9-3p are significantly inversely correlated to vascular invasion (P = 0.002), stage of advancement of Barcelona Clinical Liver Cancer (P < 0.001 and the metastatic site (P < 0.001) of the HCC group.
Endocan and miRNA 9-3p could be biomarkers with potential use for the early diagnosis of HCV-related HCC. In this regard, they are more valuable than α-fetoprotein. Moreover, miRNA 9-3p is an independent predictor of metastasis in HCC patients.
The findings of this study warrant additional investigation in prospective trials with larger cohorts and longer follow-up for confirming our results and validating the potential clinical use of these markers in early HCC detection.
We thank all medical staff and technicians of dialysis centers who agreed to participate in this study.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56690] [Article Influence: 7086.3] [Reference Citation Analysis (135)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 5051] [Article Influence: 631.4] [Reference Citation Analysis (2)] |
| 3. | Rashed WM, Kandeil MAM, Mahmoud MO, Ezzat S. Hepatocellular Carcinoma (HCC) in Egypt: A comprehensive overview. J Egypt Natl Canc Inst. 2020;32:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | El Kassas M, Elbaz T, Elsharkawy A, Omar H, Esmat G. HCV in Egypt, prevention, treatment and key barriers to elimination. Expert Rev Anti Infect Ther. 2018;16:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226-1239.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 992] [Article Influence: 90.2] [Reference Citation Analysis (2)] |
| 6. | Cancer Genome Atlas Research Network; Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017;169:1327-1341.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1578] [Cited by in RCA: 1824] [Article Influence: 202.7] [Reference Citation Analysis (1)] |
| 7. | El-Gebaly F, Abou-Saif S, Elkadeem M, Helmy A, Abd-Elsalam S, Yousef M, Elkhouly RA, Amer IF, El-Demerdash T. Study of Serum Soluble Programmed Death Ligand 1 as a Prognostic Factor in Hepatocellular Carcinoma in Egyptian Patients. Curr Cancer Drug Targets. 2019;19:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 891] [Article Influence: 89.1] [Reference Citation Analysis (17)] |
| 9. | Hu Q, Du K, Mao X, Ning S. miR-197 is downregulated in cervical carcinogenesis and suppresses cell proliferation and invasion through targeting forkhead box M1. Oncol Lett. 2018;15:10063-10069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Coolen M, Katz S, Bally-Cuif L. miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci. 2013;7:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 239] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 11. | Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1073] [Cited by in RCA: 1086] [Article Influence: 67.9] [Reference Citation Analysis (16)] |
| 12. | Higashi T, Hayashi H, Ishimoto T, Takeyama H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H, Nitta H, Hashimoto D, Chikamoto A, Beppu T, Baba H. miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br J Cancer. 2015;113:252-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006;1765:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Leroy X, Aubert S, Zini L, Franquet H, Kervoaze G, Villers A, Delehedde M, Copin MC, Lassalle P. Vascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology. 2010;56:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Cai H, Yang X, Gao Y, Xu Z, Yu B, Xu T, Li X, Xu W, Wang X, Hua L. Exosomal MicroRNA-9-3p Secreted from BMSCs Downregulates ESM1 to Suppress the Development of Bladder Cancer. Mol Ther Nucleic Acids. 2019;18:787-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2917] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 17. | Patel M, Shariff MI, Ladep NG, Thillainayagam AV, Thomas HC, Khan SA, Taylor-Robinson SD. Hepatocellular carcinoma: diagnostics and screening. J Eval Clin Pract. 2012;18:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Abudeif A. Epidemiology and Risk Factors of Hepatocellular Carcinoma in Egypt. Sohag Medical Journal. 2019;23:8-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Sun S, Poon RT, Lee NP, Yeung C, Chan KL, Ng IO, Day PJ, Luk JM. Proteomics of hepatocellular carcinoma: serum vimentin as a surrogate marker for small tumors (<or=2 cm). J Proteome Res. 2010;9:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Zen K, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3218] [Cited by in RCA: 3607] [Article Influence: 200.4] [Reference Citation Analysis (0)] |
