Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1677
Peer-review started: April 3, 2021
First decision: July 6, 2021
Revised: July 15, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: November 27, 2021
Processing time: 234 Days and 23 Hours
Drug-induced liver injury (DILI) is one of the leading causes of liver failure and withdrawal of drugs from the market. A poor understanding of the precipitating event aetiology and mechanisms of disease progression has rendered the prediction and subsequent treatment intractable. Recent literature suggests that some drugs can alter the liver’s repair systems resulting in injury. The pathop
Core Tip: This review demonstrates the critical role of the immune system in the pro
- Citation: Girish C, Sanjay S. Role of immune dysfunction in drug induced liver injury. World J Hepatol 2021; 13(11): 1677-1687
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1677.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1677
The liver plays a central role in the complex process of metabolism and elimination of drugs from the body. The liver is equipped with a wide array of detoxification systems that have evolved over time with exposure to xenobiotics. The primary role of this system is to convert a drug to a more hydrophilic form so that it can be eliminated through bile or urine. Despite the liver’s detox potential, certain drugs can still cause hepatotoxicity that can range from mild asymptomatic liver damage to liver failure[1,2].
A study showed that, out of the 462 pharmaceuticals withdrawn due to adverse drug reactions between 1953 and 2013, hepatotoxicity ranked first with 81 cases (18%). It is estimated that over 1000 drugs currently available on the market that cause liver damage[3] despite these drugs passing the safety measures of clinical trials before entering the market. Some drugs that are hepatotoxic at doses higher than the therapeutic range can also cause drug-induced liver injury (DILI) at doses within the therapeutic range[2,4-6]. This implies that the dose may not be the only contributing factor.
Despite large number of drugs known to cause liver injury, the incidence of DILI is rare. DILI is reported in 1 in every 10000 to 100000 individuals annually. This suggests that drug-host interactions in these susceptible individuals may play an important role in DILI[7-9]. Recent data shows that this interaction can result in an imbalance between damage and repair mechanisms resulting in DILI with immune dysfunction being cited as an important precipitating event in the pathophysiology