Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1208
Peer-review started: March 7, 2021
First decision: April 6, 2021
Revised: April 18, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 27, 2021
Processing time: 228 Days and 20.2 Hours
Macrovesicular Steatosis (MS) is an independent risk factor for adverse post-liver transplant (LT) outcomes. The degree of MS is intimately related to the viability of the liver graft, which in turn is crucial to the success of the operation. An ideal liver graft should have no MS and most centres would find it unacceptable to use a donor liver with severe MS for LT. While a formal liver biopsy is the gold-standard diagnostic test for MS, given the logistical and time constraints it is not universally feasible. Other tests like a frozen section biopsy are plagued by issues of fallibility with reporting and sampling bias making them inferior to a liver biopsy. Hence, the development of an accurate, non-invasive, easy-to-use, handheld, real-time device for quantification of MS would fill this lacuna in the deceased donor selection process. We present the hypothesis, design and proof-of-concept of a study, which aims to standardise and determine the feasibility and accuracy of a novel handheld device applying the principle of diffuse reflectance spectroscopy for real-time quantification of MS.
Core Tip: The degree of macrovesicular steatosis (MS) is intimately related to the viability of the liver graft, which in turn is crucial to the success of the liver transplant operation. The development of an accurate, non-invasive, easy-to-use, handheld, real-time device for quantification of MS would fill a lacuna in the deceased donor selection process. We present the hypothesis, design and proof-of-concept study for a novel handheld device for real-time quantification of MS.
- Citation: Rajamani AS, Rammohan A, Sai VR, Rela M. Non-invasive real-time assessment of hepatic macrovesicular steatosis in liver donors: Hypothesis, design and proof-of-concept study. World J Hepatol 2021; 13(10): 1208-1214
- URL: https://www.wjgnet.com/1948-5182/full/v13/i10/1208.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i10.1208
Macrovesicular Steatosis (MS) is an independent risk factor for adverse post-liver transplant (LT) outcomes. The degree of MS is intimately related to the viability of the liver graft, which in turn is crucial to the success of the operation. An ideal liver graft should have no MS and most centres would find it unacceptable to use a donor liver with severe MS for LT. While a formal liver biopsy is the gold-standard diagnostic test for MS, given the logistical and time constraints it is not universally feasible. Other tests like a frozen section biopsy are plagued by issues of fallibility with reporting and sampling bias making them inferior to a liver biopsy. Hence, the development of an accurate, non-invasive, easy-to-use, handheld, real-time device for quantification of MS would fill this much vaunted lacuna in the deceased donor selection process. We present the hypothesis, design and proof-of-concept of a study, which aims to standardise and determine the feasibility and accuracy of a novel handheld device applying the principle of diffuse reflectance spectroscopy for real-time quantification of MS.
The objective of the present investigation is to apply the principle of diffuse reflectance spectroscopy (DRS) to standardize and determine the feasibility and accuracy of a handheld device for real-time quantification of MS.
