Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1316
Peer-review started: February 26, 2021
First decision: May 3, 2021
Revised: May 10, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: October 27, 2021
Processing time: 237 Days and 18.6 Hours
Differential diagnosis of pediatric vascular liver tumors can be challenging due to inconsistent nomenclature, histologic overlap and the rarity of some entities. Here we give an up-to-date overview of the most important entities. We discuss the clinic, histology and pathophysiology of hepatic congenital and infantile heman
Core Tip: Overview of the most important pediatric hepatic vascular tumors from the point of view of the pathologist, including hepatic hemangiomas, hepatic epithelioid hemangioendothelioma and hepatic angiosarcoma.
- Citation: Cordier F, Hoorens A, Van Dorpe J, Creytens D. Pediatric vascular tumors of the liver: Review from the pathologist’s point of view. World J Hepatol 2021; 13(10): 1316-1327
- URL: https://www.wjgnet.com/1948-5182/full/v13/i10/1316.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i10.1316
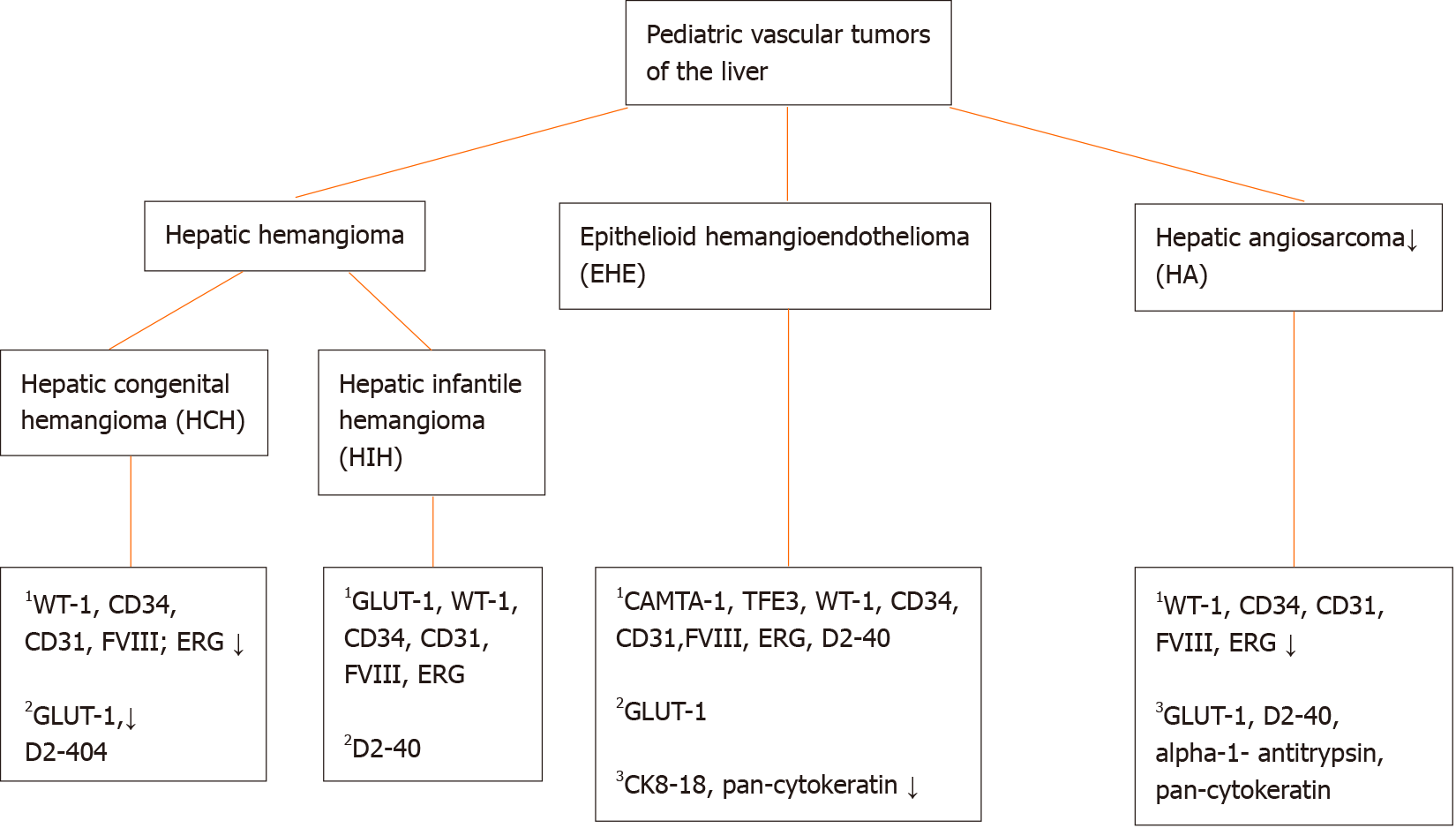
Through the years the classification of vascular anomalies in the liver has evolved due to better biological understanding with substantial contribution of molecular genetics and immunohistochemical correlates. However, terminology can be difficult due to the existence of multiple (general and organ specific) classifications and inconsistent nomenclature through the years. In 1997, vascular tumors were differentiated from vascular malformations for the first time[1]. In brief, the main difference between the above entities is that vascular tumors are considered as cellular vascular neoplastic proliferations and vascular malformations as errors in the morphogenesis lined by mature endothelium[2,3]. In 2014, The International Society for the Study of Vascular Anomalies (ISSVA) divided vascular tumors further in benign, locally aggressive or borderline and malignant entities[4]. Here, we give an overview of the most important pediatric hepatic vascular tumors, including hepatic hemangiomas, hepatic epithelioid hemangioendothelioma and hepatic angiosarcoma.
Hepatic hemangiomas belong to the group of benign vascular tumors[4]. The term “hemangioma” has been used through the years for a variety of vascular malformations of the liver. In 2018, the ISSVA reserved this term for vascular lesions that match the definition of congenital or infantile hemangiomas[5]. These benign endothelial neoplasms can occur in the liver and belong to the histologic group of “hepatic hemangioendothelioma, type 1” (Figure 1). However, the term ‘hemangioendothelioma’ has to be used with caution, due to the terminology overlap with epithelioid hemangioendothelioma (which is considered as a malignant vascular entity) and should be avoided in absence of histologic evaluation[5,6]. Further, histologic confirmation of hemangiomas is often not required, since the diagnosis can easily be made with physical examination, imaging and review of patient’s history. Still, a biopsy can be performed when the history or clinical/radiological features are atypical[5]. Heman
Hepatic congenital hemangiomas (HCH) are benign high-flow vascular tumors that proliferate in utero and are fully grown at birth with no postnatal increase in size. They are less common than hepatic infantile hemangioma (HIH) and present mostly as a solitary lesion[5,7,8]. Diagnosis can be made on prenatal imaging showing a large mass with extensive central infarction, hemorrhage, calcifications and sometimes large abnormal vessels, suggestive for arteriovenous malformation[5,9]. They can be asymp
The most important clinical differential diagnoses of a liver mass in infants include hepatic infantile hemangioma (HIH), epithelioid hemangioendothelioma, hepatoblastoma, germ cell tumors, (metastatic) neuroblastoma, mesenchymal hamartoma, cysts and abscesses[10,11].