of DILI[10-12]. This is supported by evidence from experimental studies. Some drugs that are hepatotoxic in humans do not cause liver damage in animal models, but the administration of these drugs along with low doses of lipopolysaccharide (LPS) result in a similar pattern of liver injury as observed in humans. For example, Trovafloxacin (TVX) is a broad-spectrum fluoroquinolone antibiotic, and a study reported that TVX use caused 140 severe hepatic reactions resulting in 14 cases of liver failure. Examination of the case reports suggest that the duration of TVX therapy in patients does not correlate with the toxic response, so TVX hepatotoxicity is classified as idiosyncratic. In rodent models, TVX did not cause liver damage, even at high doses. However, further studies with a normally nontoxic dose of TVX coupled with LPS induced inflammatory stress caused acute liver injury[13,14].
The upcoming sections provide a structured framework presenting DILI in three progressive stages, summarizing the interplay between drugs and the host defence networks that lead to immune system dysfunction.
Direct initiation: The metabolism of drugs by phase 1 enzymes results in the prod
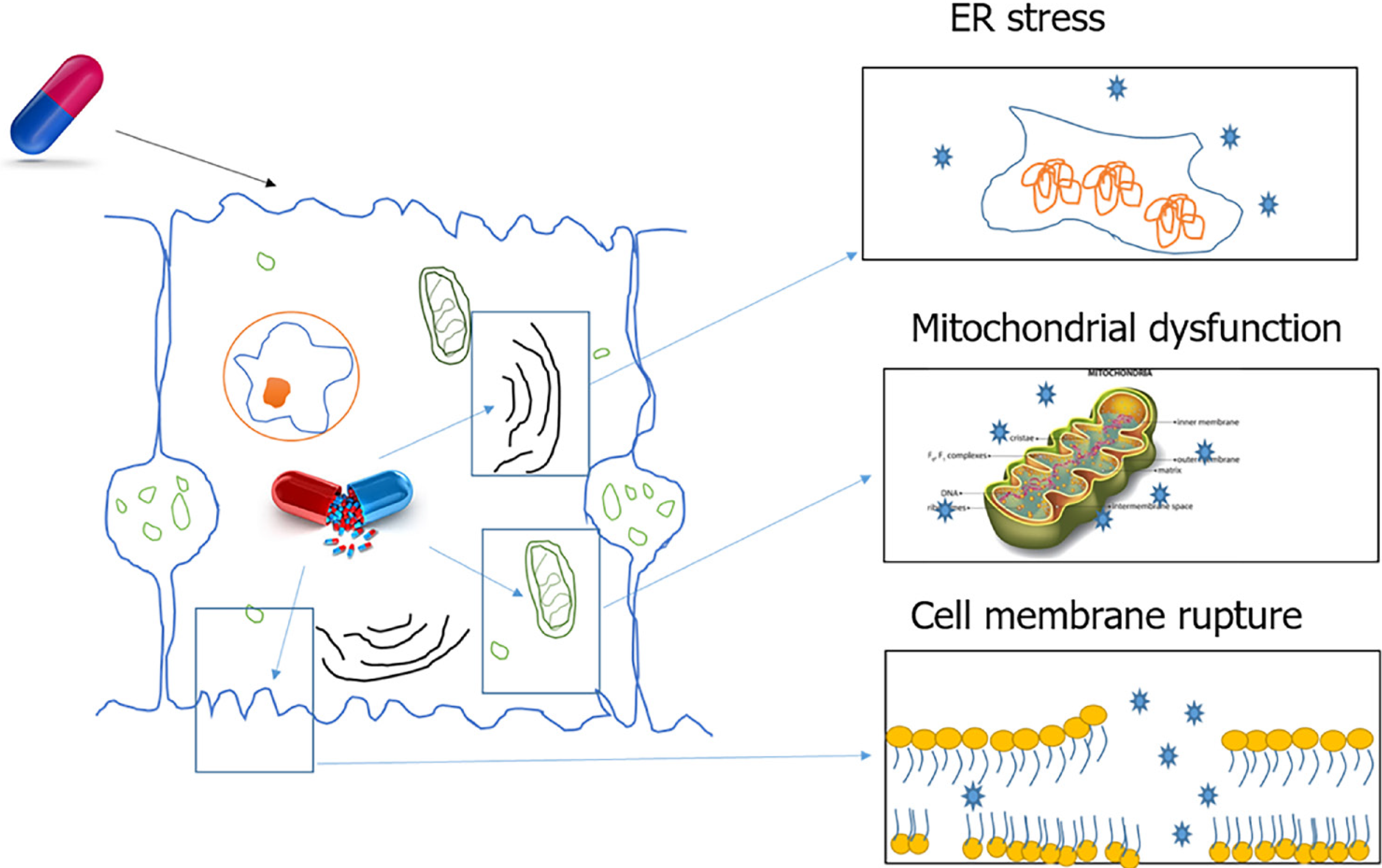
Certain drugs and reactive metabolites can bind to cellular organelles resulting in loss of function and likely cell death. One such case is the damage caused by drugs acting on the endoplasmic reticulum (ER). The ER plays an important role in protein synthesis, folding, assembly, trafficking, and regulation of intracellular calcium homeostasis. Drug related oxidative stress can disturb ER function and lead to the accumulation of unfolded proteins in the ER. This process is termed ER stress. A variety of common drugs cause ER stress, including paracetamol, lopinavir, ritonavir, saquinavir, nelfinavir, atazanavir, and amprenavir[15].
During drug metabolism, free radicals are released that are normally detoxified by cell defence mechanisms. Excessive free radical generation can be caused by enzyme induction or genetic defects in enzyme systems. Free radicals damage the cellular organelles and the lipid bilayer, which results in amplification of damage. Lipid bilayer damage can lead to the release of cytosolic components and alarmins that attract the liver’s resident immune cells. This initial immune response can amplify the sterile damage. Some of the alarmins associated with DILI are high mobility group box 1, S100 proteins, hepatoma-derived growth factor and heat shock proteins[16-20].
