Published online Jan 27, 2021. doi: 10.4254/wjh.v13.i1.132
Peer-review started: September 9, 2020
First decision: October 23, 2020
Revised: November 2, 2020
Accepted: December 4, 2020
Article in press: December 4, 2020
Published online: January 27, 2021
Processing time: 133 Days and 20.3 Hours
Abnormal liver function tests (LFTs) in post-liver transplant (LT) patients pose a challenge in the timing and selection of diagnostic modalities. There are little data regarding the accuracy of endoscopic retrograde cholangiopancreatography (ERCP) and liver biopsy (LB) in diagnosing post-transplant complications.
To evaluate the diagnostic performance of ERCP and LB in patients with non-vascular post-LT complications.
This single-center retrospective study evaluated patients undergoing both ERCP and LB for evaluation of elevated LFTs within 6 mo of LT from 2000 to 2017. Diagnostic operating characteristics including accuracy, sensitivity and specificity for various diagnoses were calculated for ERCP and LB. The R factor (ratio of alkaline phosphatase to alanine aminotransferase) was also calculated for each patient.
Of the 1284 patients who underwent LT, 91 patients (74.7% males, mean age of 51) were analyzed. Anastomotic strictures (AS, 24.2%), acute cellular rejection (ACR, 11%) and concurrent AS/ACR (14.3%) were the most common diagnoses. ERCP carried an accuracy of 79.1% (95%CI: 69.3-86.9), LB had an accuracy of 93.4% (95%CI: 86.2-97.5), and the combination of the two had an accuracy of 100% (95%CI: 96-100). There was no difference between patients with AS and ACR in mean R factor (AS: 1.9 vs ACR: 1.1, P = 0.24). Adverse events did not differ between the two tests (ERCP: 3.1% vs LB: 1.1%, P = 0.31).
In patients with abnormal LFTs after LT without vascular complications, the combination of LB and ERCP carries low risk and improves diagnostic accuracy over either test alone.
Core Tip: Patients commonly develop unexplained elevations in liver function tests after liver transplantation. After cross sectional imaging and basic lab tests, endoscopic retrograde cholangiopancreatography (ERCP) and liver biopsy (LB) are both performed in arbitrary fashion since the diagnostic capacity of each test remains unclear. In this study we found that ERCP and LB are both effective diagnostic tests in the setting of the 2 most common diagnoses, anastomotic biliary stricture and acute cellular rejection. Combining these tests increases the overall diagnostic accuracy to 100%, and both tests carried adverse event rates of < 5%. This study justifies combining ERCP and LB when the diagnosis remains elusive.
- Citation: Attwell A, Han S, Kriss M. Endoscopic retrograde cholangiopancreatography and liver biopsy in the evaluation of elevated liver function tests after liver transplantation. World J Hepatol 2021; 13(1): 132-143
- URL: https://www.wjgnet.com/1948-5182/full/v13/i1/132.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i1.132
Since 2012, the number of liver transplants (LTs) performed annually in the United States has increased each year, reaching a record number of 8250 in 2018[1]. Just as the field of transplantation has evolved over the past 5 decades, so too have the nuances of post-transplant clinical care. Clinicians commonly face the conundrum of abnormal liver function tests (LFTs) soon after LT which often indicates a transplant-related complication. Practice guidelines provided by the American Association for the Study of Liver Diseases (AASLD), American Society of Transplantation, and the European Association for the Study of the Liver note that the frequency of monitoring LFTs after LT and the subsequent work-up should be individualized to the patient and time after LT, prior complications, stability of serial testing, and the suspected underlying pathology[2,3].
The underlying cause, however, can be challenging to discern. Depending on the pattern of abnormal LFTs, evaluation of the biliary system with transabdominal ultrasound, MRI, CT, and/or endoscopic retrograde cholangiopancreatography (ERCP) may be most appropriate when the LFT pattern is cholestatic, whereas liver biopsy (LB) should be performed first when parenchymal injury is suspected[2]. To date, there are insufficient data regarding the relative accuracy of ERCP and LB in diagnosing specific post-LT complications. Current societal guidelines strongly support both of these tests (Grade 1A recommendations) but provide little guidance on which should be performed initially[2]. The decision to choose LB, ERCP, or both (and in which order) is therefore left to the discretion of the transplant surgeon, hepatologist, or interventional endoscopist. The primary aim of this study was to evaluate the diagnostic performance of ERCP and LB in patients with non-vascular post-LT complications.
This was a single-center, retrospective review of all patients who underwent LT followed by both LB and ERCP at the University of Colorado Hospital from January 2000 to June 2017.
Patients undergoing deceased or living donor LT at our center during the study period were identified using the LT database. Inclusion criteria included adult patients post-LT who underwent both LB and ERCP within 6 mo after LT with a primary indication of elevated LFTs. Patients with a clearly identifiable cause of elevated LFTs–such as drug or medication-related hepatitis, vascular liver disease or infectious hepatitis based on the initial history, labs, or imaging studies-were excluded from the analysis. Patients who did not receive post-LT care at our institution were also excluded. Post-LT biliary anatomy types included duct-to-duct (DD) anastomosis and Roux-en-Y hepaticojejunostomy (RYHJ).
Patients with a mixed pattern of liver injury based on LFTs underwent either LB or ERCP initially at the discretion of the provider. ERCP was the first invasive diagnostic test performed when patients had symptoms suggestive of cholangitis or a predominantly cholestatic pattern of elevated LFTs. LB was performed after labs and cross-sectional imaging when hepatocellular disease was suspected. It is our practice to monitor immunosuppressant levels on all post-LT patients. Approval from the Colorado Multi-Institutional Review Board was obtained prior to beginning the study.