| 21. | Köberle V, Kronenberger B, Pleli T, Trojan J, Imelmann E, Peveling-Oberhag J, Welker MW, Elhendawy M, Zeuzem S, Piiper A, Waidmann O. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013;49:3442-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | El-Abd NE, Fawzy NA, El-Sheikh SM, Soliman ME. Circulating miRNA-122, miRNA-199a, and miRNA-16 as Biomarkers for Early Detection of Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. Mol Diagn Ther. 2015;19:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Naz Z, Usman S, Saleem K, Ahmed S, Bashir H, Bilal M, Sumrin A. Alpha-fetoprotein: A fabulous biomarker in hepatocellular, gastric and rectal cancer diagnosis. Biomed Res. 2018;29:2478-2483. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Chu CW, Hwang SJ, Luo JC, Lai CR, Tsay SH, Li CP, Wu JC, Chang FY, Lee SD. Clinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol. 2001;32:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Massironi S, Fraquelli M, Paggi S, Sangiovanni A, Conte D, Sciola V, Ciafardini C, Colombo M, Peracchi M. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig Liver Dis. 2009;41:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Metwaly K, Sameea EA, El-Azab G, Assem M, Abbas M, Zakareya T, Abo Raia G. Mean platelet volume and mean platelet volume/platelet count ratio as markers for hepatocellular carcinoma in patients with chronic hepatitis C virus related cirrhosis. J Cancer Res Clin Oncol. 2016;8:33-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Nault JC, Guyot E, Laguillier C, Chevret S, Ganne-Carrie N, N'Kontchou G, Beaugrand M, Seror O, Trinchet JC, Coelho J, Lasalle P, Charnaux N, Delehedde M, Sutton A, Nahon P. Serum proteoglycans as prognostic biomarkers of hepatocellular carcinoma in patients with alcoholic cirrhosis. Cancer Epidemiol Biomarkers Prev. 2013;22:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Ozaki K, Toshikuni N, George J, Minato T, Matsue Y, Arisawa T, Tsutsumi M. Serum endocan as a novel prognostic biomarker in patients with hepatocellular carcinoma. J Cancer. 2014;5:221-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Huang GW, Tao YM, Ding X. Endocan expression correlated with poor survival in human hepatocellular carcinoma. Dig Dis Sci. 2009;54:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Chen LY, Liu X, Wang SL, Qin CY. Over-expression of the Endocan gene in endothelial cells from hepatocellular carcinoma is associated with angiogenesis and tumour invasion. J Int Med Res. 2010;38:498-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Yang L, Mu Y, Cui H, Liang Y, Su X. MiR-9-3p augments apoptosis induced by H2O2 through down regulation of Herpud1 in glioma. PLoS One. 2017;12:e0174839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Tang J, Li Y, Liu K, Zhu Q, Yang WH, Xiong LK, Guo DL. Exosomal miR-9-3p suppresses HBGF-5 expression and is a functional biomarker in hepatocellular carcinoma. Minerva Med. 2018;109:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Sun Z, Han Q, Zhou N, Wang S, Lu S, Bai C, Zhao RC. MicroRNA-9 enhances migration and invasion through KLF17 in hepatocellular carcinoma. Mol Oncol. 2013;7:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Schraivogel D, Weinmann L, Beier D, Tabatabai G, Eichner A, Zhu JY, Anton M, Sixt M, Weller M, Beier CP, Meister G. CAMTA1 is a novel tumour suppressor regulated by miR-9/9* in glioblastoma stem cells. EMBO J. 2011;30:4309-4322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Zhang J, Jia J, Zhao L, Li X, Xie Q, Chen X, Wang J, Lu F. Down-regulation of microRNA-9 Leads to activation of IL-6/Jak/STAT3 pathway through directly targeting IL-6 in HeLa cell. Mol Carcinog. 2016;55:732-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author’s Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology, 001 Founder and President.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goenka MK, Moldogazieva NT S-Editor: Wang LL L-Editor: Filipodia P-Editor: Yu HG