Free radicals can also damage the mitochondrial membrane leading to cell dysfunction and death. Mitochondrial dysfunction includes disruption or disturbance to different metabolic pathways and damage to mitochondrial components. In addition, these mitochondrial alterations can have several deleterious consequences, such as oxidative stress, ATP depletion, triglycerides accumulation, and necrotic cell death[21].
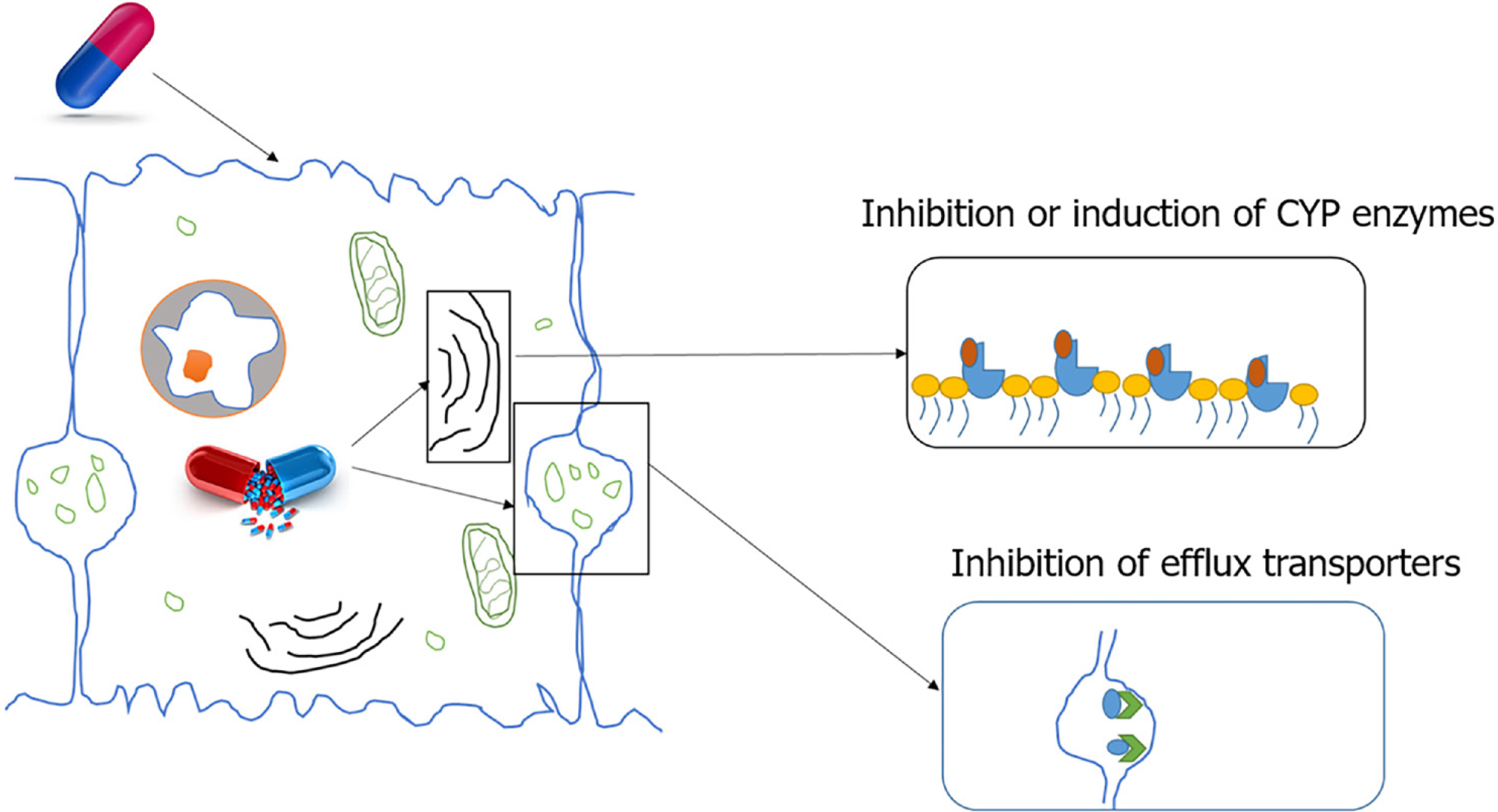
Indirect initiation of DILI: There are two main mechanisms of indirect initiation of DILI. Inhibition of efflux transporters. Bile salt export pump (BSEP) is a member of the ABC transporter superfamily located in the canalicular membrane of hepatocytes. BSEP is responsible for the biliary excretion of bile acids. Drug metabolites inhibit BSEP function, resulting in toxicity. One such metabolite, Troglitazone sulphate, a metabolite of troglitazone, inhibits BSEP mediated taurocholate transport which contributes to troglitazone toxicity. Other potent BSEP inhibitors with the potential to cause DILI include cyclosporin A, bosentan, sulindac, rifamycin, and glibenclamide[2,22].
Enzyme induction: Paracetamol is known to cause liver injury through enzyme induction due to CYP2E1 induction by ethanol. A minor percentage of ethanol is metabolised by CYP2E1. When ethanol and paracetamol are taken simultaneously, ethanol slows the degradation of the CYP enzyme increasing its half-life from 7 h to 37 h. Until ethanol is present in the body more CYP2E1 is induced and a portion is blocked from paracetamol for ethanol metabolism. Once ethanol is completely removed, CYP2E1 enhances paracetamol metabolism resulting in the excess production of toxic intermediary metabolite, NAPQI, causing liver injury[2,23] (Figure 2).
The initiation of DILI does not necessarily result in adverse outcomes. In experimental models, the progression of DILI mainly depends on the persistent and recurrent assault by the toxins that deplete the liver’s resources leading to irreversible damage. This is unlikely at the therapeutic dose of most drugs, as the liver has highly developed protective and regenerative mechanisms. Experimental and clinical data suggest that a myriad of host and drug-related factors contribute to the progressive dysfunction of survival mechanisms that lead to DILI. This is further complicated by the fact that each drug can cause multiple patterns of liver disease, implying an important role for host-drug interactions in the progression of DILI. Immune dysfunction is a major determinant of hepatic cell death and DILI progression[2,4,6,24-26].