There are 3 clinical subtypes depending on the pattern of evolution: rapidly involuting congenital hemangioma (RICH), partially involuting congenital hemangioma (PICH) and noninvoluting congenital hemangioma (NICH)[4,5,10]. These subtypes share common histopathologic features and have to be seen as part of a single entity with differences in their clinical behavior[12,13].
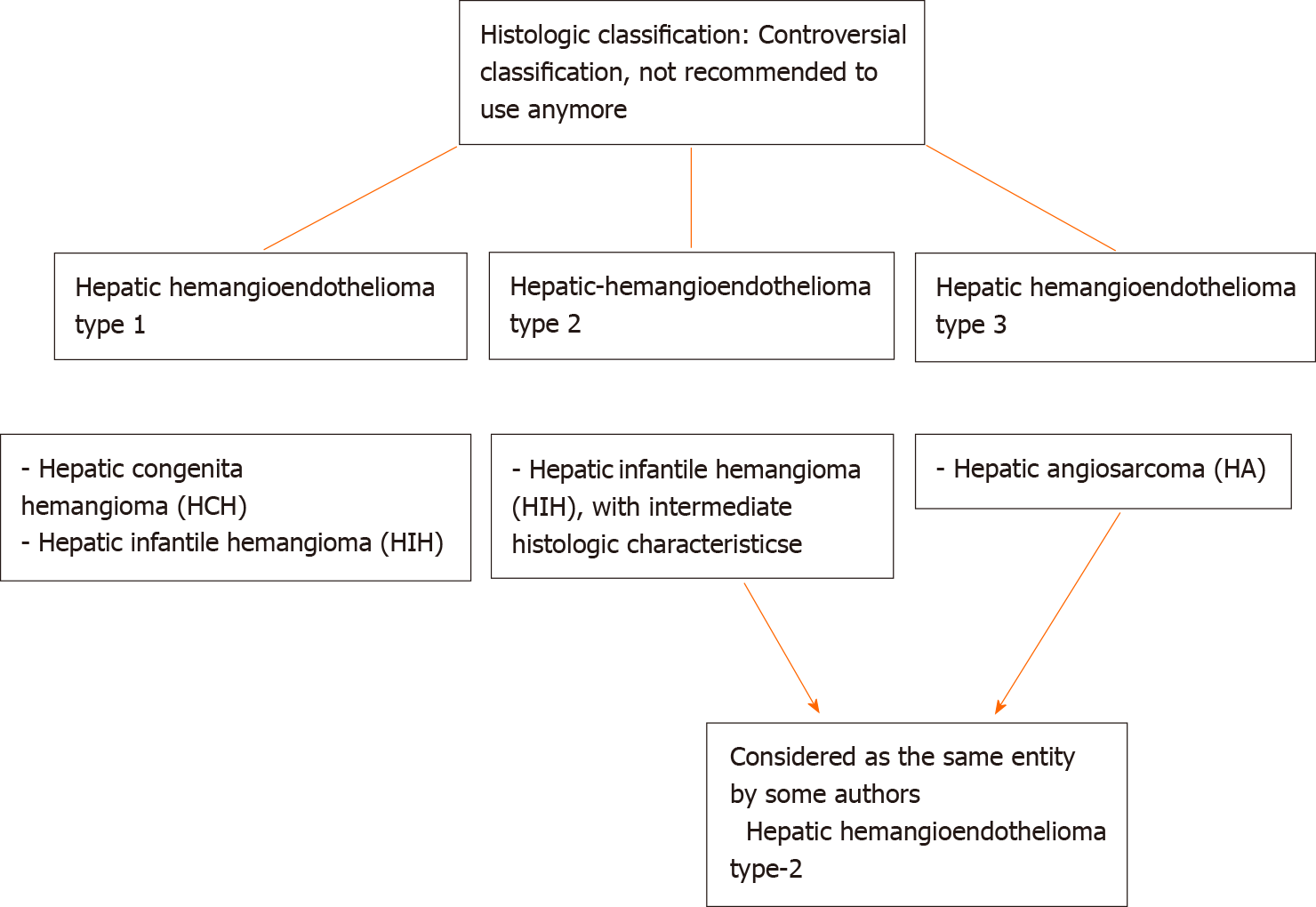
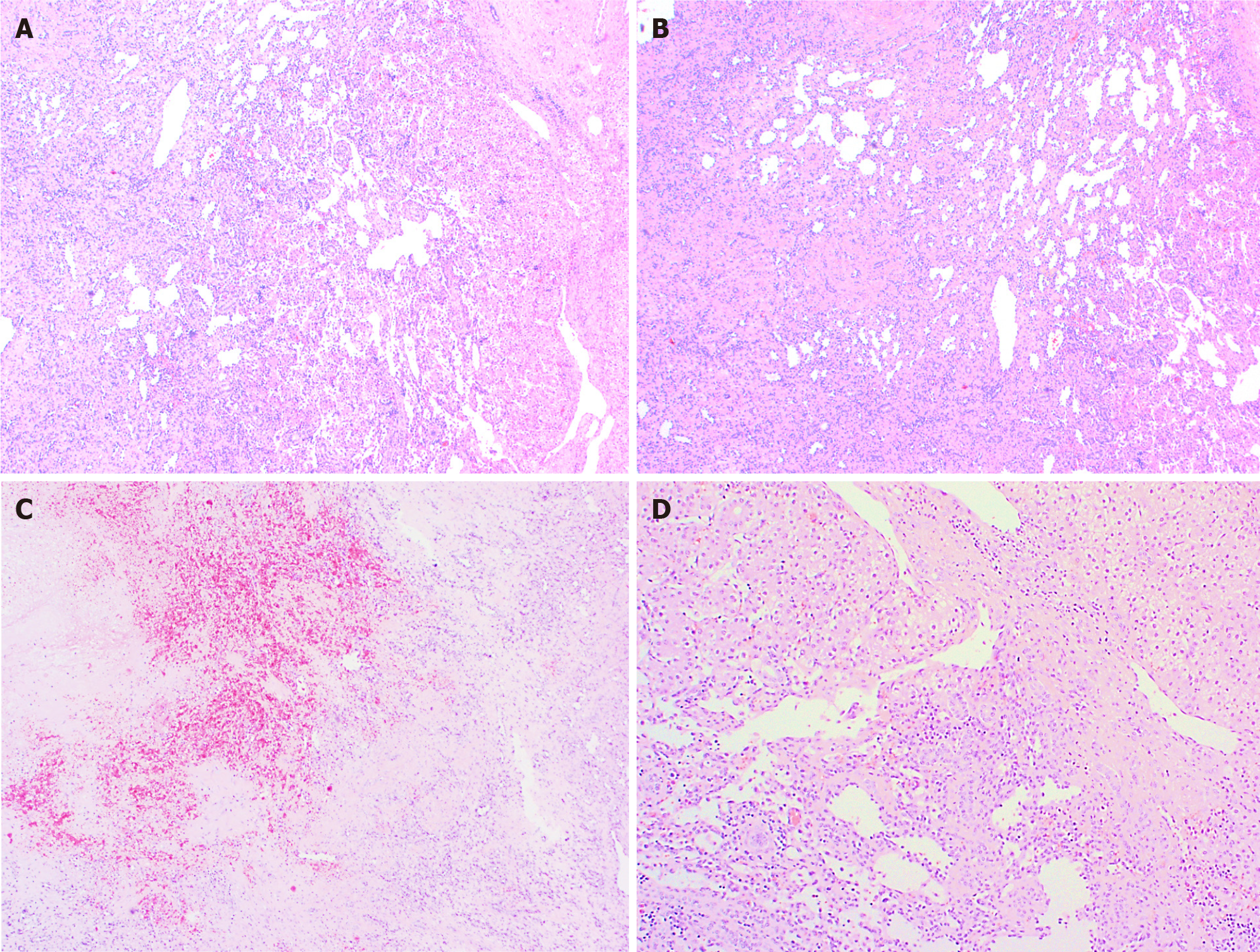
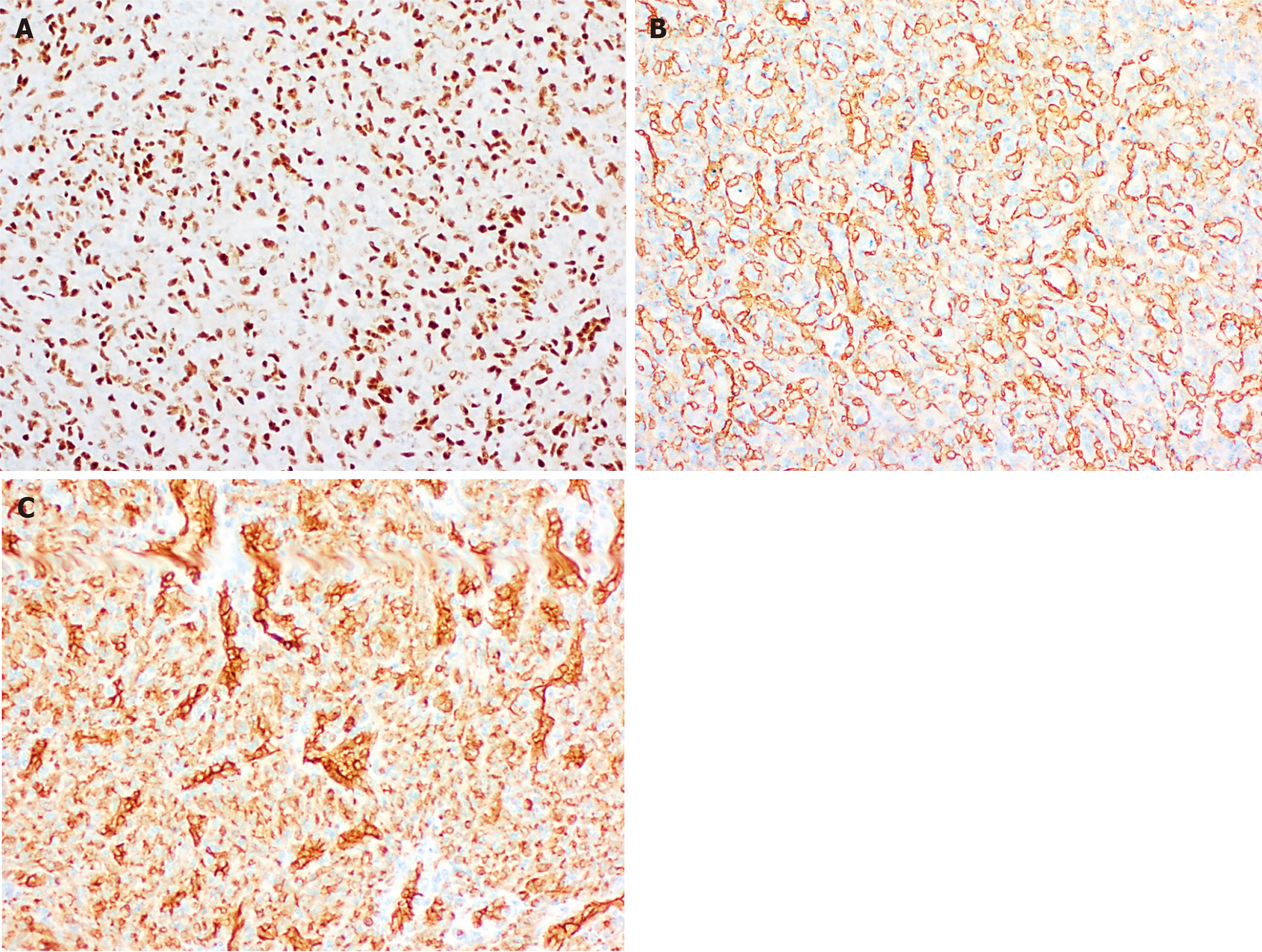
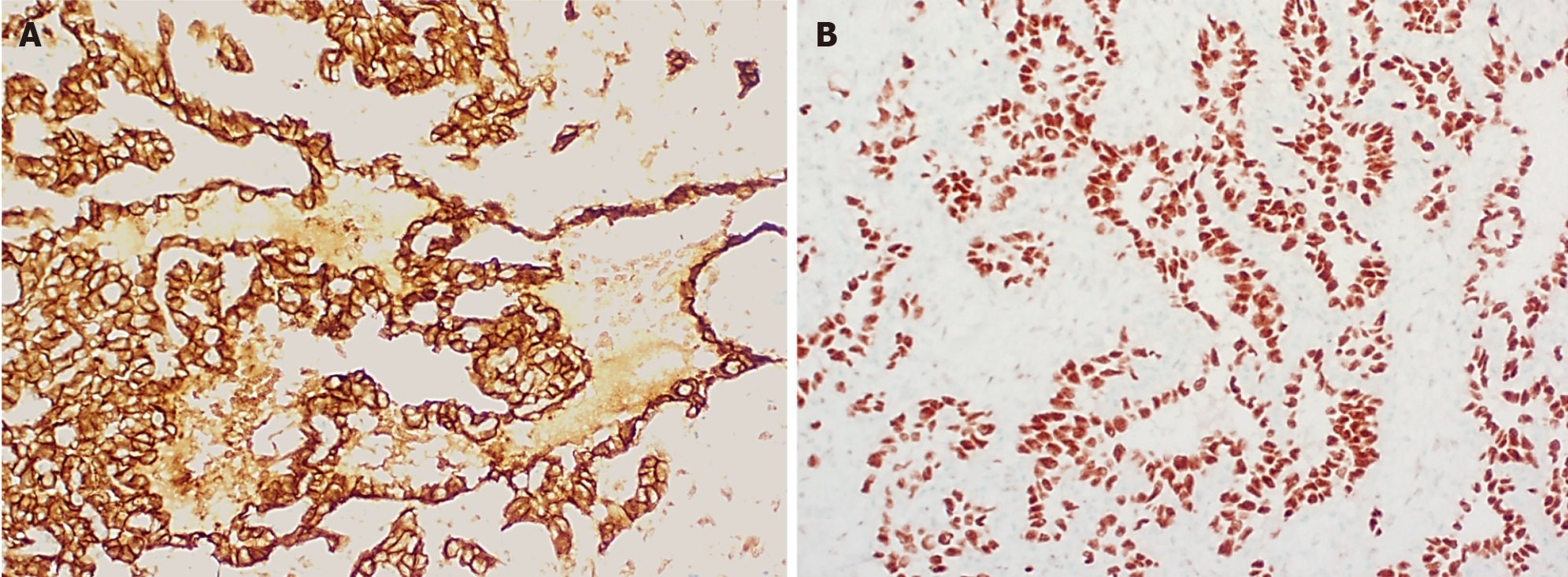
Histologically (Figure 2), HCHs are usually well-demarcated vascular lesions which can show entrapment of hepatocytes and bile ducts in interface areas[9]. RICH is composed of lobules of variable sized, mostly small thin-walled vessels lined by plump endothelium without cytonuclear atypia[7,10]. There may be evidence of thrombosis and the central part (i.e., the first area of involution) may contain necrotic and hemorrhagic areas, fibrosis and focal dystrophic calcifications. Extramedullary hematopoiesis can also be observed. At the periphery of the lesion abundant larger vessels occur, sometimes associated with aneurysmal changes[7]. In contrast to RICH, NICH shows lobules of small vessels with interlobular fibrosis but without signs of involution. Arteriovenous microfistulae with large irregular vessels in the center can occur[10]. PICH shows histologic overlap between RICH and NICH and cannot be distinguished histologically[12,13]. Endothelial cells show immunoreactivity for Wilms’ Tumor 1 (WT-1), CD34, CD31, factor VIII and Erythroblast transformation-specific [ETS]-related gene (ERG)[13-15]. Triana et al[16] showed there was no expression of podoplanin (D2-40) in HCH. However, El Zein et al showed focal positivity for podoplanin in congenital hemangiomas of the skin, mainly in abnormal extralobular lymphatic vessels or in patients with concomitant thrombocytopenia (with decrease of intensity when platelet count normalized)[13]. The endothelial cells of HCH do not stain for glucose transporter-1 (GLUT-1), which is an important hallmark in the differentiation of HCH with HIH (Figure 3)[5,10].