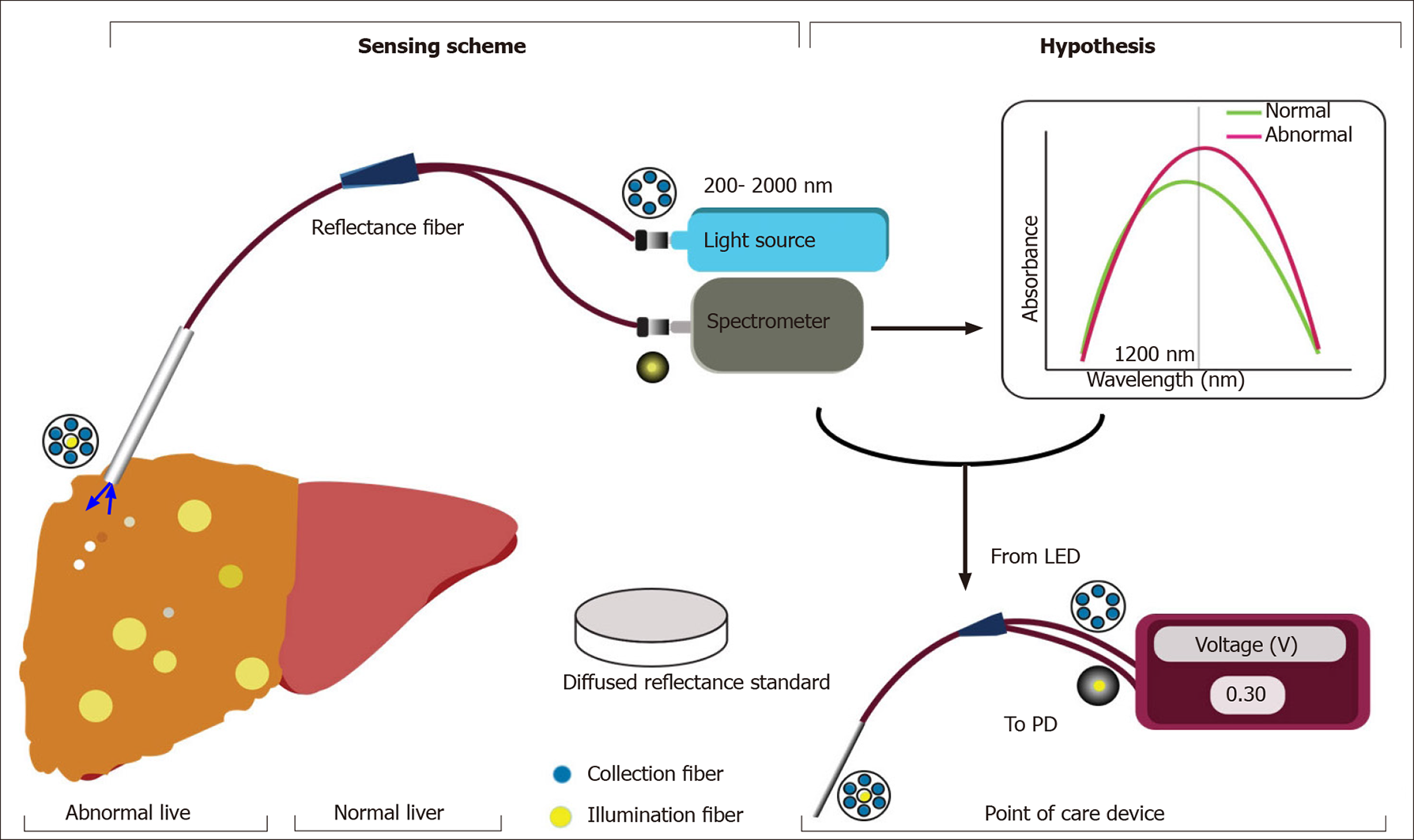
DRS is an optical measurement method which is based on the principle of tissue illumination and the measurement of reflectance[8]. Briefly, the tissue is illuminated with light from a broadband light source, and after interacting with the tissue the diffusely reflected light is collected and analyzed. By fitting the analyzed data to a mathematical model, tissue characteristics such as its structure and composition can be estimated. Quantification of MS with infrared (IR) spectroscopy directly depends on the absorption of IR light due to vibrational excitation in molecular groups[9]. In liver tissues, the absorption in the visible wavelength range is dominated by bile and hemoglobin, whereas lipid, water and collagen are the main source of absorption in the near-infrared wavelength range. Hence IR spectra is the wavelength of interest for this study. Recent studies on the human liver show that the absorption of light around 1200 nm is dominated by the lipid and this can be used for the assessment of steatosis[9-11].
We hypothesize that the broadband light source can be replaced with a narrow band light emitting diode (LED) of 1200 nm and the spectrometer with a highly sensitive photodetector. Using the absorption characteristics, a calibration curve can be determined based on the fat content on the liver; allowing for the development of a mathematical model and a real-time quantitative analysis of MS. We also hypothesize that once the difference in absorbance spectrum between normal and MS liver is established, the optical device can be miniaturized further. This novel optic-based handheld device for MS detection will retain its accuracy whilst being portable and affordable as well.
