Published online Aug 27, 2020. doi: 10.4254/wjh.v12.i8.506
Peer-review started: April 13, 2020
First decision: April 29, 2020
Revised: July 9, 2020
Accepted: July 26, 2020
Article in press: July 26, 2020
Published online: August 27, 2020
Processing time: 133 Days and 14.9 Hours
Non-alcoholic fatty liver disease (NAFLD) has a heterogeneous distribution across racial and ethnic groups, with a disproportionate burden among Hispanics. Although there are currently no approved therapies for treatment of NAFLD, several therapies have been investigated in clinical trials.
To analyze the inclusion of racial and ethnic minority groups in clinical trials for NAFLD.
We performed a systematic review of North American, English-language, prospective studies for NAFLD therapies published from 2005 to 2019. Racial and ethnic enrollment data were recorded for each eligible study. Meta-analysis was performed to compute pooled prevalence of different racial and ethnic groups, followed by further subgroup analyses. These analyses were based on diagnosis of non-alcoholic steatohepatitis (NASH) and timing of study on enrollment by ethnicity. Descriptive statistics were performed to compare racial and ethnic study enrollment to previously reported NAFLD population prevalence.
Thirty-eight studies met criteria for inclusion in the systematic review. When reported, median age of enrolled subjects was 49 years (range 41.5-58) with 56% female participants. NAFLD was defined through biopsy findings in 79% (n = 30) of the studies. Of the included articles, treatment modalities ranged from medications (n = 28, 74%), lifestyle interventions (n = 5, 13%), bariatric surgery (n = 4, 11%) and phlebotomy (n = 1, 2%). Twenty-eight studies (73%) included racial and/or ethnic demographic information, while only 17 (45%) included information regarding Hispanic participation. Of the 2983 patients enrolled in all eligible trials, a total of only 346 (11.6%) Hispanic participants was reported. Meta-analysis revealed a pooled Hispanic prevalence of 24.3% (95% confidence interval 16.6-32.0, I2 94.6%) among studies documenting Hispanic enrollment. Hispanic enrollment increased over time from 15% from 2005-2014 to 37% from 2015-2019.
In a meta-analysis of NAFLD trials, documentation of racial/ethnic demographic data occurred in less than half of studies. Standardization of reporting of race/ethnicity and targeted interventions toward minority recruitment are needed to improve diversity of enrollment.
Core tip: The Hispanic population in the United States is disproportionately affected by non-alcoholic fatty liver disease (NAFLD). Currently, there is no Food and Drug Administration approved treatment for this disease, but several clinical trials are investigating new potential therapies. This study evaluates the inclusion of race and ethnicity in the enrollment of these trials. In a systemic review and meta-analysis of clinical trials for treatment of NAFLD, 44% of eligible trials reported data on race and ethnicity. Despite a high burden of disease, Hispanic participation remained low. Future targeted interventions must take place to increase the enrollment of diverse and representative study populations in clinical trials.
- Citation: Patel P, Muller C, Paul S. Racial disparities in nonalcoholic fatty liver disease clinical trial enrollment: A systematic review and meta-analysis. World J Hepatol 2020; 12(8): 506-518
- URL: https://www.wjgnet.com/1948-5182/full/v12/i8/506.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i8.506
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States, affecting up to 25% of the global adult population[1]. NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), with some patients experiencing eventual cirrhosis. Given the rising incidence, NAFLD is poised to become the leading indication for liver transplantation in the coming years[2].
Risk factors for the development of NAFLD include insulin resistance and metabolic syndrome (encompassing elevated fasting glucose levels, hypertension, dyslipidemia, and central obesity). However, not all individuals with these risk factors develop NAFLD. In a recent systematic review and meta-analysis, heterogeneity in NAFLD burden between racial and ethnic groups was noted, with the highest prevalence seen in Hispanic populations (pooled prevalence 22.9%)[3].
Although it remains unclear why Hispanics are at a higher risk of developing NAFLD and NASH, there is likely an interplay of multifactorial causes. Genetic risk factors play a large role in the pathogenesis of NAFLD. Studies have shown the single nucleotide polymorphisms in patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), and membrane bound O-acyl transferase (MBOAT) play various roles in different races[4]. For example, the isoleucine to methionine substitution at position 148 (I148M) variant in PNPLA3, has been strongly linked to hepatic fat content. This variant occurs more frequently in Hispanics (49%) compared to non-Hispanic whites (23%) or African Americans
Although weight loss through lifestyle interventions or bariatric surgery can reverse the effects of NAFLD, there are currently no Food and Drug Administration (FDA) approved therapies for the treatment of NAFLD. Several promising therapies are currently being investigated in clinical trials. Although the burden of NAFLD on Hispanics is significant, it is unknown if this population is represented in these clinical trials. Identifying possible racial disparities is the first step in improving targeted interventions for patient subgroups. The aim of this study was to evaluate the enrollment of Hispanics in NAFLD trials conducted in the United States and Canada. We hypothesized that the expected rate of Hispanics in NAFLD therapy trials should be proportionate to the burden of disease among Hispanics within the NAFLD population.