ERCP was performed under conscious sedation, monitored anesthesia care, or general anesthesia by one of 7 advanced endoscopists who have performed > 1000 ERCPs each. Endoscopists utilized the standard technique in cannulating the bile duct and performing cholangiography. Occlusion cholangiography was used to visualize the entire native and donor biliary tree with particular attention paid to the anastomosis. Biliary sphincterotomy was performed in select cases at the discretion of the endoscopist. If present, strictures were treated with the placement of plastic or fully covered metal stents were placed across strictures according to the endoscopist’s judgment. Dilation of strictures via balloon or catheter was performed prior to stenting in select cases.
Conventional techniques such as balloon and basket sweeping were used to remove bile duct stones and/or casts, and single or multiple stents were placed across anastomotic bile duct leaks. For patients with DD biliary anastomosis, a standard duodenoscope was used to reach the ampulla. For patients with RYHJ anatomy either a pediatric colonoscope or small bowel enteroscope (single-balloon, double-balloon, or rotational overtube) was used to reach the biliary anastomosis.
While percutaneous (ultrasound-guided) LB represented the preferred route of biopsy, transjugular LB was generally performed in patients with an International Normalized Ratio > 1.5, when intravascular pressure measurements were needed, or when the abdominal anatomy precluded a safe percutaneous approach. Both percutaneous and transjugular LB were performed under conscious sedation. LB techniques are described in detail in an AASLD position paper[4]. Board certified GI pathologists examined all histology samples.
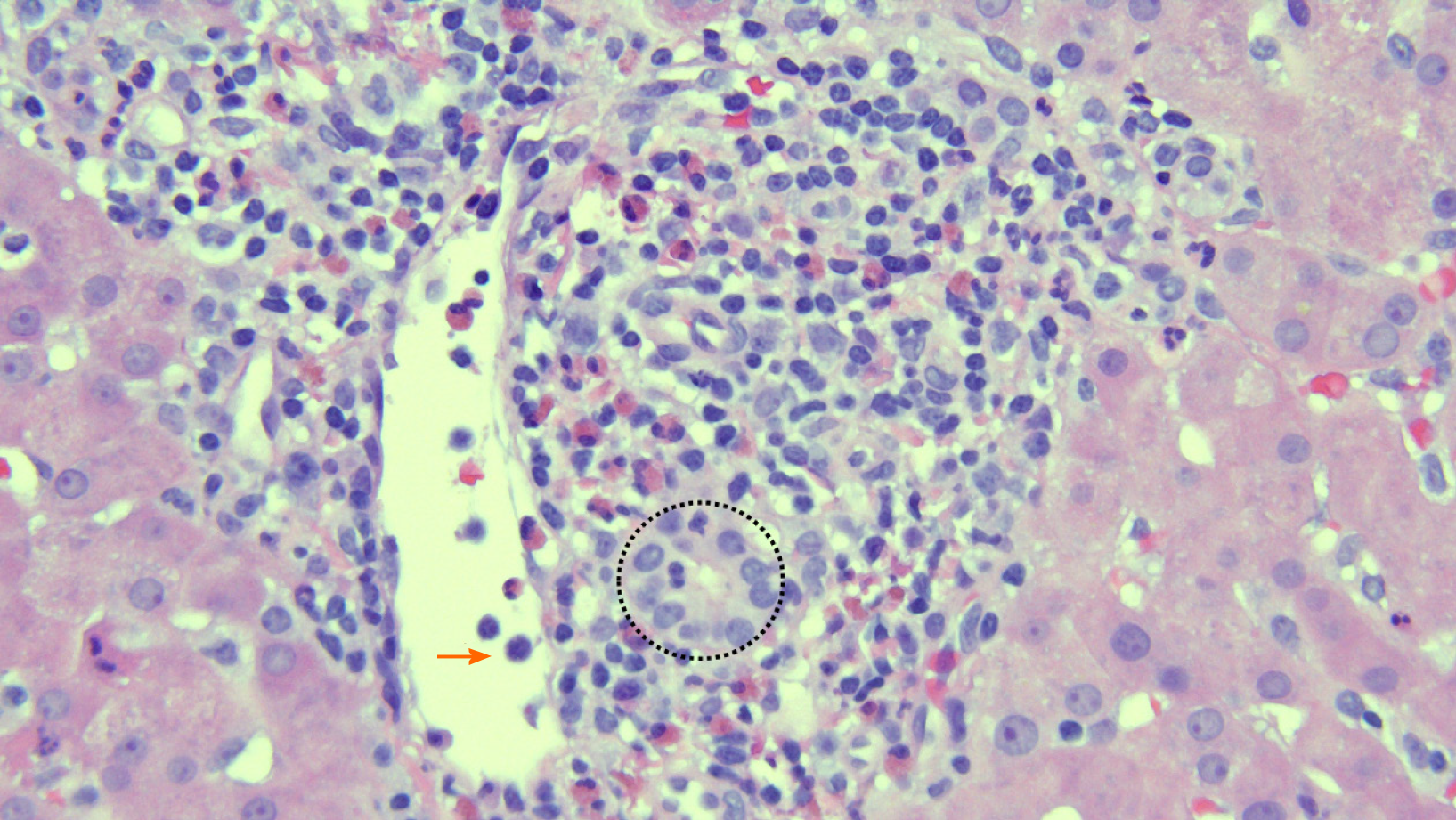
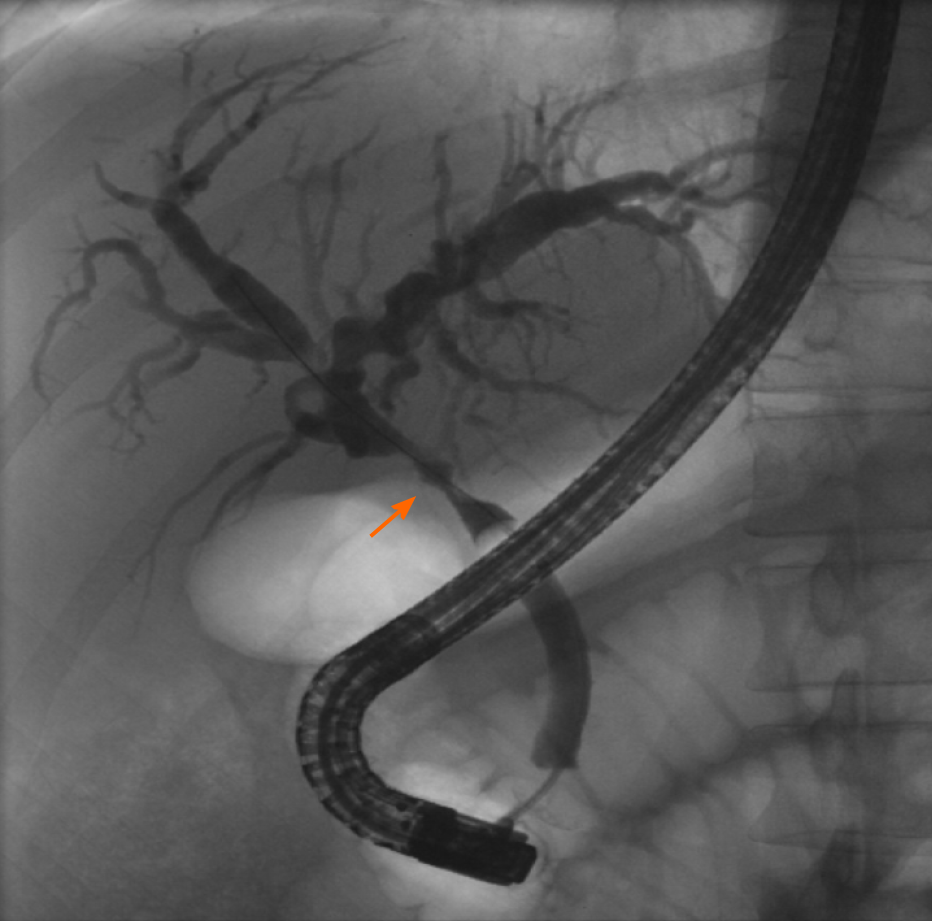
The study’s primary outcome was the accuracy of ERCP and LB in making the ultimate final diagnosis or diagnoses driving the abnormal LFTs, as determined by the GI and Hepatology services. Secondary outcomes included sensitivity and specificity for ERCP and LB in the final diagnosis. Acute cellular rejection (ACR) was defined and graded using a 1-9 scale based on histopathologic findings using the rejection activity index, which was based on inflammatory changes