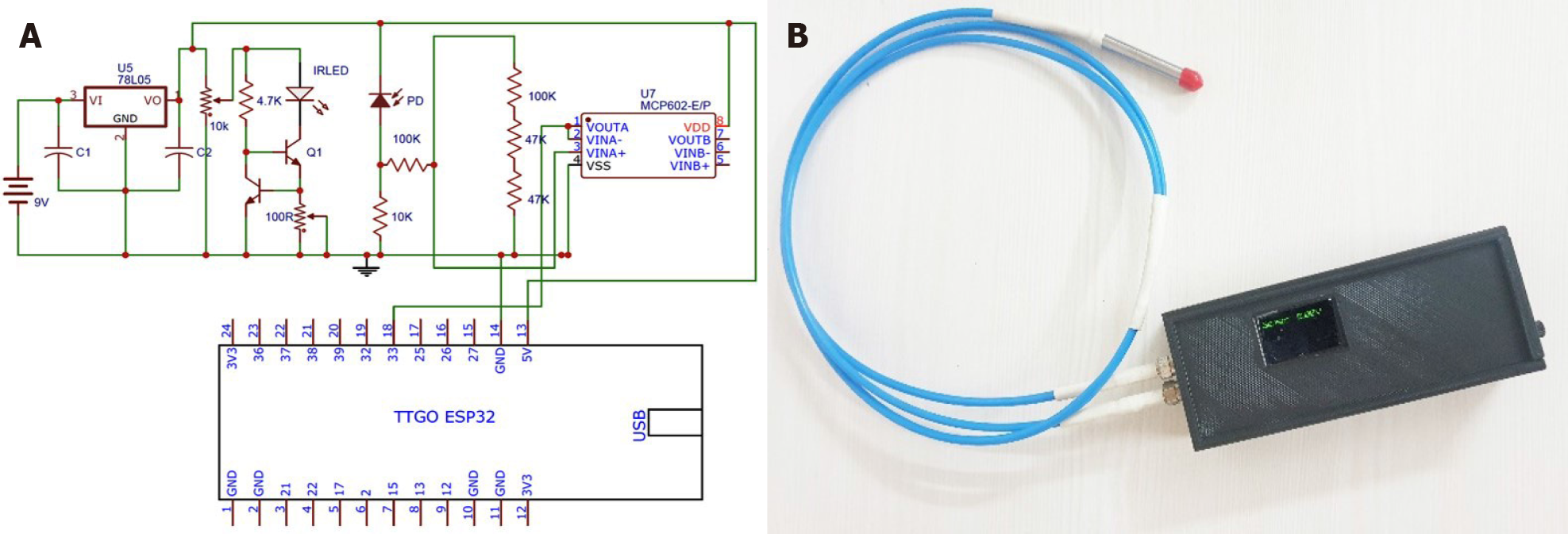
A handheld device was designed and developed with a single infrared LED (IR-LED)-photodetector (PD) arrangement coupled through a fibre optic reflection probe bundle. One end of the reflection probe was coupled to a LED, and the other end to a highly sensitive photodetector. These optoelectronic components were placed in a custom-made plastic block to avoid ambient noise or cross-coupling between the LED and PD. The optoelectronic circuitry comprised of a 5 V linear voltage regulator followed by a constant current circuit using two bipolar junction transistors to drive IR-LED and a trans-impedance amplifier circuit for the PD to convert photocurrent into photo
The measurement was carried out with the handheld device employing a mathematical model. With the device powered on, the fiberoptic reflection probe was placed on the diffuse reflectance standard (WS-1 ocean optics, United States) and the initial voltage value made note of. This was taken as the reference value; the probe was then placed on the test sample to record its voltage value. An algorithm was formulated to calculate the resultant fat absorbance value (Af) with this reference (Vr) and test (Vt) voltage values from the below equation.