The literature search was performed using the PubMed (United States National Institutes of Health, Bethesda, MD, United States) database from January 1, 2005 to March 31, 2019. Three index search terms for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and fatty liver were combined. Other potential studies were identified from reference lists of previously published review articles. The search was restricted to English-language articles. Conference abstracts were excluded. Three investigators (Patel P, Muller C and Paul S) reviewed articles for study inclusion. Discrepancies were resolved by consensus.
Published studies of patients with NAFLD or NASH receiving any therapeutic intervention were included. NAFLD and NASH were independently defined by each study, usually either by imaging or histology.
Randomized controlled trials (RCTs) or prospective cohort studies conducted in the United States and Canada with human subjects aged 18 years or older were included. Retrospective studies, case-control, case series, case reports, reviews, and studies with non-human subjects or non-English language were excluded. Three investigators (Patel P, Muller C and Paul S) reviewed articles for study inclusion with discrepancies resolved by consensus. All data were extracted by 1 researcher and verified by another independent researcher and included study author, country, publication date, study design, intervention, sex, age, and race and/or ethnicity. Enrollment demographic information regarding race and ethnicity, when available, was recorded as defined in each individual study. For the purposes of analysis, ethnicity referred to designations of “Hispanic” or “non-Hispanic”, reported along with an independent racial designation for each participant.
An assessment of risk of bias was not performed as we had a heterogenous inclusion criteria, and a risk assessment is not applicable to our study design. Additionally, given the framework of our research question, we have demonstrated that these studies are, in fact, biased towards patient selection.
NAFLD prevalence data was obtained using a recent systematic review and meta-analysis that examined racial and ethnic disparities in NAFLD prevalence among adult patients in the United States through August 2, 2016[3]. In this study, the prevalence of NAFLD in the Hispanic population was 22.9% compared to 14.4% in white persons and 13.0% in black persons[3]. Additionally, the prevalence of NASH followed similar trends in this analysis with Hispanics disproportionately affected with a prevalence of 45.4%[3].
Descriptive statistics were performed with frequencies and proportions reported. Two-tailed z-test was performed to compare differences in proportions. All meta-analyses were performed using random effects models and results were pooled using the maximum likelihood estimation. The arcsine transformation was used to estimate the absolute proportion of Hispanics participating in each study. Study heterogeneity was assessed using the Cochrane I2 statistic. All statistical analyses were performed using OpenMeta software. The statistical methods of this study were reviewed by Dr. Sonali Paul.