in the portal triads, bile ducts, and venous endothelium (with scores of 1-3 for each of the 3 categories)[5]. A score of 3 or more was classified as definite ACR (Figure 1)[5]. Recurrent hepatitis C infection (HCV) after LT was defined by detectable serum HCV RNA. Anastomotic stricture (AS) was defined as a benign-appearing narrowing in the region of the biliary anastomosis during ERCP, typically within 5-6 mm from the suture line, usually associated with delayed contrast drainage and/or moderate resistance to passage of an inflated 12 mm balloon (Figure 2).
True positive results for LB or ERCP were defined by findings supportive of at least one of the final diagnosis/es as defined above. True negative results were defined by ERCP or LB results that failed to support the final diagnosis/es with or without supporting an alternative diagnosis. For example, if LB showed signs of a large bile duct obstruction or cholangitis, this was considered a true positive for a final diagnosis of anastomotic stricture or cholangitis, respectively. Conversely, if ERCP did not show biliary pathology, this was considered a false negative when the final diagnosis was a hepatocellular disorder such as ACR or recurrent HCV.
Descriptive statistics were used to depict patient demographics, symptoms and laboratory data. An R factor was calculated as the ratio between the degree of elevation of alkaline phosphatase and the degree of elevation of alanine aminotransferase[6]. R factors > 5 were considered to be consistent with hepatocellular damage and R factors < 2 suggested cholestatic patterns of injury, with R factors between 2 and 5 suggesting a mixed pattern of injury. Diagnostic operating characteristics including sensitivity, specificity, and accuracy [(true positive + true negative)/(true positive + false negative + false positive + true negative)] were calculated for both ERCP and LB. Fisher’s exact test or the chi square test were used to compare categorical variables between patients with ACR and AS. The student’s t-test was used to compare continuous variables between patients with ACR and AS. Adverse event rates were compared between ERCP and LB using the Fisher’s exact test. All statistical analysis was performed using STATA 15.1 (StataCorp, College Station, TX, United States).