This section covers the two main mechanisms of immune reactions induced by drugs and the influence of host factors on them.
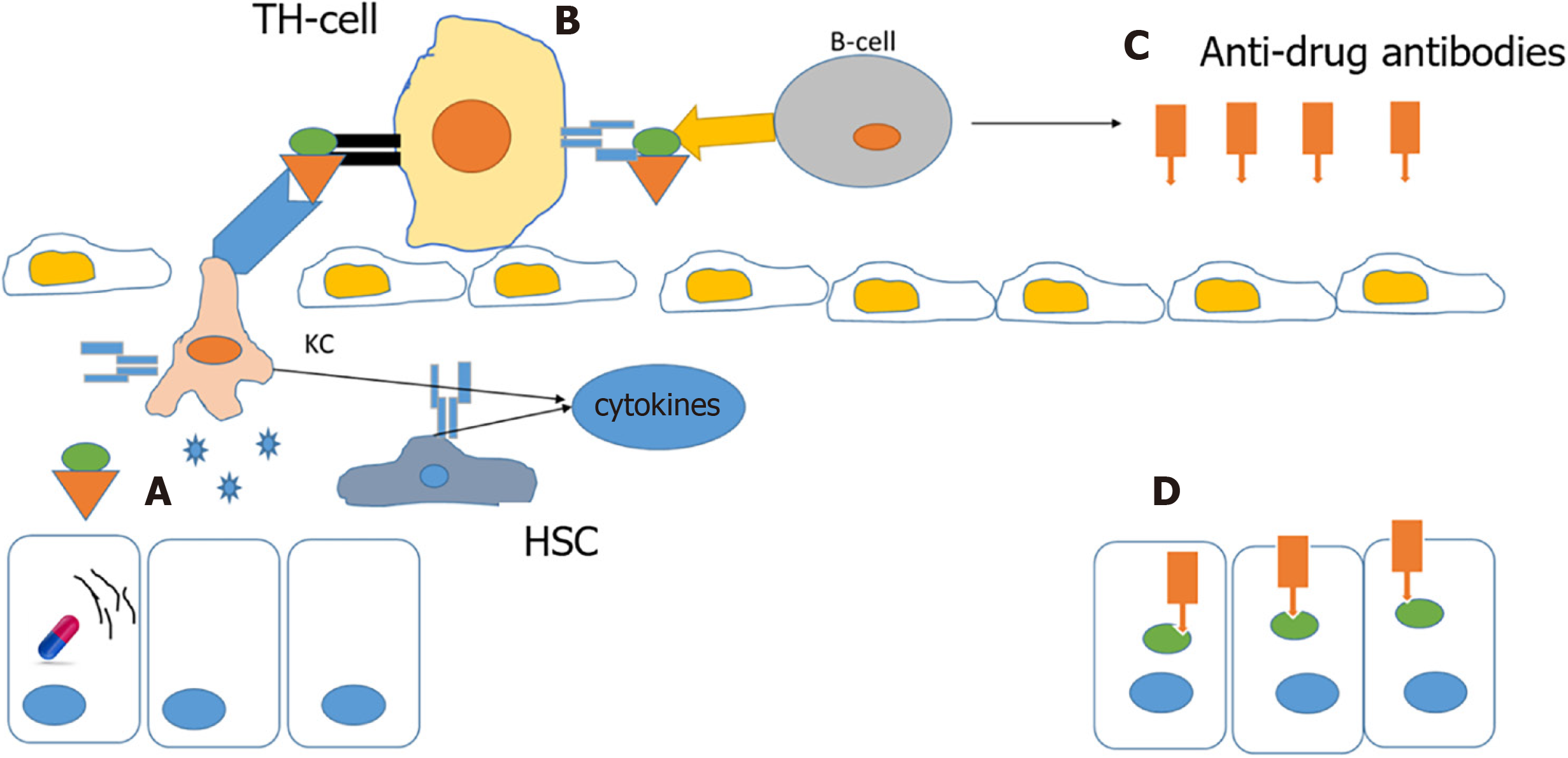
A drug or its metabolites alone cannot activate an immune response due to their small size, but a drug’s reactive metabolites or the drug itself can bind to cellular proteins and form protein-drug adducts that elicit an immune response. In normal individuals, this complex is degraded by cellular detoxification but in susceptible individuals, these adducts act as immunogens and are taken up by antigen-presenting cells and presented by major histocompatibility complexes to helper T cells, and further activation by cytokines stimulates an immune response and anti-drug antibodies are also produced, resulting in extensive death of cells where the drug has accumulated[6,27-29] (Figure 3).
It is hypothesized that ER stress is a contributing factor for this type of reaction. Accumulation of drug/metabolite causes ER stress, which results in misfolding of proteins. These misfolded proteins are more susceptible to drug-protein adduct formations that elicit an immune response[15].
An example of this type of reaction is abacavir, a reverse transcriptase inhibitor employed in the treatment of AIDS, which causes a rare, but serious hypersensitivity reaction that resembles an immune allergic drug reaction. Several genetic variants in the HLA regions are identified as risk factors for DILI, the incidence of hypersensitivity reactions to abacavir is markedly elevated in subjects who carry the B*57:01 variant in the human leukocyte antigen B (HLA-B) gene. Furthermore, carriers of this genotype are at increased risk of flucloxacillin-induced DILI. Studies have shown an association between HLA-B1*15:01 and amoxicillin/clavulanate DILI. The HLA-B*35:02 allele is reported to have a significant association with minocycline DILI[10,25,30,31]. DILI caused by other drugs such as amoxicillin-clavulanate, lumiracoxib, ticlopidine, lapatinib, and ximelagatran is also associated with HLA genotypes, suggesting an important role of the immune system in DILI[25,31].
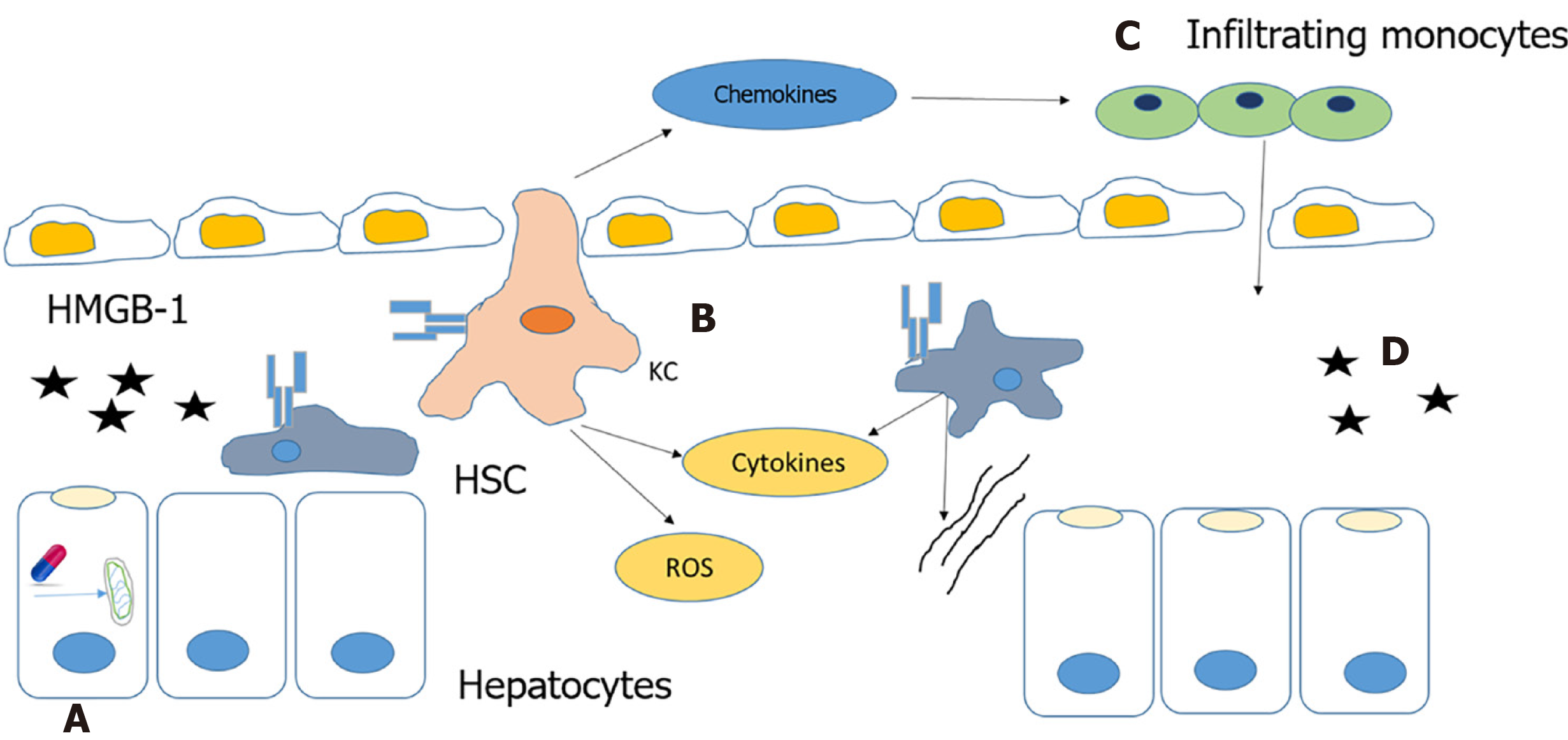
Autoimmune DILI is caused by the release of alarmins from necrotic cells or cells with leaky cell membranes. This results in the activation of innate immune cells. Alarmins are rapidly released following necrotic cell death that are not released by apoptotic cells. The immune system also can be induced to produce and release alarmins to recruit and activate innate immune cells[19,32] (Figure 4).