Genetic studies revealed that almost all HCHs have mutually exclusive, missense mutations that alter glutamine at amino acid 209 (Gln209) in the alleles which code for guanine nucleotide-binding protein G(q)alpha (GNAQ) and guanine nucleotide-binding protein subunit alpha-11 (GNA11), regardless of subtype. This implies that also other genetic, epigenetic and/or environmental factors may influence the behaviour of these lesions[10,17]. A subset shows missense mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) (c.3140A > T; p.His1047Leu)[16].
HIH is the most common benign hepatic tumor in infancy, with female predominance[7]. It proliferates rapid after birth, reaching a maximal size at 6 to 12 mo, and then it gradually involutes until 3 to 9 years[5,6]. Most hemangiomas are asymptomatic and remain undetected or are incidental findings on postnatal imaging. Still, a subset can be symptomatic due to their size, location or hemodynamic effects[8]. The high flow within the tumor or presence of shunts can cause cardiac failure. Also, thrombocytopenia and anemia can be observed when intralesional thrombosis occurs[5,8,18,19]. Due to high expression of type 3 iodothyronine deiodinase in these vascular lesions, which inactivates thyroid hormone, acquired consumptive hypothyroidism occurs. All of these complications are detected after birth during the proliferation phase and can be missed initially on newborn screening[5]. Further, HIH can occur in association with Beckwith-Wiedemann syndrome[6].
The clinical differential diagnosis of HIH is broad and includes arteriovenous malformations, arterioportal fistula, mesenchymal hamartoma, hepatoblastoma, angiosarcoma and (metastatic) neuroblastoma[8].