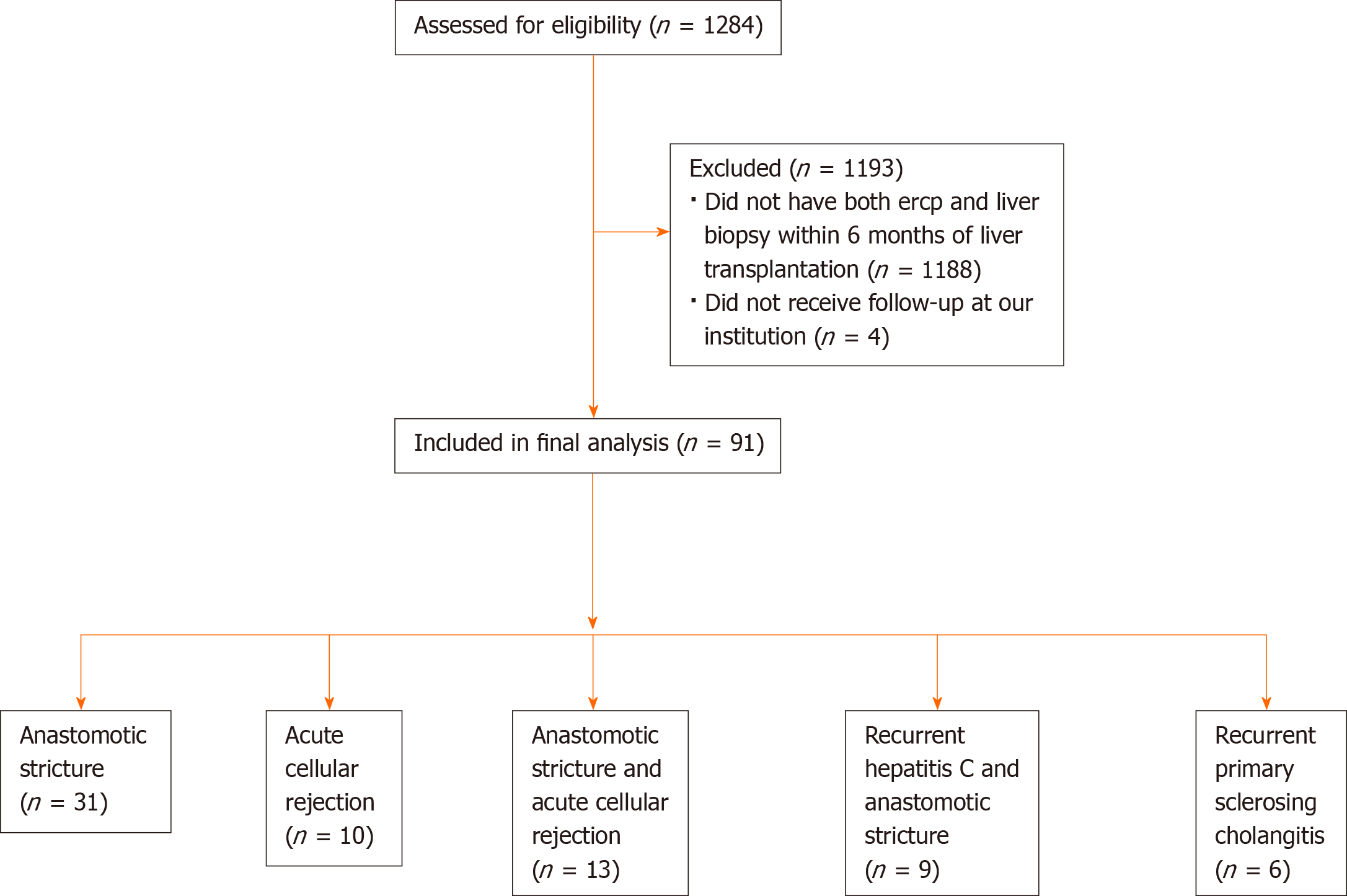
A total of 1284 patients underwent LT at our center during the study period (Figure 3). Of these, 96 patients (7.5%) received both an ERCP and LB for evaluation of persistently elevated LFTs within the first 6 mo after LT. Ninety-one patients received long-term follow-up at our institution and were included in the final analysis. The mean time interval between the 2 procedures was 9.1 d (SD 6.9).
The mean age of the cohort was 51 (SD 12.1) and 74.7% (n = 68) were male (Table 1). Deceased donor transplants (n = 73, 80.2%) accounted for the majority of transplants, and 73.6% (n = 67) had DD biliary anatomy. Presenting symptoms included jaundice (23.1%, n = 21), abdominal pain (15.4%, n = 14), and fever (12.1%, n = 11), and 21 (25%) patients were asymptomatic. Initial imaging consisted of ultrasound (74.7%), CT (18.7%), and magnetic resonance cholangiopancreatography (MRCP, 6.6%) with a mean donor bile duct diameter of 4.6 (SD 1.9) mm. Imaging revealed a dilated duct in 9 (9.9%, 8 with ultrasound, 1 with MRCP) of patients. LB was performed as the first of the 2 tests in 51 (56%) patients, and 71.4% (n = 65) of LBs were performed via the percutaneous route. Nearly 75% of patients were on dual immunosuppression therapy (n = 68) with 22% of patients on monotherapy (n = 20) with the combination of tacrolimus and mycophenolate sodium being the most common combination therapy (n = 21).