Mitochondrial dysfunction is reported to play a critical role in the pathogenesis of autoimmune DILI. NSAIDs, such as diclofenac and nimesulide, and other drugs can cause mitochondrial dysfunction that leads to the formation of the mitochondrial permeability transition pore (MPTP). MPTP formation is induced by increased oxidative stress that results in a dissipation of membrane potential, uncoupling of oxidative phosphorylation leading to necrotic cell death and the release of alarmins[18,21,33].
HMGB-1 is an alarmin released by necrotic cells that binds to TLR4 receptors of kupffer cells (KCs) and hepatic stellate cells (HSC), and activates them. Activated KCs produce mediators that directly induce cell death, such as tumor necrosis factor (TNF)-α, Fas ligand and reactive oxygen species, or indirectly cause death through the recruitment of neutrophils by cytokines and chemokines like IL-1β and CXCL2. Production of chemokine, CCL2 (MCP-1) recruits monocytes from the bone marrow to the liver. These infiltrating monocytes produce inflammatory chemokines resulting in the activation of HSCs and the promotion of fibrosis[18,34].
Host sex and sex hormones influence immune response. Studies have shown that female patients with DILI are at higher risk of developing acute liver failure (ALF) with more severe hepatitis and higher levels of pro-inflammatory cytokines. In a halothane-induced experimental DILI model, oestrogen reduced liver injury while progesterone increased liver damage, both hormones influenced immune response. Another important factor affecting DILI is race. A study reported that African-Americans are at a higher risk of developing chronic DILI, while Asian individuals are at increased risk of ALF, liver-related death, or damage that precipitates a need for liver transplantation[4,7,10,24,35].
In normal individuals, DILI resolves completely without any residual liver injury. But there are three major exceptions. They are ALF, cirrhosis and acute-on-chronic liver failure (ACLF). These conditions are relatively rare but severe and may result in death or require a liver transplant.
Even in the absence of pre-existing liver disease, drugs can cause a rapid loss of liver function either directly, as seen in overdoses, or through inflammatory cell mediated mechanisms such as cytokine overproduction. Drug-induced ALF is defined by the signs or symptoms of hepatic failure and encephalopathy during the course of acute DILI. The time to onset of ALF after the start of a medication can vary from a few days to months, but not exceeding six months[4,24,36-38].
In Western countries, paracetamol overdose is the most common reason behind ALF. In India, anti-TB regimens with isoniazid, rifampicin and pyrazinamide are reported as the leading cause of ALF. Other drugs that are reported to cause ALF include phenytoin, carbamazepine, valproate, nitrofurantoin, propylthiouracil, disulfiram, diclofenac, ketoconazole, flutamide, sulphonamides, terbinafine, fluoroquinolone antibiotics and macrolide antibiotics. Drug-induced ALF is a major cause for withdrawal from the market or restricted use of a medication (troglitazone, bromfenac, nefazodone, halothane, telithromycin). ALF occurs in cases with acute hepatocellular injury with characteristics similar to acute viral hepatitis[10,23,39-41].
Paracetamol is responsible for more than 50% of drug related ALF and about 20% of liver transplant cases in the United States[42]. In case of paracetamol overdose, the drug metabolite NAPQ1 depletes GSH and causes organelle damage, the most significant resulting in mitochondrial stress. Thereby the NAPQ1 accumulation triggers necrosis[43,44]. Hepatocyte necrosis passively releases various DAMPs such as HMGB-1, HSP and DNA fragments. These DAMPs activate the resident immune cells such as Kupffer cells and natural killer (NK) cells. Cytokines and chemokines such as TNF-α, IL-1β and CCL2 produced by the activated immune cells and the DAMPs enter systemic circulation and cause infiltration of neutrophils and monocytes into the liver. In conditions of sterile injury, the immune cells function to clear the dead cells by producing chemokines and free radicals to digest it. Once the cellular debris is cleared the immune cells undergo phenotypic change and support in liver regeneration. However, in case of paracetamol overdose, the overwhelming amount of cellular debris and DAMPs causes excess immune activation, whose products such as superoxide, nitric oxide and peroxynitrite result in further amplification of liver injury leading to massive necrosis and organ failure[45-48].