HIH presents clinically/macroscopically as white-tan nodules with occasionally degenerative changes in the centre[9]. They can be divided into 3 categories based on degree of unaffected liver parenchyma: focal, multifocal or diffuse disease. Focal HIH shows overlap with RICH, as it does not express GLUT-1 and can be found on prenatal imaging[8,18,19]. Therefore, focal HIH is not considered as a true HIH[8]. Multifocal HIH presents as areas of hemangioma with intervening segments of normal hepatic parenchyma, whereas a diffuse pattern is defined as innumerable tumors with nearly complete hepatic parenchymal replacement[5]. Diffuse HIH shows a higher risk of complications, e.g., abdominal compartment syndrome, heart failure, profound hypothyroidism, and even mortality[5,8]. Associated cutaneous infantile hemangioma is often present in patients with multifocal or diffuse HIH and increases with prema
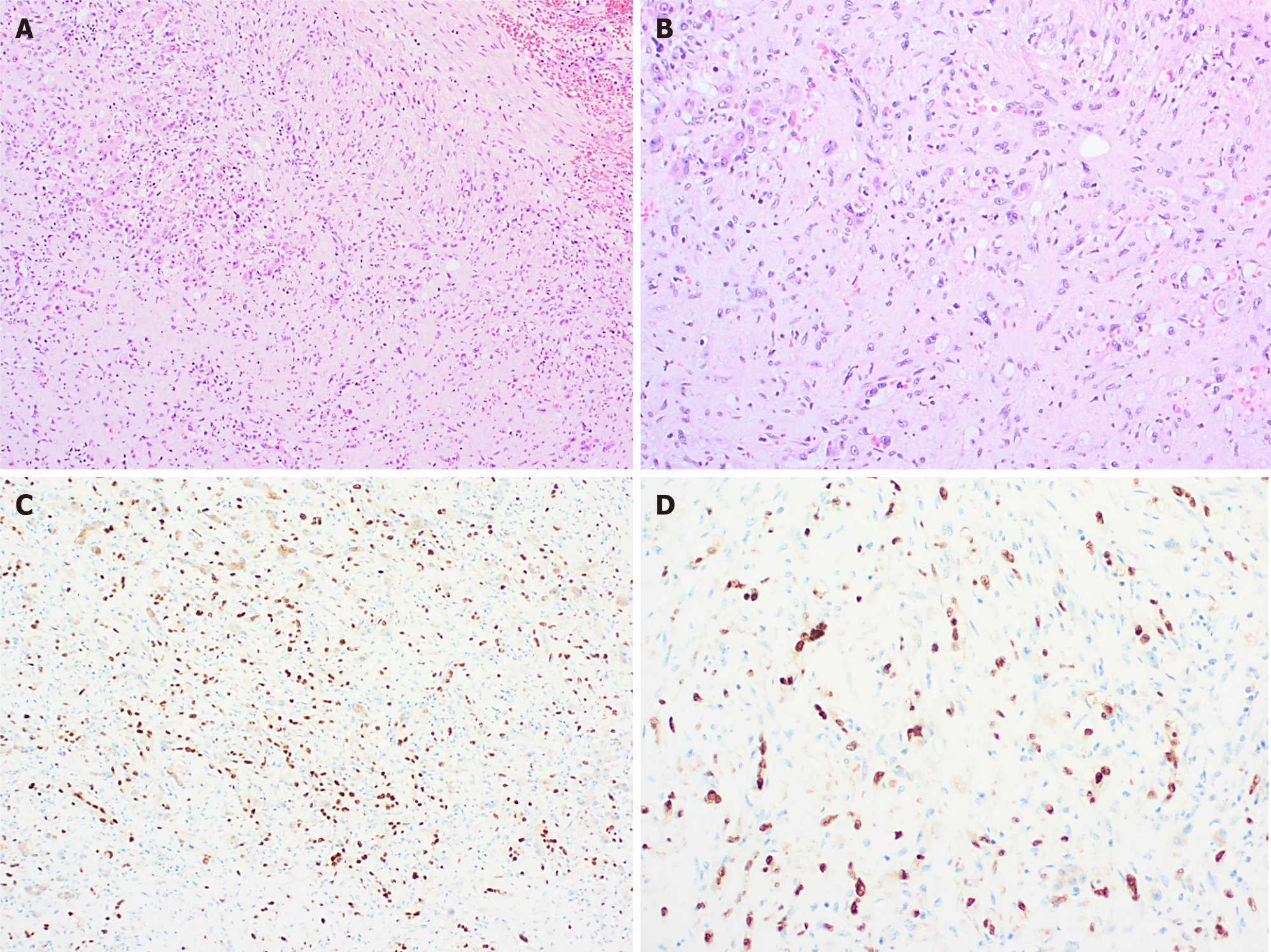
Histologically (Figure 4), HIH are well-demarcated, non-encapsulated vascular lesions composed of lobular, mostly small-sized vessels (capillary-like) with a pericytic cuff, highlighted by the immunohistochemical staining smooth muscle actin (SMA) (Figure 4)[6,9,11,20]. The periphery of these vascular lesions is cellular and mitotic active, with plump endothelial cells (suggesting active growth). Involution is particularly prominent in the center of the lesion and is characterized by reduced cellularity and enlarged vascular spaces lined by flat, mitotically inactive endothelium. The interstitium is fibrotic or fibromyxoid[9]. Bile ducts and hepatocytes are often entrapped within the advancing edge of the tumor. Areas of extra
Multifocal and diffuse HIH show positive staining for GLUT-1, which correlates with a high cell-proliferation and distinguish them from other types of vascular liver tumors (Figure 5)[5,10,22]. The endothelial cells are also positive for ERG, CD31, CD34 and factor VIII but do not express the lymphatic marker podoplanin [D2-40 (Figure 5)][5,15,21].