| Variable | Overall cohort (n = 91) |
| Age | 51 (12.1) |
| Sex (male) | 68 (74.7) |
| Presenting symptom | |
| Jaundice | 21 (23.1) |
| Fever | 11 (12.1) |
| Abdominal pain | 14 (15.4) |
| Asymptomatic | 21 (25) |
| Liver biopsy performed first | 51 (56) |
| Percutaneous liver biopsy | 65 (71.4) |
| Bile duct diameter (mm) | 4.6 (1.9) |
| R factor | 2 (2.4), Range: 0.1-6.4 |
| Alkaline phosphatase (international units/liter) | 392.6 (248.4) |
| AST (units/liter) | 200.5 (674.8) |
| ALT (units/liter) | 205.4 (444.2) |
| Total bilirubin (mg/dL) | 4.5 (5.4) |
| Deceased donor | 73 (80.2) |
| Transplant biliary anatomy | |
| Duct-to-duct | 67 (73.6) |
| Roux-en-Y hepaticojejunostomy | 24 (26.4) |
| Tacrolimus | 66 (73.3) |
| Sirolimus | 20 (22.2) |
| Everolimus | 6 (6.6) |
| Mycophenolate sodium | 28 (31.1) |
| Mycophenolate mofetil | 13 (14.4) |
| Cyclosporine | 16 (17.8) |
| Prednisone | 20 (22.2) |
| Immunosuppression monotherapy | 20 (22) |
| Dual immunosuppression therapy | 68 (74.7) |
| Triple immunosuppression therapy | 3 (3.3) |
Technically, all LB and ERCP procedures were performed successfully. The most common single diagnosis ultimately was AS (34.1%), followed by ACR (11%) with all diagnoses displayed in Table 2. A total of 29 (31.9%) patients had multiple concurrent diagnoses contributing to the elevation in LFTs (and included as final diagnoses), and the most common was a dual diagnosis of AS with ACR (14.3%, n = 13). Four (4.4%) patients had 3 concurrent diagnoses, all of which included ACR and AS (Table 2).
| Single diagnosis | n (%) |
| Anastomotic stricture | 31 (34.1) |
| Acute cellular rejection | 10 (11) |
| Recurrent primary sclerosing cholangitis | 6 (19.4) |
| Recurrent HCV | 5 (5.5) |
| Biliary cast syndrome | 3 (3.3) |
| Ischemic cholangiopathy | 2 (2.2) |
| Papillary stenosis | 1 (1.1) |
| Posterior reversible encephalopathy syndrome | 1 (1.1) |
| Cholestatic hepatitis | 1 (1.1) |
| Recurrent PBC | 1 (1.1) |
| Venous outflow obstruction | 1 (1.1) |
| Two diagnoses | |
| Anastomotic stricture and acute cellular rejection | 13 (14.3) |
| Recurrent HCV and anastomotic stricture | 6 (19.4) |
| Bile leak and acute cellular rejection | 2 (2.2) |
| Congestive hepatopathy and anastomotic stricture | 1 (1.1) |
| Anastomotic stricture and suprahepatic cava stenosis | 1 (1.1) |
| Recurrent PBC and anastomotic stricture | 1 (1.1) |
| CMV hepatitis and bile leak | 1 (1.1) |
| Three diagnoses | |
| Acute cellular rejection, anastomotic stricture, and recurrent HCV | 2 (2.2) |
| Acute cellular rejection, anastomotic stricture, and de novo autoimmune hepatitis | 1 (1.1) |
| Acute cellular rejection, anastomotic stricture, and CMV hepatitis | 1 (1.1) |
The diagnostic operating characteristics of LB and ERCP are shown in Table 3. The overall accuracy of ERCP was 79.1% (95%CI: 69.3-86.9). The overall accuracy of LB was 93.4% (95%CI: 86.2-97.5). Combined, the 2 tests had an overall accuracy of 100% (95%CI: 96-100).
| ERCP | LB | ERCP + LB | |
| Overall accuracy % (95%CI) | 79.1 (69.3-86.9) | 93.4 (86.2-97.5) | 100 (96-100) |
| Overall sensitivity % (95%CI) | 79.1 (69.3-86.9) | 93.4 (86.2-97.5) | 100 (96-100) |
| Acute cellular rejection accuracy % (95%CI) | 0 (0-30.9) | 100 (69.2-100) | 100 (91.9-100) |
| Anastomotic stricture accuracy % (95%CI) | 100 (84.6-100) | 72.7 (49.8-89.3) | 100 (89.4-100) |
For AS, ERCP had an accuracy of 100% (95%CI: 84.6-100) while LB had an accuracy of 72.7% (95%CI: 49.8-89.3). For ACR, LB had an accuracy of 100% (95%CI: 69.2-100) while ERCP had an accuracy of 0% (95%CI: 0-30.9). Sensitivities carried the same values as the accuracy in all cases due to the lack of false positive results. For the same reason, specificity could not be calculated for any of the diagnostic tests.
The mean R factor (ratio of alkaline phosphatase and alanine aminotransferase) was 2 (SD 2.4), with a mean alkaline phosphatase (AP) level of 392.6 (SD 248.4) IU/L and mean total bilirubin (TB) level of 4.5 (SD 5.4) mg/dL. The mean aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were 200.5 (SD 674.8) and 205.4 (444.2), respectively. Between patients with AS and patients with ACR, there was no significant difference in R factor (AS: 1.9 vs ACR: 1.1, P = 0.24), AP (AS: 376.3 vs ACR: 452.2, P = 0.48), TB (AS: 4.1 vs ACR: 5.5, P = 0.41), AST (AS: 130.9 vs ACR: 127.9, P = 0.94), or ALT (AS: 203.1 vs ACR: 169.5, P = 0.58). There was also no difference between the 2 diagnoses in terms of bile duct diameter (AS: 4.8 mm vs ACR: 3.8 mm, P = 0.36). Patients with concurrent AS and ACR had a mean R factor of 1.06 (0.7).
A total of 3 adverse events occurred after 96 ERCPs (3.1%): 1 case of mild post-ERCP pancreatitis treated conservatively, and 2 cases of post-procedure abdominal pain requiring overnight hospitalization and supportive care. One adverse event occurred after LB, a hepatoportal fistula that required hospitalization and angiography with embolization by Interventional Radiology. There was no significant difference in the adverse event rates due to ERCP or LB (3.1% vs 1.1%, P = 0.31).