Cirrhosis is characterized by islands or nodules of regenerative parenchymal cells surrounded by excessive deposition of fibrous tissue and portal hypertension. Cirrhosis is rarely the initial manifestation of DILI and is most often a cumulative response to long-term exposure to hepatotoxic drugs. It usually occurs at least six months after starting the drug treatment. The time to onset of cirrhosis due to medications is typically long; at least 6 month after starting the medication but usually several years afterwards. The drugs that are most commonly cause cirrhosis are vitamin A, amiodarone, statins, tamoxifen, valproic acid, fibrates, and methotrexate[4,25,26,49-51]. Drugs such as dantrolene, phenytoin, trazadone and nitrofurantoin are also associated with chronic hepatitis with autoimmune features that may lead to cirrhosis[52-54].
Amiodarone is a benzofuran derivative mainly used in the treatment of arrhythmia. The safety of long-term use of amiodarone is well established however there are several reports of reversible and irreversible liver injury from its long-term use. Even though rare amiodarone can cause asymptomatic continuous liver injury that has histological features similar to alcoholic hepatitis such as nodular formation, fibrosis, steatosis and neutrophil infiltration[55-61]. Due to its lipophilic nature and long half-life, amiodarone accumulates in the hepatocytes affecting cellular organelles such as ER and mitochondria causing misfolding of proteins. Amiodarone affects the cholesterol metabolism by blocking enzymes emopamil binding protein and dehydrocholesterol reductase 24. As cholesterol plays an important role in maint
Acute-on-chronic liver failure (ACLF) as the name suggests is characterized by ALF due to a different cause in patients with chronic liver disease (compensated) resulting in short term mortality. It consists of two components: a chronic underlying liver disease and an acute trigger[70,71]. Devarbhavi et al[72] reported that drugs contributed to 10.5% cases in the Asia-Pacific region. Among these drugs, the most common culprits were complementary and alternative medications (71.7%), followed by anti-TB drug combination therapies (27.3%). Anti-TB drug isoniazid is also observed to cause severe hepatitis that leads to liver failure[72-74].
Studies suggest that excessive focal liver and systemic inflammatory response play a significant role in the development of ACLF. Reports have shown high levels of cytokines in patients with ACLF. This may be due to the activation of monocytes and macrophages in response to DAMPs, microbial toxins or drug adducts[19,75,76].
Paracetamol induced liver failure in patients with alcoholic hepatitis is a typical example of drug induced ACLF. Alcoholic hepatitis is reported in approximately 25% of the cases of ACLF. The trigger due to paracetamol toxicity can occur in two ways- the first is due to direct toxicity by paracetamol and the second due to immune response that is secondary to the hepatocellular damage due to the direct toxicity. The activation of innate immune response due to the paracetamol acute toxicity results in upregulation of cytokine and chemokine production that initiates severe systemic inflammation, liver damage and mortality[70,75,77,78].
The dysregulation in innate immune response plays important roles in disease progression as well as disease severity. In the liver, systemic inflammation plays a significant role in the development and course of chronic alcoholic hepatitis. Similar to the acute toxicity, immune activation in alcoholic liver disease results in activation of resident Kupffer cells and dendritic cells as well as the infiltrating immune cells- monocytes and neutrophils lead to progression towards fibrosis and cirrhosis. This disrupts the liver architecture and function setting stage for liver failure, that can be actuated by an acute trigger[75,78,79].
Drugs and their metabolic products can cause liver damage through multiple mechanisms. Under normal conditions, the liver is well equipped to neutralize potential drug-related damage, but in susceptible individuals, this same drug use can result in severe liver injury. This is further amplified by a dysfunctional immune responses that is influenced by host factors like genetics, age and sex. The severe adverse outcomes of DILI are ALF, cirrhosis and acute-on-chronic liver injury. All these injuries are associated with concurrent immune dysfunction. A better understanding of immune mediators may offer new targets for the management of DILI. Individualized therapy that focuses on early detection of risk factors, triggers and stage of the liver injury may play a significant role in effectively attenuating this disorder.
The authors wish to thank Anthony J DeSana, Spinal Cord and Brain Injury Research Center, Department of Physiology, University of Kentucky, United States, for English editing of this manuscript.