There are several hypotheses for the pathophysiology of HIH and its cutaneous counterpart. Clinical observations suggested hypoxia as a trigger for infantile hemangioma (IH). Hypoxia may be due to maternal events as well as the infant’s own hypoxia-induced factors and is associated with GLUT-1, as GLUT-1 is a downstream target of hypoxia-inducible factor-1-alpha (HIF-1α), along with vascular endothelial growth factor A (VEGF-A) and insulin-like growth factor 2 (IGF-2). Also, the renin-angiotensin system (RAS) may play a role because high concentrations of angiotensin II (ATII), due to local expression of angiotensin-converting enzyme (ACE) in IH, stimulate cell proliferation. Further, IH expresses GLUT-1 and vascular antigens like Fc-gamma-receptor II, merosin, and Lewis Y antigen, which are also expressed in placental tissue. Another study found that IH endothelial cells share a similar immunophenotype (CD34 and CD133 positive) with embryonic veins, suggesting IH endothelial cells are arrested in an early stage of vascular differentiation[23]. Further, Takahashi et al observed an imbalance of vasculogenic factors in IH. During the proliferating phase, IH shows a high expression off type IV collagenase and vascular endothelial growth factor (VEGF) and when involuting there is an increase in tissue metalloproteinases, inhibiting new vessel formation[24]. Moreover, Walter et al show
Epithelioid hemangioendothelioma (EHE) is a rare malignant vascular tumor, which can occur anywhere in the body but typically arises in liver and lung[4,26]. It is mostly seen in adults, but can be diagnosed in children (estimated prevalence of 1/1000000, mean age 13,8 years)[27]. Hepatic EHEs show a more aggressive course than when arising in bone/soft tissue and are mostly multifocal. Hepatic EHE presents in most cases as a tumoral mass and has an unpredictable clinical course. It may be indolent, stable or aggressive[26,27]. Size > 3 cm and high mitotic index (> 3 mitoses/50 HPF) are poor prognostic factors in elderly[26].