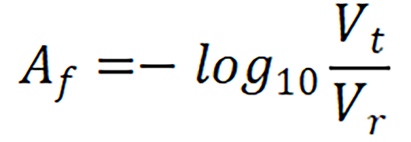
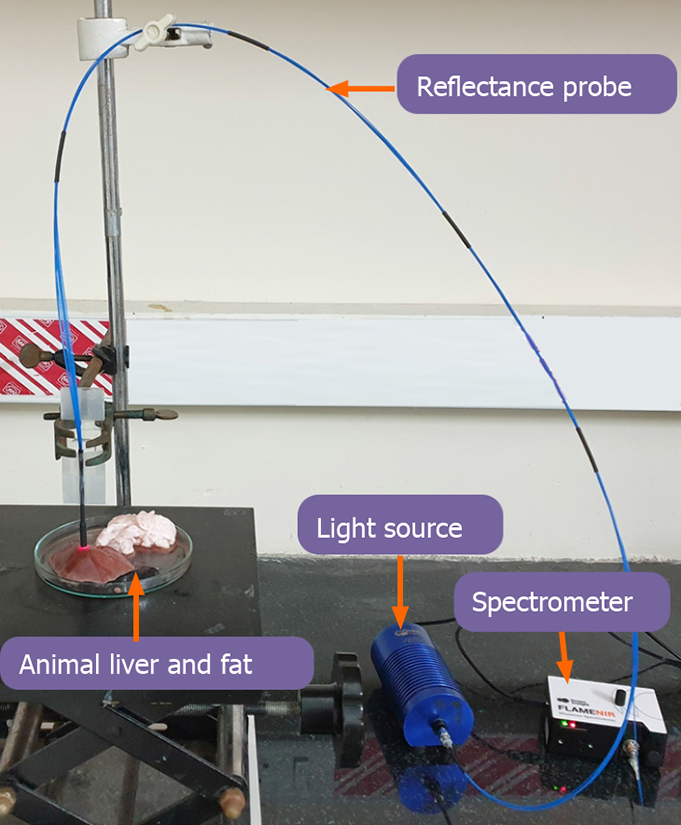
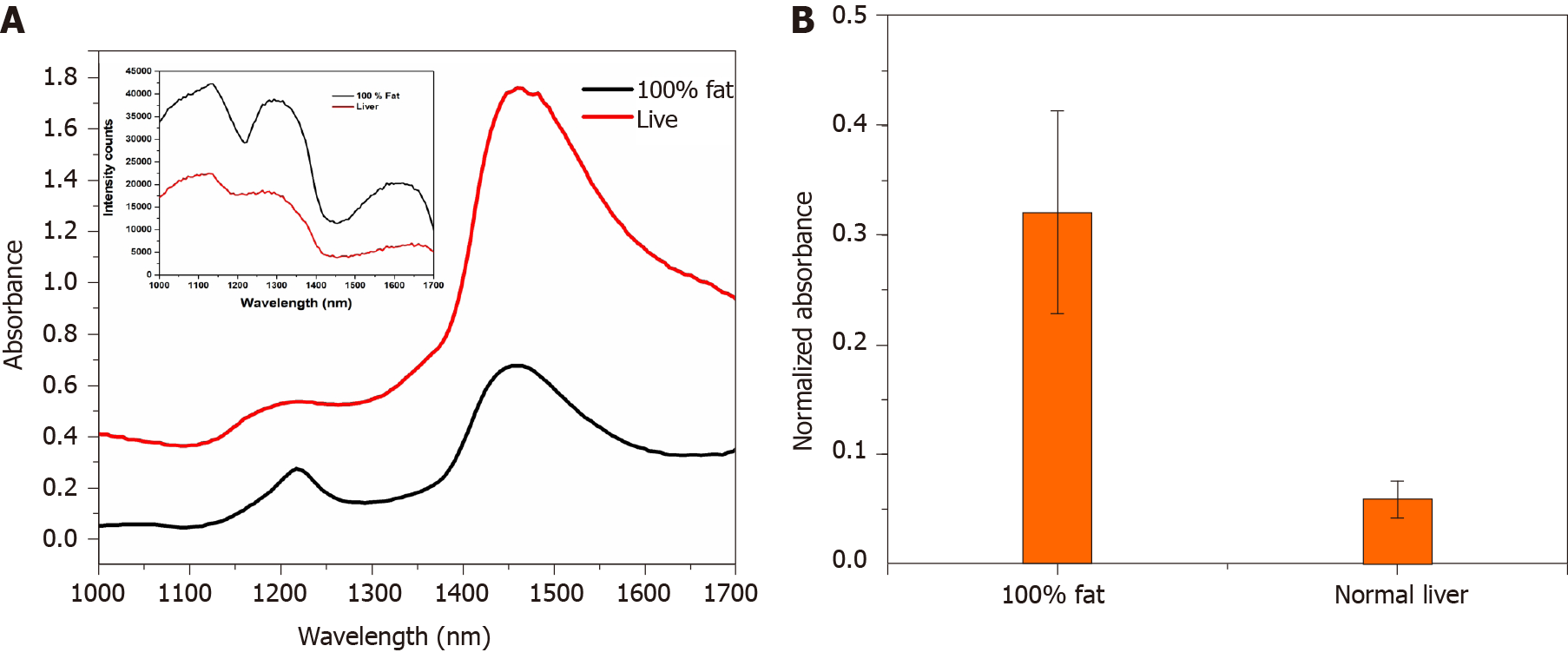
For a practical assessment of the above hypothesis, an initial proof of concept analysis was done using 50 abattoir retrieved large animal livers, with varying percentage of fat (Figure 2). Calibration of the device was initially done with 100% fat and normal liver. The results from fat and normal liver were compared to determine the fat composition. Absorbance data was normalized by taking the closest valley to 1300 nm to improve its sensitivity towards estimation of fat percentage [13,14].
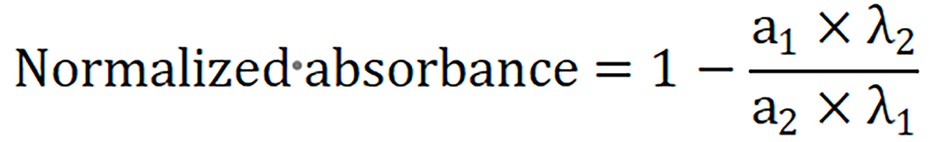
The above equation was used to calculate the normalized absorbance value. This was done by taking the ratio of absorbance responses (a1, a2) at two wavelengths (λ1 and λ2) and subtracting it from 1. The specific absorption spectrum of fat peaked at approximate 1200 nm and the normal liver had a Gaussian response at 1200 nm (Figure 3A). Figure 3B shows the calculated absorbance response of fat and liver was noted to be 0.3203 ± 0.09 and 0.058 ± 0.01 respectively. The absorbance values obtained were evaluated against the gold standard biopsy results of these animal livers.
It is an observational study where the point-of-care device is used to assess MS in a non-invasive manner. The study is to be conducted at organ retrieval centres across the city of Chennai, India. The study design is presented in Figure 4.
Initial calibration of the device is to be conducted on 50 livers. Fifteen live liver donors will be assessed for levels of MS. Ten recordings with the device per liver will be noted across the right lobe. As a standard unit protocol, all live liver donors undergo an intraoperative liver biopsy which will be used for comparison. 100% fat as a baseline calibration will be used by analyzing the excised falciform ligament from each of these patients. 35 livers in the real-world deceased donor situation will be analyzed using the device to correlate the estimated MS content with a standard biopsy estimation. These observations will enable the development of a calibrated algorithm based on the reflectance for MS. Optimum conditions for use, including lighting, temperature, distance from the liver, will also be standardized.
Analysis will be performed on 50 deceased donor livers to test and evaluate the accuracy of the developed algorithm and the point-of-care device.