| 1. | Kirchain WR, Allen RE. Drug-Induced Liver Disease. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A pathophysiologic approach, 10e. McGraw-Hill, 2016. |
| 2. | Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 825] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 3. | Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. 2016;14:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 4. | Kaplowitz N. Avoiding idiosyncratic DILI: two is better than one. Hepatology. 2013;58:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Kaplowitz N. Drug-induced liver injury. Clin Infect Dis. 2004;38 Suppl 2:S44-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Waddington JC, Meng X, Naisbitt DJ, Park BK. Immune drug-induced liver disease and drugs. Curr Opin Toxicol. 2018;10:46-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 7. | Bell LN, Chalasani N. Epidemiology of idiosyncratic drug-induced liver injury. Semin Liver Dis. 2009;29:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Björnsson ES. Incidence and outcomes of DILI in Western patients. Clin Liver Dis (Hoboken). 2014;4:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 10. | Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: Interactions between drug properties and host factors. J Hepatol. 2015;63:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 283] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 11. | Williams CD, Jaeschke H. Role of innate and adaptive immunity during drug-induced liver injury. Toxicol Res. 2012;1:161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Shaw PJ, Ganey PE, Roth RA. Idiosyncratic drug-induced liver injury and the role of inflammatory stress with an emphasis on an animal model of trovafloxacin hepatotoxicity. Toxicol Sci. 2010;118:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Oda S, Yokoi T. [Establishment of animal models of drug-induced liver injury and analysis of possible mechanisms]. Yakugaku Zasshi. 2015;135:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol Sci. 2012;33:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Wang X, Cueto R, Effi C, Zhang Y, Tan H, Qin X, Ji Y, Yang X, Wang H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019;26:101284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 17. | Stanger BZ. Cellular homeostasis and repair in the mammalian liver. Annu Rev Physiol. 2015;77:179-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Yang R, Tonnesseen TI. DAMPs and sterile inflammation in drug hepatotoxicity. Hepatol Int. 2019;13:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1972] [Cited by in RCA: 2090] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 20. | Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2251] [Cited by in RCA: 2923] [Article Influence: 224.8] [Reference Citation Analysis (0)] |
| 21. | Smith RA, Hartley RC, Cochemé HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci. 2012;33:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 22. | Schuetz JD, Swaan PW, Tweedie DJ. The role of transporters in toxicity and disease. Drug Metab Dispos. 2014;42:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1295] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 24. | Tujios SR, Lee WM. Acute liver failure induced by idiosyncratic reaction to drugs: Challenges in diagnosis and therapy. Liver Int. 2018;38:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Russmann S, Kullak-Ublick GA, Grattagliano I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr Med Chem. 2009;16:3041-3053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 26. | Kuzu UB, Öztaş E, Turhan N, Saygili F, Suna N, Yildiz H, Kaplan M, Akpinar MY, Akdoğan M, Kaçar S, Kiliç ZM, Köksal AŞ, Ödemiş B, Kayaçetin E. Clinical and histological features of idiosyncratic liver injury: Dilemma in diagnosis of autoimmune hepatitis. Hepatol Res. 2016;46:277-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Liu ZX, Kaplowitz N. Immune Mechanisms in Drug-Induced Hepatotoxicity. In: Gershwin ME, Vierling JM, Manns MP, eds. Liver Immunology. Totowa, NJ: Humana Press; 2007: 363–374. [DOI] [Full Text] |
| 28. | Uetrecht J. Mechanistic Studies of Idiosyncratic DILI: Clinical Implications. Front Pharmacol. 2019;10:837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146:914-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 30. | Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A; PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1336] [Cited by in RCA: 1264] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 31. | Russmann S, Jetter A, Kullak-Ublick GA. Pharmacogenetics of drug-induced liver injury. Hepatology. 2010;52:748-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Sebode M, Schulz L, Lohse AW. "Autoimmune(-Like)" Drug and Herb Induced Liver Injury: New Insights into Molecular Pathogenesis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, Liu ZX, Kaplowitz N. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci. 2013;34:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1978] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 35. | Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, Aithal GP. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 36. | Larrey D, Pageaux GP. Drug-induced acute liver failure. Eur J Gastroenterol Hepatol. 2005;17:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17:575-586, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Argo CK, Caldwell SH. Editorial: Severe Acute Liver Injury: Cause Connects to Outcome. Am J Gastroenterol. 2017;112:1397-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, Stravitz RT, Larson AM, Liou I, Fix O, Schilsky ML, McCashland T, Hay JE, Murray N, Shaikh OS, Ganger D, Zaman A, Han SB, Chung RT, Brown RS, Munoz S, Reddy KR, Rossaro L, Satyanarayana R, Hanje AJ, Olson J, Subramanian RM, Karvellas C, Hameed B, Sherker AH, Lee WM, Reuben A. The Natural History of Severe Acute Liver Injury. Am J Gastroenterol. 2017;112:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 273] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 41. | Yang Q, Shi Y, He J, Chen Z. The evolving story of macrophages in acute liver failure. Immunol Lett. 2012;147:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J Clin Transl Hepatol. 2016;4:131-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 43. | Tittarelli R, Pellegrini M, Scarpellini MG, Marinelli E, Bruti V, di Luca NM, Busardò FP, Zaami S. Hepatotoxicity of paracetamol and related fatalities. Eur Rev Med Pharmacol Sci. 2017;21:95-101. [PubMed] |
| 44. | Rotundo L, Pyrsopoulos N. Liver injury induced by paracetamol and challenges associated with intentional and unintentional use. World J Hepatol. 2020;12:125-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (8)] |
| 45. | Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr. 2014;3:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 112] [Reference Citation Analysis (0)] |