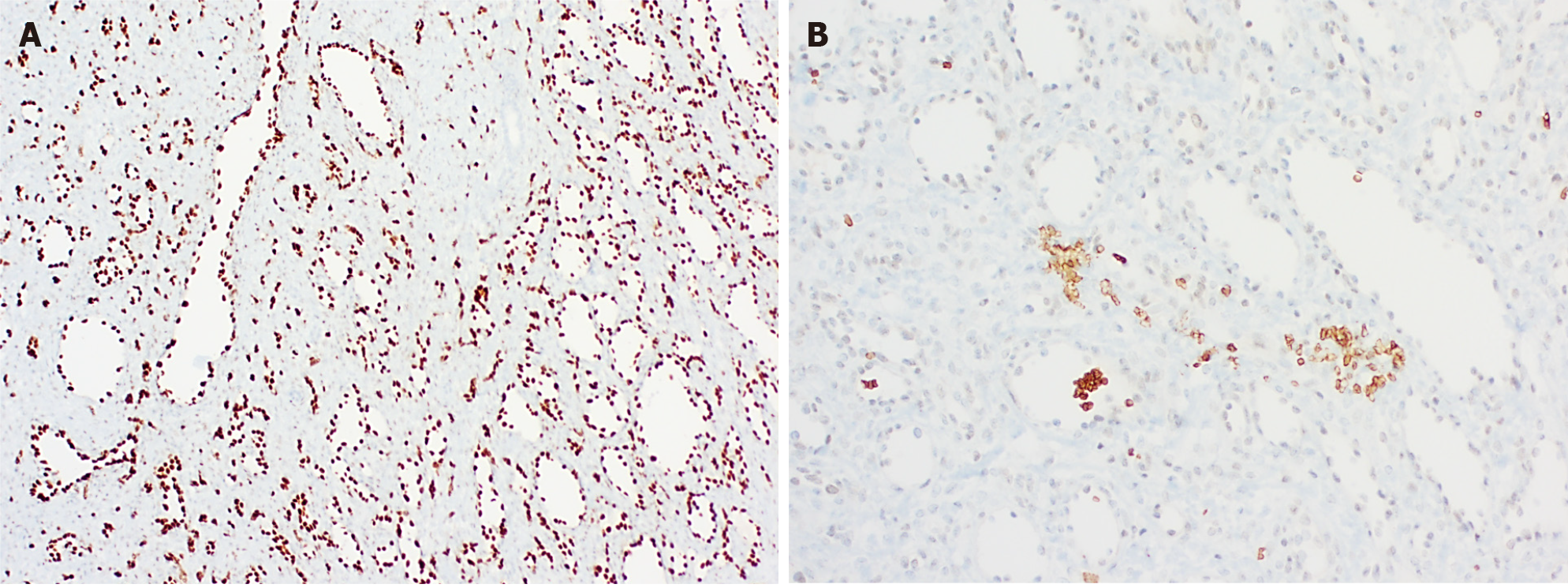
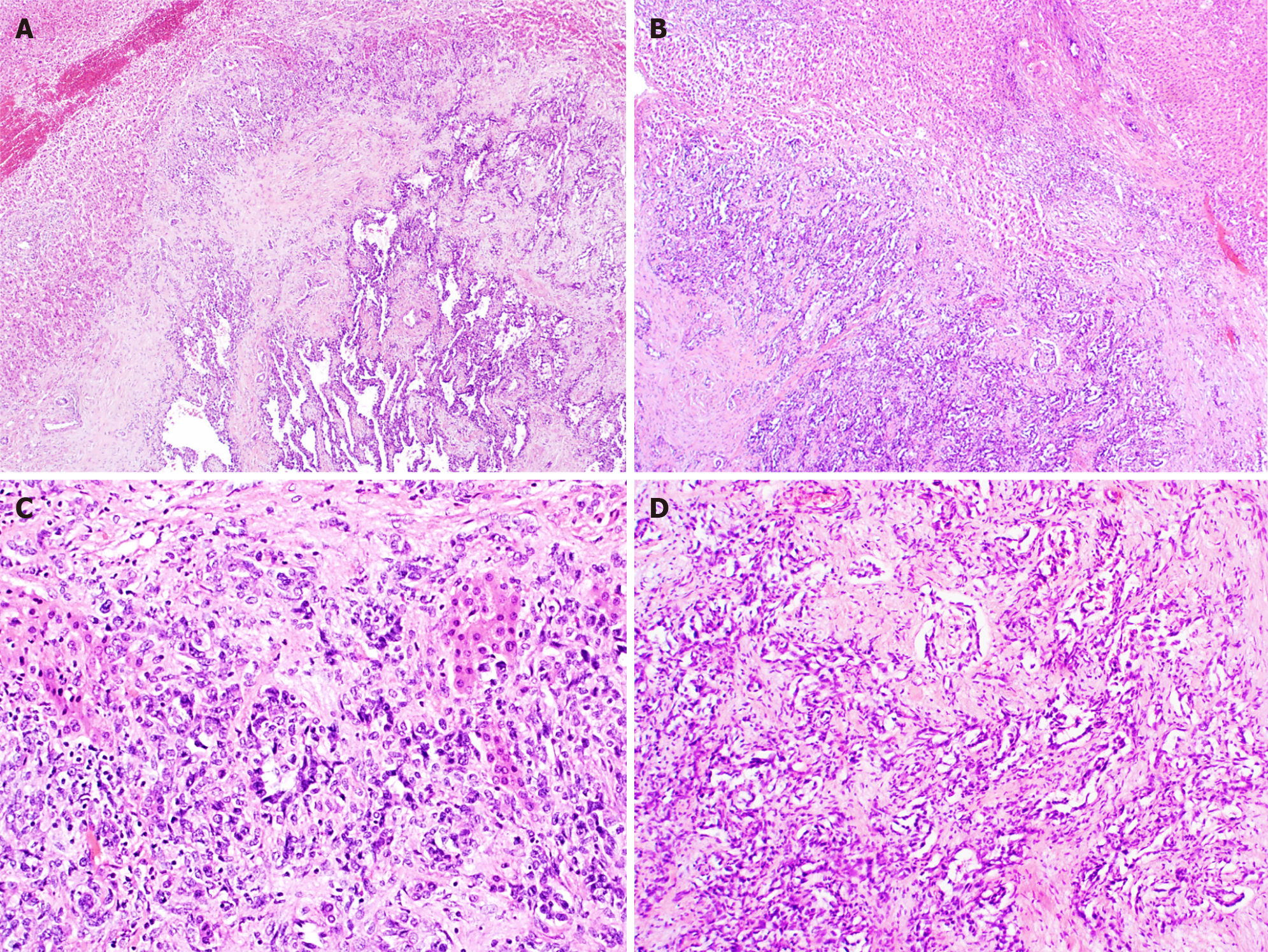
EHEs appear macroscopically as solid, white lesions with some hemorrhagic changes[20]. Histologically (Figure 6), EHEs are relatively distinctive from the normal liver parenchyma and are composed of nests, cords, strands or single infiltrative epithelioid cells set in a myxohyaline stroma. The cells in HEH are epithelioid with eosinophilic cytoplasm and frequently show intracytoplasmic vacuoles (so-called “blister cells”)[20,28]. Occasionally, there are tufts or papillary projections into the vessels. A subset of EHE shows histologic overlap with hepatic angiosarcoma (HA) containing necrosis or moderate to severe cytonuclear atypia (with large hyper
Most of the EHEs are characterized by chromosomal translocations involving 1p36.3 and 3q25 resulting in WW domain-containing transcription regulator1 (WWTR1, also known as TAZ) – CAMTA1 fusion genes. A small subset shows Yes-associated protein 1(YAP1)-TFE3 gene fusions[26,28]. TAZ and YAP are transcriptional coactivators and effectors, which are downregulated by the Hippo tumor suppressor pathway. WWTR1-CAMTA1 fusion genes therefore induce oncogenic transformation due to constitutive nuclear localization and activation of TAZ independent of the Hippo pathway[26].
Hepatic angiosarcoma (HA) is a rare high-grade malignant vascular tumor that occurs mostly in elderly[5,29,30]. Seldom they occur in children and the majority of pediatric angiosarcoma cases arises in the heart/pericardium and mediastinum[29]. When occurring in the liver angiosarcoma presents as a rapid enlargement of the liver associated with jaundice, abdominal pain, vomiting, fever, tachypnea, dyspnea and anemia[30]. Consumptive coagulopathy, disseminated intravascular coagulation and congestive heart failure are known complications[31]. In children HA has a female predominance and occurs mostly around 40 mo. It represents 1%-2% of all pediatric liver tumors and has the potential to metastasize, even at the onset of the disease. Metastasis is commonly found in the lungs[30,32]. HA can occur in the background of a HIH or can develop 4 to 5 years after primary diagnosis of HIH. Therefore, HIH in patients older than 1 year, should be followed carefully[30]. Also, in the past, several chemical carcinogens, including vinyl chloride monomer (VCM), thorotrast, radium and arsenic, have been associated with HA formation[33,34]. Pediatric HA has a poor prognosis with an average survival of 16 mo and a 5-year overall survival of 20%-35%[30,32].