For the calibration cohort, all living liver donors will be included. The standard selection criteria for these living donors are include: (1) Age 18-50 years; (2) ABO compatible blood group with the recipient; (3) No comorbidities, or 1 comorbidity; (4) Liver attenuation index ≥ +6; (5) Body mass index < 30 kg/m2; (6) Graft to recipient weight ratio > 0.8; (7) Functional liver remnant volume > 30%; (8) Anatomically suitable for donation; and (9) Any other donor who beyond the above criteria but approved for donation based on the decision of the multi-disciplinary team meeting.
The deceased donors include all brain-dead donors consented for organ donation: (1) Adults between 18 years and 75 years of age; and (2) Donation after brain death. For the validation cohort, all brain-dead donors consented for organ donation will be included: (1) Adults between 18 years and 75 years of age; and (2) Donation after brain death.
As the device analyses the fat content of the donor liver, no specific recipient-based inclusion criteria were defined. Donors of all recipients who underwent the LT operation and recipients of all etiologies were included.
(1) Paediatric deceased donors; (2) Donation after cardiac death; (3) Donations where a frozen section/standard biopsy could not be performed; and (4) Discarded organs.
Liver with underlying fibrosis, cholestasis, sinusoidal obstruction syndrome (blue color) and those which could possibly bias the spectral analyses.
The study will be conducted in accordance with the principles of the Declaration of Helsinki and “good clinical practice” guidelines. Approval from the institutional ethics committee has been obtained. As a testimonial to its bona fide nature, the study has also been registered with the Clinical Trials Registry of India, National Institute of Medical Statistics, Indian Council of Medical Research, India. CTRI No: CTRI/ 2021/01/030223.
Statistical analysis will be performed with the SPSS V.20.0. To compare specific variables, the extended χ2 test will be used. For non-parametric analysis of continuous distributed variables, the Mann-Whitney U test and the Kruskal-Wallis test will be used. P < 0.05 is considered statistically significant.
The need for a quick, portable, efficacious and economical device to diagnose MS is evident by the number of proof-of-concept studies available in this regard[7,9,11,15]. DRS as a diagnostic modality has been used in endoluminal studies of upper and lower gastrointestinal endoscopies[16,17]. Using the absorption and scattering patterns of biological tissues, DRS allows for accurate differentiation of polyps and subendothelial pathology. Reports on the use of DRS in the identification of MS in murine and porcine liver models show promising results[10,11]. Clinical studies are however sparse and those attempted involve using a micro-spectrometer placed directly over the liver graft[8,10,15]. Nonetheless, there are several drawbacks to these devices. The micro-spectrometers require a sophisticated optical setup, which included an optical spectrometer and other expensive optical components. In addition, due to concerns of sterility, a spectrometer cannot be used on multiple patients. Moreover, these devices require network access, without which the diagnostic algorithm may not be useful. Put together these devices have proved cumbersome to the organ-retrieving surgeon.
To overcome the pitfalls of these prototype models, our device uses IR light guided via an optical fibre, and the diffuse reflections are obtained from the tissue sample by measuring the steady-state spectrum. The broadband light source is replaced with a narrow band LED and the spectrometer with a photodetector. Once the algorithm is standardized this optical setup can be miniaturized further, and linked to the internet allowing for remote viewing by the concerned teams.
To push the envelope further, should our device be validated in the current study, we propose that there is potential to link our device with a smartphone application incorporating the algorithm and make use of the current generation of high-resolution smartphone cameras. This would allow for a real-time high-resolution image along with MS percentage to be remotely transmitted using the mobile network to the concerned senior members of the transplant team.
We hypothesize that once validated, our device can potentially prove to be an invaluable apparatus at the hands of the organ retrieving surgeon. It will be non-invasive, portable (hand-held), economical, provide real-time readings of the percentage of MS with image reference and be efficaciously handled by junior surgeons, while not requiring any special network capabilities apart from the presence of the now ubiquitous smartphone. This will dramatically ease the currently available circuitous and subjective process of determining MS and decision making in selecting deceased donor organs for LT. Nonetheless, ours is a hypothesis and initial proof-of-concept study which requires real-world validation across multiple centres and in a large cohort of patients before it can become an integral part of the liver retrieval algorithm.