| 46. | Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, Heymann F, Kalthoff S, Lefebvre E, Eulberg D, Luedde T, Marx G, Strassburg CP, Roskams T, Trautwein C, Tacke F. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology. 2016;64:1667-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 47. | Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 476] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 48. | Guo H, Chen S, Xie M, Zhou C, Zheng M. The complex roles of neutrophils in APAP-induced liver injury. Cell Prolif. 2021;54:e13040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Dakhoul L, Ghabril M, Chalasani N. Drug-induced chronic liver injury. J Hepatol. 2018;69:248-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Ortega-Alonso A, Stephens C, Lucena MI, Andrade RJ. Case Characterization, Clinical Features and Risk Factors in Drug-Induced Liver Injury. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Vaja R, Ghuman N. Drugs and the liver. Anaesth Intensive Care Med. 2018;19:30-34. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Chang CC, Petrelli M, Tomashefski JF Jr, McCullough AJ. Severe intrahepatic cholestasis caused by amiodarone toxicity after withdrawal of the drug: a case report and review of the literature. Arch Pathol Lab Med. 1999;123:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Fisher K, Vuppalanchi R, Saxena R. Drug-Induced Liver Injury. Arch Pathol Lab Med. 2015;139:876-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 54. | Fernandes NF, Martin RR, Schenker S. Trazodone-induced hepatotoxicity: a case report with comments on drug-induced hepatotoxicity. Am J Gastroenterol. 2000;95:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Oikawa H, Maesawa C, Sato R, Oikawa K, Yamada H, Oriso S, Ono S, Yashima-Abo A, Kotani K, Suzuki K, Masuda T. Liver cirrhosis induced by long-term administration of a daily low dose of amiodarone: a case report. World J Gastroenterol. 2005;11:5394-5397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Raja K, Thung SN, Fiel MI, Chang C. Drug-induced steatohepatitis leading to cirrhosis: long-term toxicity of amiodarone use. Semin Liver Dis. 2009;29:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Buggey J, Kappus M, Lagoo AS, Brady CW. Amiodarone-Induced Liver Injury and Cirrhosis. ACG Case Rep J. 2015;2:116-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Dees A. A Late Presentation of Amiodarone -Induced Hepatotoxicity. MOJ Clin Med Case Rep. 2016;4. |
| 59. | Tsuda T, Tada H, Tanaka Y, Nishida N, Yoshida T, Sawada T, Sakata K, Hayashi K, Kawashiri MA, Oyama T, Sasaki M, Kurose N, Yamagishi M. Amiodarone-induced reversible and irreversible hepatotoxicity: two case reports. J Med Case Rep. 2018;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Kocak MZ. Oral Amiodarone-induced liver Injury, especially Gamma Glutamyl Transferase Elevation: A Case Report. EJMO. 2018;2:117-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Bratton H, Alomari M, Al Momani LA, Aasen T, Young M. Prolonged Jaundice Secondary to Amiodarone Use: A Case Report and Literature Review. Cureus. 2019;11:e3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Erez N, Hubel E, Avraham R, Cohen R, Fishman S, Bantel H, Manns M, Tirosh B, Zvibel I, Shibolet O. Hepatic Amiodarone Lipotoxicity Is Ameliorated by Genetic and Pharmacological Inhibition of Endoplasmatic Reticulum Stress. Toxicol Sci. 2017;159:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Rutkowski DT. Liver function and dysfunction - a unique window into the physiological reach of ER stress and the unfolded protein response. FEBS J. 2019;286:356-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Mansouri A, Gattolliat CH, Asselah T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology. 2018;155:629-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 605] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 65. | Maiers JL, Malhi H. Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis. Semin Liver Dis. 2019;39:235-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 66. | Simonen P, Li S, Chua NK, Lampi AM, Piironen V, Lommi J, Sinisalo J, Brown AJ, Ikonen E, Gylling H. Amiodarone disrupts cholesterol biosynthesis pathway and causes accumulation of circulating desmosterol by inhibiting 24-dehydrocholesterol reductase. J Intern Med. 2020;288:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Allen LB, Genaro-Mattos TC, Anderson A, Porter NA, Mirnics K, Korade Z. Amiodarone Alters Cholesterol Biosynthesis through Tissue-Dependent Inhibition of Emopamil Binding Protein and Dehydrocholesterol Reductase 24. ACS Chem Neurosci. 2020;11:1413-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Njoku DB. Drug-induced hepatotoxicity: metabolic, genetic and immunological basis. Int J Mol Sci. 2014;15:6990-7003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | European Association for the Study of the Liver. Clinical Practice Guideline Panel: Chair:; Panel members; EASL Governing Board representative:. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 768] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 70. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 456] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 71. | Blasco-Algora S, Masegosa-Ataz J, Gutiérrez-García ML, Alonso-López S, Fernández-Rodríguez CM. Acute-on-chronic liver failure: Pathogenesis, prognostic factors and management. World J Gastroenterol. 2015;21:12125-12140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 72. | Devarbhavi H, Choudhury AK, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Chawla YK, Dhiman RK, Duseja A, Taneja S, Ning Q, Jia JD, Duan Z, Yu C, Eapen CE, Goel A, Tan SS, Hamid SS, Butt AS, Jafri W, Kim DJ, Hu J, Sood A, Midha V, Shukla A, Ghazinian H, Sahu MK, Treeprasertsuk S, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Kalal C, Abbas Z, Sollano JD, Prasad VGM, Payawal DA, Dokmeci AK, Rao PN, Shrestha A, Lau GK, Yuen MF, Saraswat VA, Shiha G, Yokosuka O, Kedarisetty CK, Jain P, Bhatia P, Sarin SK; APASL ACLF working party. Drug-Induced Acute-on-Chronic Liver Failure in Asian Patients. Am J Gastroenterol. 2019;114:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 73. | Wang P, Pradhan K, Zhong XB, Ma X. Isoniazid metabolism and hepatotoxicity. Acta Pharm Sin B. 2016;6:384-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 74. | Metushi I, Uetrecht J, Phillips E. Mechanism of isoniazid-induced hepatotoxicity: then and now. Br J Clin Pharmacol. 2016;81:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 75. | Khanam A, Kottilil S. Abnormal Innate Immunity in Acute-on-Chronic Liver Failure: Immunotargets for Therapeutics. Front Immunol. 2020;11:2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 76. | Chen P, Wang YY, Chen C, Guan J, Zhu HH, Chen Z. The immunological roles in acute-on-chronic liver failure: An update. Hepatobiliary Pancreat Dis Int. 2019;18:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 77. | Kamath PS. Acute on chronic liver failure. Clin Liver Dis (Hoboken). 2017;9:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Gustot T, Jalan R. Acute-on-chronic liver failure in patients with alcohol-related liver disease. J Hepatol. 2019;70:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 79. | Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Navarro-Alvarez N S-Editor: Ma YJ L-Editor: A P-Editor: Yu HG