Diagnosis of a HA can be really challenging, as it is an extremely rare tumor and there are no specific radiographic characteristics that differentiate malignant vascular hepatic tumors from benign ones[33,35]. Histologic diagnosis can only be obtained by adequate and representative tissue biopsies, received by laparotomy[35].
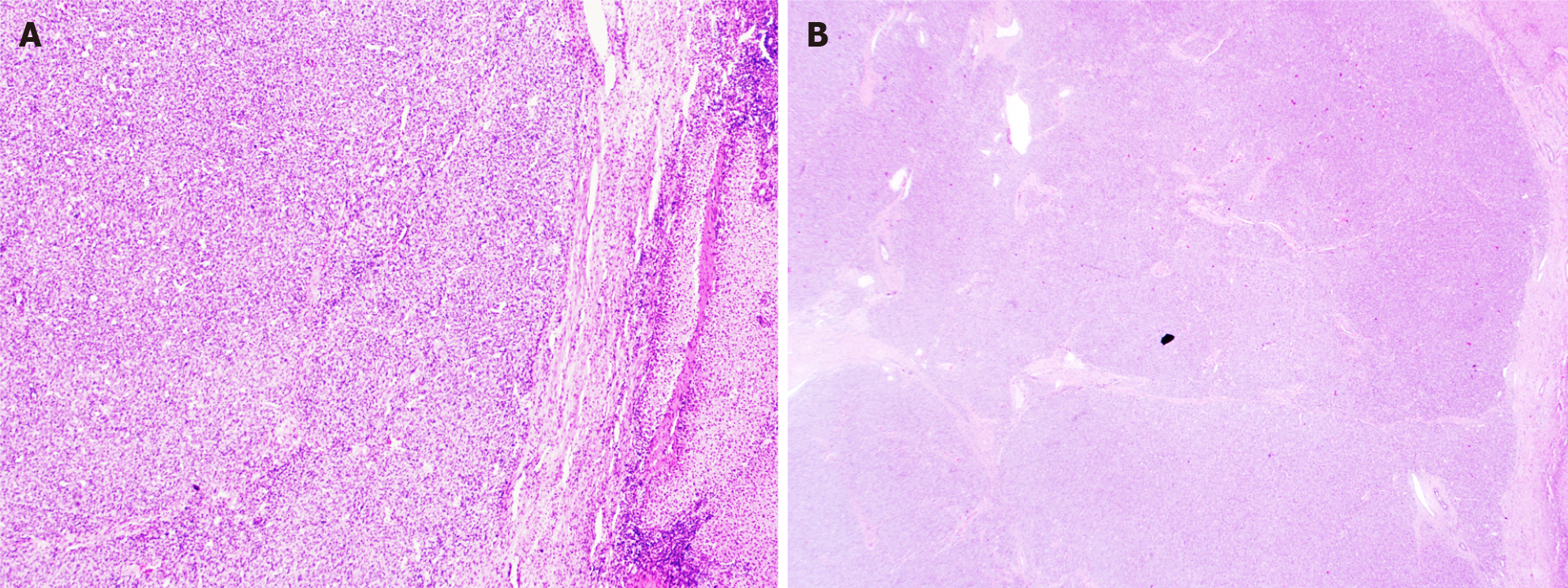
Macroscopically, HA presents as a large solitary mass, or as multiple or diffuse nodules in the centre and periphery of the liver. Often sponge-like hemorrhagic areas alternate with solid gray-white nodules, surrounded by normal liver parenchyma (Figure 7)[31,36]. Commonly, both liver lobes are affected[35,36]. Histologically (Figures 8 and 9), HA shows an unencapsulated vascular tumoral lesion composed of anastomosing vascular spaces and sinusoids lined by endothelial cells with marked cytological atypia and multilayering[29,31,33,35]. The cells are plump, pleomorphic with hyperchromatic nuclei and show brisk mitotic activity[33]. Focally infiltrative whorls or glomeruloid foci of sarcomatoid cells or kaposiform spindle cells with intracytoplasmic PAS positive eosinophilic globules can be seen[30,32,33,35]. Tumor necrosis can be observed[29]. Histologically, HA is classified as hepatic hemangioendothelioma, type 3 (Figure 10)[5]. HA shows immunoreactivity for ERG, CD31, CD34 and factor VIII[15,28,33]. A small percentage expresses pan-cytokeratin[33]. Ki-67 shows a proliferation of more than 10%[36]. HAs are occasionally positive for GLUT-1 and podoplanin (D2-40)[15,22,32]. The spindle cell component may show cytoplasmic immunopositivity for alpha-1-antitrypsin[30].
Uptil now, little is known about the genetics of HA, due to examination of small cohorts with a selected gene panel[34]. KRAS mutations have been described in sporadic and thorotrast-induced HA, and TP53 mutations in VCM-related HA[37,38]. Also alterations in the RAS-RAF-MAPK pathway, CDKN2A/p16 and PTEN gene have been found[34,39]. Recently a ROS1-GOPC/FIG (Fused In Glioblastome) fusion has been found in 1 case[34,37]. This fusion gene can act as a potential target for therapy. Further, upregulation of VEGF-receptor and consistent increased expression of VEGF are commonly seen[34].
Diagnosis of a pediatric hepatic vascular tumor can be challenging, not only for the clinici/radiologist, but for the pathologist as well. Throughout the years immunohistochemical markers[10] and molecular genetics have been proven very helpful in the differential diagnosis of vascular tumors. Here we gave an overview of the most important pediatric hepatic vascular tumors and their histology and pathophysiology. Still there is a lot to discover about these vascular lesions.