It is common to encounter asymptomatic patients with abnormal LFTs in the post-LT setting, as well as symptomatic patients with normal LFTs. It is also common for patients to undergo multiple invasive diagnostic tests as part of the work-up. Abnormal LFTs post-LT are a major cause of unplanned hospital readmissions, and the ensuing work-up may consume significant resources[7]. ERCP is the accepted diagnostic and therapeutic test for suspected biliary pathology and LB is the accepted test for suspected hepatocellular pathology. But in reality, because of the poor specificity of LFT patterns and the limitations of cross-sectional imaging, patients with post-LT LFT elevations will too often undergo both procedures. The timing and order of these procedures is left to the discretion of the transplant surgeon, hepatologist and advanced endoscopist, with little evidence to guide them. Despite the high incidence of immune-mediated and biliary complications following LT, the usual clinical tools (e.g., clinical history, LFT patterns, bile duct diameter on imaging) are poorly specific for any single diagnosis. Besides the main finding of our study, this study demonstrated that patients with AS had no significant difference from patients with ACR in terms of R factor, alkaline phosphatase level, total bilirubin level, AST level, ALT level, or bile duct diameter. Hence, additional testing with LB and ERCP was justified.
Ultrasound and MRCP have variable accuracy in diagnosing biliary pathology post-LT, since obstructive ductal dilation in the transplanted liver is variable. Several studies have demonstrated poor sensitivity and specificity of bile duct diameter post-LT[8-11]. While both modalities can detect biliary dilatation, MRCP offers an advantage over ultrasound in being able to detect biliary strictures with a sensitivity ranging from 64%-79%[9,12]. While both of these modalities are first-line options for imaging in the diagnostic work-up of elevated LFTs after LT, we have found that MRCP both under-estimates and over-estimates stenosis size and severity. Additionally, ERCP permits a real-time accurate assessment of strictures, based on contrast drainage and balloon passage, and the ability to perform stricture therapy. For these reasons, we generally go straight to ERCP and bypass MRCP when there is significant ductal dilation, a cholestatic pattern of LFTs, or a negative LB.
To our knowledge, this is the largest study evaluating the diagnostic performance of combined LB and LT in patients with abnormal LFTs after LT. Our novel finding in this study is the high diagnostic accuracy for ERCP and LB, in contrast to standard laboratory tests or cross-sectional imaging. Diagnostic accuracy was 79.1% overall for ERCP and 93.4% overall for LB. Combined, the 2 tests study had an overall diagnostic accuracy of 100%.
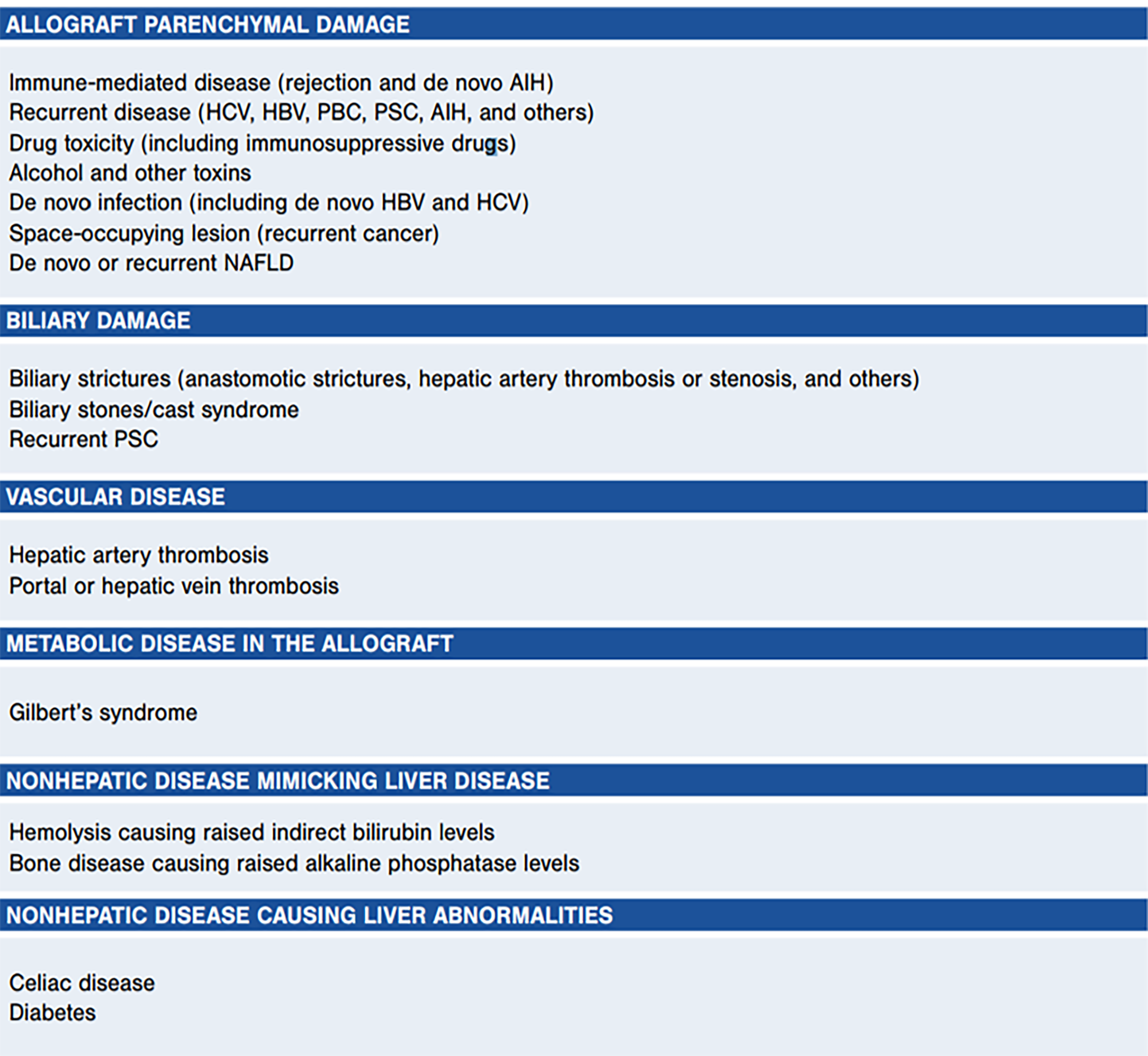
ACR and AS were the most frequent final diagnoses in our patients. These are commonly encountered diseases in the LT population, but the differential diagnosis remains broad (Figure 4) and includes de novo autoimmune hepatitis, recurrent liver disease (HCV, PSC, others), drug toxicity, de novo infection, biliary stones or casts, hepatic artery thrombosis, and more[2]. We recognize that a previously common clinical dilemma–differentiating recurrent HCV from ACR or other etiologies–is less common in the current direct-acting antiviral (DAA) era, and our study included patients in the current and pre-DAA eras.