| 1. | Zhang QY, Zhang QF, Zhang DZ. The Impact of Steatosis on the Outcome of Liver Transplantation: A Meta-Analysis. Biomed Res Int. 2019;2019:3962785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8184] [Article Influence: 682.0] [Reference Citation Analysis (0)] |
| 3. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5072] [Cited by in RCA: 4754] [Article Influence: 528.2] [Reference Citation Analysis (2)] |
| 4. | McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Yersiz H, Lee C, Kaldas FM, Hong JC, Rana A, Schnickel GT, Wertheim JA, Zarrinpar A, Agopian VG, Gornbein J, Naini BV, Lassman CR, Busuttil RW, Petrowsky H. Assessment of hepatic steatosis by transplant surgeon and expert pathologist: a prospective, double-blind evaluation of 201 donor livers. Liver Transpl. 2013;19:437-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Hołówko W, Mazurkiewicz M, Grąt M, Koperski L, Lewandowski Z, Smoter P, Ziarkiewicz-Wróblewska B, Górnicka B, Zborowska H, Krawczyk M. Reliability of frozen section in the assessment of allograft steatosis in liver transplantation. Transplant Proc. 2014;46:2755-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 561] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Nilsson JH, Reistad N, Brange H, Öberg CF, Sturesson C. Diffuse Reflectance Spectroscopy for Surface Measurement of Liver Pathology. Eur Surg Res. 2017;58:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Le Naour F, Gadea L, Danulot M, Yousef I, Vibert E, Wavelet M, Kaščáková S, Castaing D, Samuel D, Dumas P, Guettier C. Quantitative assessment of liver steatosis on tissue section using infrared spectroscopy. Gastroenterology. 2015;148:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Evers DJ, Westerkamp AC, Spliethoff JW, Pully VV, Hompes D, Hendriks BH, Prevoo W, van Velthuysen ML, Porte RJ, Ruers TJ. Diffuse reflectance spectroscopy: toward real-time quantification of steatosis in liver. Transpl Int. 2015;28:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Westerkamp AC, Pully VV, Karimian G, Bomfati F, Veldhuis ZJ, Wiersema-Buist J, Hendriks BH, Lisman T, Porte RJ. Diffuse reflectance spectroscopy accurately quantifies various degrees of liver steatosis in murine models of fatty liver disease. J Transl Med. 2015;13:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Rajamani AS, M D, Sai VVR. Plastic fiber optic sensor for continuous liquid level monitoring. Sensors Actuators A Phys. 2019;296:192-199. [DOI] [Full Text] |
| 13. | Cho MJ, Pernarowski M. Application of absorbance ratios to analysis of pharmaceuticals. VI. Analysis of binary mixture using a reference spectrum. J Pharm Sci. 1970;59:1333-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Cleverley B, Dolby R. Absorbance Ratio Calculations from the Infrared Spectra of Thick Liquid Films. Appl Spectrosc. 1978;32:187-189. [DOI] [Full Text] |
| 15. | Golse N, Cosse C, Allard MA, Laurenzi A, Tedeschi M, Guglielmo N, Fernandez-Sevilla E, Robert M, Tréchot B, Pietrasz D, Pittau G, Ciacio O, Sa Cunha A, Castaing D, Cherqui D, Adam R, Samuel D, Sebagh M, Vibert E. Evaluation of a micro-spectrometer for the real-time assessment of liver graft with mild-to-moderate macrosteatosis: A proof of concept study. J Hepatol. 2019;70:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Coda S, Siersema PD, Stamp GW, Thillainayagam AV. Biophotonic endoscopy: a review of clinical research techniques for optical imaging and sensing of early gastrointestinal cancer. Endosc Int Open. 2015;3:E380-E392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Mundo AI, Greening GJ, Fahr MJ, Hale LN, Bullard EA, Rajaram N, Muldoon TJ. Diffuse reflectance spectroscopy to monitor murine colorectal tumor progression and therapeutic response. J Biomed Opt. 2020;25:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li SW S-Editor: Wu YXJ L-Editor: A P-Editor: Liu JH