| 1. | Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol. 1997;24:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Gampper TJ, Morgan RF. Vascular anomalies: hemangiomas. Plast Reconstr Surg. 2002;110:572-85; quiz 586; discussion 587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Yang B, Li L, Zhang LX, Sun YJ, Ma L. Clinical Characteristics and Treatment Options of Infantile Vascular Anomalies. Medicine (Baltimore). 2015;94:e1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJ, Mitchel S, Powell J, Prendiville J, Vikkula M; ISSVA Board and Scientific Committee. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:e203-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 831] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 5. | Iacobas I, Phung TL, Adams DM, Trenor CC 3rd, Blei F, Fishman DS, Hammill A, Masand PM, Fishman SJ. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J Pediatr. 2018;203:294-300.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Zavras N, Dimopoulou A, Machairas N, Paspala A, Vaos G. Infantile hepatic hemangioma: current state of the art, controversies, and perspectives. Eur J Pediatr. 2020;179:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Roebuck D, Sebire N, Lehmann E, Barnacle A. Rapidly involuting congenital haemangioma (RICH) of the liver. Pediatr Radiol. 2012;42:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 8. | Kulungowski AM, Alomari AI, Chawla A, Christison-Lagay ER, Fishman SJ. Lessons from a liver hemangioma registry: subtype classification. J Pediatr Surg. 2012;47:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Mo JQ, Dimashkieh HH, Bove KE. GLUT1 endothelial reactivity distinguishes hepatic infantile hemangioma from congenital hepatic vascular malformation with associated capillary proliferation. Hum Pathol. 2004;35:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Lewis D, Hachey K, Fitzgerald S, Vaidya R. Rapidly involuting congenital haemangioma of the liver. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Regier TS, Ramji FG. Pediatric hepatic hemangioma. Radiographics. 2004;24:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Nasseri E, Piram M, McCuaig CC, Kokta V, Dubois J, Powell J. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | El Zein S, Boccara O, Soupre V, Vieira AF, Bodemer C, Coulomb A, Wassef M, Fraitag S. The histopathology of congenital haemangioma and its clinical correlations: a long-term follow-up study of 55 cases. Histopathology. 2020;77:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Trindade F, Tellechea O, Torrelo A, Requena L, Colmenero I. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Fujii T, Zen Y, Sato Y, Sasaki M, Enomae M, Minato H, Masuda S, Uehara T, Katsuyama T, Nakanuma Y. Podoplanin is a useful diagnostic marker for epithelioid hemangioendothelioma of the liver. Mod Pathol. 2008;21:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Triana P, Rodríguez-Laguna L, Giacaman A, Salinas-Sanz JA, Martín-Santiago A, López-Santamaría M, Palacios E, Beato MJ, Martinez-González V, López-Gutierrez JC. Congenital hepatic hemangiomas: Clinical, histologic, and genetic correlation. J Pediatr Surg. 2020;55:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Ayturk UM, Couto JA, Hann S, Mulliken JB, Williams KL, Huang AY, Fishman SJ, Boyd TK, Kozakewich HPW, Bischoff J, Greene AK, Warman ML. Somatic Activating Mutations in GNAQ and GNA11 Are Associated with Congenital Hemangioma. Am J Hum Genet. 2016;98:1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Gnarra M, Behr G, Kitajewski A, Wu JK, Anupindi SA, Shawber CJ, Zavras N, Schizas D, Salakos C, Economopoulos KP. History of the infantile hepatic hemangioma: From imaging to generating a differential diagnosis. World J Clin Pediatr. 2016;5:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 19. | Christison-Lagay ER, Burrows PE, Alomari A, Dubois J, Kozakewich HP, Lane TS, Paltiel HJ, Klement G, Mulliken JB, Fishman SJ. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62-7; discussion 67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN, Huysentruyt CJ, Verdijk MA, van Asseldonk MM, Suurmeijer AJ, Bras J, Palmedo G, Groenen PJ, Mentzel T. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Zhang Z, Chen HJ, Yang WJ, Bu H, Wei B, Long XY, Fu J, Zhang R, Ni YB, Zhang HY. Infantile hepatic hemangioendothelioma: a clinicopathologic study in a Chinese population. World J Gastroenterol. 2010;16:4549-4557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 22. | Hernández F, Navarro M, Encinas JL, López Gutiérrez JC, López Santamaría M, Leal N, Martínez L, Patrón M, Tovar JA. The role of GLUT1 immunostaining in the diagnosis and classification of liver vascular tumors in children. J Pediatr Surg. 2005;40:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Smith CJF, Friedlander SF, Guma M, Kavanaugh A, Chambers CD. Infantile Hemangiomas: An Updated Review on Risk Factors, Pathogenesis, and Treatment. Birth Defects Res. 2017;109:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 454] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Walter JW, North PE, Waner M, Mizeracki A, Blei F, Walker JW, Reinisch JF, Marchuk DA. Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma. Genes Chromosomes Cancer. 2002;33:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Hettmer S, Andrieux G, Hochrein J, Kurz P, Rössler J, Lassmann S, Werner M, von Bubnoff N, Peters C, Koscielniak E, Sparber-Sauer M, Niemeyer C, Mentzel T, Busch H, Boerries M. Epithelioid hemangioendotheliomas of the liver and lung in children and adolescents. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Cournoyer E, Al-Ibraheemi A, Engel E, Chaudry G, Stapleton S, Adams DM. Clinical characterization and long-term outcomes in pediatric epithelioid hemangioendothelioma. Pediatr Blood Cancer. 2020;67:e28045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Jung H, Kim HN, Jang Y, Park CK, Ha SY. CAMTA-1 Expression in 24 Cases of Hepatic Epithelioid Hemangioendothelioma in a Single Institute: Diagnostic Utility for Differential Diagnosis from Hepatic Angiosarcoma. In Vivo. 2019;33:2293-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Deyrup AT, Miettinen M, North PE, Khoury JD, Tighiouart M, Spunt SL, Parham D, Weiss SW, Shehata BM. Angiosarcomas arising in the viscera and soft tissue of children and young adults: a clinicopathologic study of 15 cases. Am J Surg Pathol. 2009;33:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Dimashkieh HH, Mo JQ, Wyatt-Ashmead J, Collins MH. Pediatric hepatic angiosarcoma: case report and review of the literature. Pediatr Dev Pathol. 2004;7:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Chavhan GB, Siddiqui I, Ingley KM, Gupta AA. Rare malignant liver tumors in children. Pediatr Radiol. 2019;49:1404-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Grassia KL, Peterman CM, Iacobas I, Margolin JF, Bien E, Padhye B, Meyers RL, Adams DM. Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Marletta S, Cavallo E, Ammendola S, Stefanizzi L, Mastrosimini MG, D'Onofrio M, Brunelli M, Caliò A, Pecori S, Dalbeni A, Ruzzenente A, Capelli P. Multifocal Hepatic Angiosarcoma with Atypical Presentation: Case Report and Literature Review. J Gastrointest Cancer. 2021;52:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Marks EI, Pamarthy S, Dizon D, Birnbaum A, Yakirevich E, Safran H, Carneiro BA. ROS1-GOPC/FIG: a novel gene fusion in hepatic angiosarcoma. Oncotarget. 2019;10:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Awan S, Davenport M, Portmann B, Howard ER. Angiosarcoma of the liver in children. J Pediatr Surg. 1996;31:1729-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Wang ZB, Wei LX. [Primary hepatic angiosarcoma: a clinical and pathological analysis]. Zhonghua Bing Li Xue Za Zhi. 2013;42:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Gigante E, Paradis V, Ronot M, Cauchy F, Soubrane O, Ganne-Carrié N, Nault JC. New insights into the pathophysiology and clinical care of rare primary liver cancers. JHEP Rep. 2021;3:100174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Przygodzki RM, Finkelstein SD, Keohavong P, Zhu D, Bakker A, Swalsky PA, Soini Y, Ishak KG, Bennett WP. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest. 1997;76:153-159. [PubMed] |
| 39. | Tate G, Suzuki T, Mitsuya T. Mutation of the PTEN gene in a human hepatic angiosarcoma. Cancer Genet Cytogenet. 2007;178:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Anatomy and morphology
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Monsereenusorn C, Sira AM S-Editor: Ma YJ L-Editor: A P-Editor: Wu RR