In the early days of LT, ACR was a near-universal complication resulting in long-term graft failure[13,14]. Advances in immunosuppression have subsequently led to reduced rates of allograft rejection, though the incidence still ranges from 20% to 40% after LT, with most occurring within the first month[15-17]. In addition, ACR remains clinically significant, impacting long-term graft survival and mortality[18]. The incidence of biliary complications after LT is highly variable but still relatively common. The estimated incidence of AS post-LT is up to 20% for patients following deceased donor LT and 19%-40% after living donor liver transplantation. Risk factors include graft ischemia, DD anastomosis, reperfusion injury, deceased donor, and hepatic artery thrombosis. The incidence of non-anastomotic stricture is 0.5% to 10%, while stones/sludge are seen post-LT in approximately 5% of patients. Biliary cast syndrome is less common (2.5%-3%)[19-22].
It is critical to make a prompt and diagnosis when a transplanted patient presents with abnormal LFTs, since graft survival depends on timely and appropriate treatment. While ACR is successfully treated with various combinations of immunosuppressive medication, the management of biliary complications is procedural. AS may be treated successfully with endoscopic placement of multiple plastic stents or a covered metal stent. Recent data suggests that metal stents incur fewer procedures and costs while leading to stricture resolution similarly to plastic stents[23].
Our study sheds light on the frequency of dual diagnoses in patients with abnormal LFTs post-LT, which is an under-studied phenomenon. In this study, 34 (37.4%) patients had multiple diagnoses, of which the most common combination was AS plus ACR (14.3%). Four patients (4.4%) ultimately received 3 final diagnoses. In practice, patients receive therapy for multiple diseases concurrently (e.g. stenting for AS plus corticosteroid bursts for AS), so knowing which diagnosis is dominant can be challenging. Previous studies assessing abnormal LFTs in the post-LT population mostly included patients undergoing LB or ERCP but not both, so our study may represent more complex, sicker patients[7]. Alternatively, some of the various diagnoses in our patients may be clinically silent. AS, for example, is quite subjective and may be diagnosed or treated by endoscopists even though the stricture may not be high-grade or impede bile flow.
Our findings suggest that physicians managing post-LT patients can have a lower threshold to perform both LB and ERCP when evaluating abnormal LFTs within the context of the patient’s clinical presentation. While one modality alone has high diagnostic accuracy over lab tests and imaging, LB and ERCP combined have a very high diagnostic accuracy. Ultimately the decision to perform one test over the other depends on clinician experience, but both tests improve the diagnostic accuracy over one test alone. However, despite the high prevalence of multiple final diagnoses (37.4%), only 96 of 1284 transplanted patients at our center underwent both ERCP and LB during the study period, suggesting they are used sparingly overall. Finally, the adverse event rates of ERCP and LB are low, and we demonstrated no significant difference between the two.
This study was limited by its size and design. It was performed at a single, United States tertiary care hospital with experienced endoscopists and transplant hepatologists, so the results may not be generalizable to other centers. The final diagnosis was determined by review of the medical record and hence may be affected by bias or subjectivity amongst the various treating physicians. Moreover, a reproducible, objective grading score for AS has not been established. The study was also limited by its retrospective nature and by limiting the analysis to patients undergoing ERCP and LB early after LT during the 17-year study period. An additional limitation is the variable time gap between ERCP and LB, although across the entire study population the mean time interval between both procedures was relatively short (9.1 d) suggesting that the diagnostic evaluation typically occurred during a single clinical episode. Despite these limitations, our cohort represents the modern-day practice of ERCP and LB after LT, and the study permits a comparison between the 2 key diagnostic tests in the most common clinical scenarios. Future studies may include a prospective evaluation of abnormal LFTs post-LT or outcomes of post-LT patients who undergo empiric treatment without LB or ERCP.
In summary, these results offer insight into the diagnostic and etiology of abnormal LFTs after LT, in which standard lab and imaging studies have poor specificity. Our study shows that LB and ERCP improve diagnostic accuracy over either test alone and carry low risk. Dual diagnoses are relatively common in this population. In the future, prospective and multicenter studies should include patients undergoing LB and ERCP beyond the early post-LT period and establish reproducible, objective criteria for the ultimate diagnosis.
Elevated liver function tests (LFTs) are commonly encountered in the post-liver transplant (LT) setting. When a diagnosis is not made by history, labs, and cross-sectional imaging, endoscopic retrograde cholangiopancreatography (ERCP) and liver biopsy (LB) are commonly performed. However, the diagnostic performance of each of these tests individually and in combination remains unknown.
We first hoped to determine what are the most common diagnoses in the population of patients with elevated LFTs after LT. At the same time, we want to assess the diagnostic performance of both ERCP and LB in these patients so that we can decide which of these tests is safer and more effective at clinching the diagnosis.
We aimed to assess the diagnostic accuracy and safety of ERCP and LB together and in isolation for a final diagnosis in patients with unexplained LFT elevations after LT.
In this single-center, retrospective study we evaluated patients undergoing both ERCP and LB for the evaluation of elevated LFTs within 6 mo of LT based on review of existing medical records. Diagnostic accuracy, sensitivity and specificity for the various final diagnoses were calculated for each test.
Anastomotic strictures (AS), acute cellular rejection (ACR) and concurrent AS and ACR were the most common diagnoses. ERCP carried an accuracy of 79.1%, LB had an accuracy of 93.4%, and the combination of the 2 had an accuracy of 100% (95%CI: 96-100). The pattern of liver chemistries (R Factor) did not diagnostic accuracy of either test. Adverse event rates did not differ between the 2 tests.
While LB had a higher accuracy than ERCP, the combination of the 2 tests had an accuracy of 100% and a low adverse event rate, suggesting that physicians can have a low threshold in utilizing both modalities for the evaluation of elevated LFTs.
In patients with elevated LFTs after LT without a diagnosis, neither LB nor ERCP is clearly superior. Both tests can be used and the decision to use one over the other will depend on the clinical context and physician preference. However, when necessary both tests can be used safely together to reach a final diagnosis in nearly all patients.
| 1. | Services USDoHH. Transplants by Donor Type, Organ Procurement and Transplantation Network (OPTN). 2019. Available from: https://optn.transplant.hrsa.gov/. |
| 2. | Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 746] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 4. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1632] [Article Influence: 96.0] [Reference Citation Analysis (2)] |
| 5. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1014] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 6. | DeLeve LD, Kaplowitz N. Mechanisms of drug-induced liver disease. Gastroenterol Clin North Am. 1995;24:787-810. [PubMed] |
| 7. | Russell TA, Angarita SAK, Showen A, Agopian V, Busuttil RW, Kaldas FM. Optimizing the Management of Abnormal Liver Function Tests after Orthotopic Liver Transplant: A Systems-Based Analysis of Health Care Utilization. Am Surg. 2017;83:1152-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | St Peter S, Rodriquez-Davalos MI, Rodriguez-Luna HM, Harrison EM, Moss AA, Mulligan DC. Significance of proximal biliary dilatation in patients with anastomotic strictures after liver transplantation. Dig Dis Sci. 2004;49:1207-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Akbar A, Tran QT, Nair SP, Parikh S, Bilal M, Ismail M, Vanatta JM, Eason JD, Satapathy SK. Role of MRCP in Diagnosing Biliary Anastomotic Strictures After Liver Transplantation: A Single Tertiary Care Center Experience. Transplant Direct. 2018;4:e347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, Singal AG, Taylor JR, Elmunzer BJ. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? Gastrointest Endosc. 2011;73:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Beswick DM, Miraglia R, Caruso S, Marrone G, Gruttadauria S, Zajko AB, Luca A. The role of ultrasound and magnetic resonance cholangiopancreatography for the diagnosis of biliary stricture after liver transplantation. Eur J Radiol. 2012;81:2089-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Nacif LS, Pinheiro RS, Pécora RA, Ducatti L, Rocha-Santos V, Andraus W, D'Albuquerque LC. Late acute rejection in liver transplant: a systematic review. Arq Bras Cir Dig. 2015;28:212-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Thurairajah PH, Carbone M, Bridgestock H, Thomas P, Hebbar S, Gunson BK, Shah T, Neuberger J. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation. 2013;95:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Campsen J, Zimmerman MA, Mandell S, Kaplan M, Kam I. A Decade of Experience Using mTor Inhibitors in Liver Transplantation. J Transplant. 2011;2011:913094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Wang YC, Wu TJ, Wu TH, Lee CF, Chou HS, Chan KM, Lee WC. The risk factors to predict acute rejection in liver transplantation. Transplant Proc. 2012;44:526-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | González MG, Madrazo CP, Rodríguez AB, Gutiérrez MG, Herrero JI, Pallardó JM, Ortiz de Urbina J, Paricio PP. An open, randomized, multicenter clinical trial of oral tacrolimus in liver allograft transplantation: a comparison of dual vs. triple drug therapy. Liver Transpl. 2005;11:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Levitsky J, Goldberg D, Smith AR, Mansfield SA, Gillespie BW, Merion RM, Lok AS, Levy G, Kulik L, Abecassis M, Shaked A. Acute Rejection Increases Risk of Graft Failure and Death in Recent Liver Transplant Recipients. Clin Gastroenterol Hepatol 2017; 15: 584-593. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 19. | Albert JG, Filmann N, Elsner J, Moench C, Trojan J, Bojunga J, Sarrazin C, Friedrich-Rust M, Herrmann E, Bechstein WO, Zeuzem S, Hofmann WP. Long-term follow-up of endoscopic therapy in stenosis of the bilio-biliary anastomosis associated with orthotopic liver transplantation. Liver Transpl. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (4)] |
| 22. | Moy BT, Birk JW. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J Clin Transl Hepatol. 2019;7:61-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 23. | Martins FP, De Paulo GA, Contini MLC, Ferrari AP. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation: a randomized controlled trial. Gastrointest Endosc 2018; 87: 131.e1-131. e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 485] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy; American College of Gastroenterology; and American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Reichert MC, Tajiri K S-Editor: Fan JR L-Editor: A P-Editor: Wang LL