Prespecified subgroup analyses explored differences in Hispanic trial participation by specifically a diagnosis of NASH, mode of NAFLD diagnosis, and type of therapeutic intervention. Further subgroup analyses examined the effect of study design (RCT versus prospective cohort) on enrollment by ethnicity.
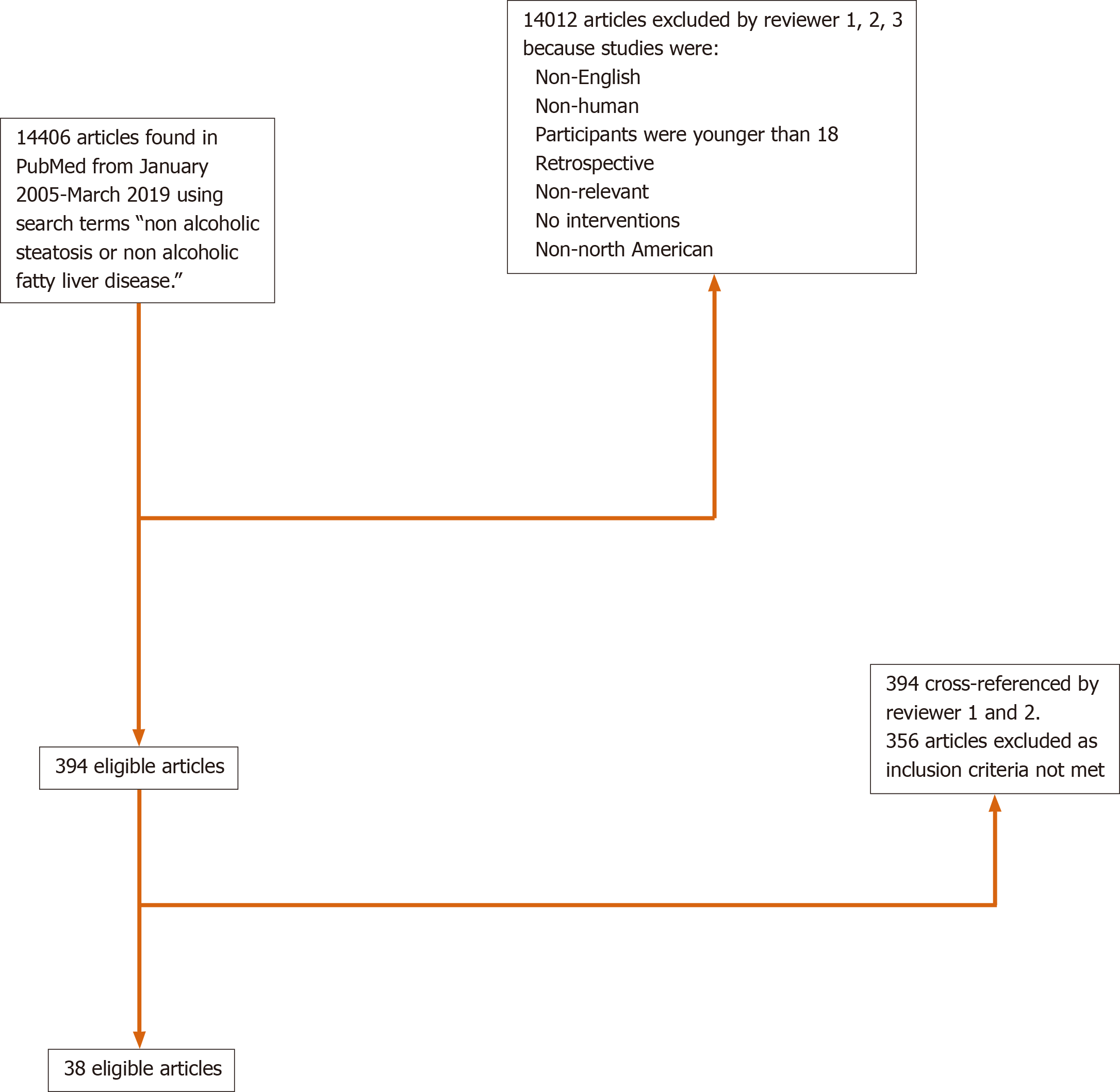
The search strategy yielded 14406 citations using the relevant search terms, with 38 meeting eligibility criteria (Figure 1). Thirty-two studies (84%) were conducted in the United States, 4 studies (11%) performed in Canada, and 2 studies (5%) were multinational (Table 1). Twenty-six (68%) studies were randomized controlled trials and 12 (32%) were prospective cohort or open label studies. When reported, median age of enrolled subjects was 49 years old (range 41.5-58) with 56% female participants. NAFLD was defined through biopsy findings in 79% (n = 30) of the studies. Of the included articles, treatment modalities ranged from medications (n = 28, 74%), lifestyle interventions (n = 5, 13%), bariatric surgery (n = 4, 11%) and phlebotomy (n = 1, 2%).
| Year | Author | Study design | NAFLD or NASH | How NAFLD defined (ultrasound/biopsy) | Intervention | Total enrolled | % Men | Median age (yr) | Reporting of Race | Reporting of ethnicity | % White | % Black | % Hispanic | % Asian | % Other | Unknown |
| 2005 | Huang et al | Uncontrolled, open-label trial | NASH | Biopsy | Dietary intervention/ counseling | 23 | 47.8 | 48 | N | Y | 87% | 0% | 13% | 0% | 0% | 0% |
| 2005 | Clark et al | Prospective cohort | NAFLD | Biopsy | Roux-en-Y | 16 | 50.0 | 43 | Y | N | 88% | NR | NR | NR | NR | 12%3 |
| 2006 | Barker et al | Retrospective cohort | NASH | Biopsy | Roux-en-Y | 19 | 10.5 | 49 | N | N | NR | NR | NR | NR | NR | NR |
| 2006 | Browning et al | Prospective cohort | NAFLD | Imaging (MRI)2 | Statins | 268 | 44.0 | 54 | Y | Y | 38% | 50% | 10% | 0% | 2% | 0% |
| 2006 | Belfort et al | RCT | NASH | Biopsy | Pioglitazone | 47 | 44.7 | 51 | N | N | NR | NR | NR | NR | NR | NR |
| 2007 | Balas et al | RCT | NASH | Biopsy | Pioglitazone | 35 | 54.3 | 48 | N | N | NR | NR | NR | NR | NR | NR |
| 2007 | Lutchman et al | Uncontrolled, open-label trial | NASH | Biopsy | Discontinuation of pioglitazone | 13 | 54.0 | 41.5 | Y | Y | 84% | 0% | 8% | 8% | 0% | 0% |
| 2009 | Loomba et al | Uncontrolled, open-label trial | NASH | Biopsy | Metformin | 26 | 50.0 | 44 | Y | Y | 65% | 0% | 15% | 19% | 0% | 0% |
| 2010 | Chalasani et al | RCT | NASH | Biopsy | Pioglitazone/ Vitamin E | 247 | 40.0 | 46 | Y | Y | NR | NR | 15% | NR | NR | 85%4 |
| 2011 | Foster et al | RCT | NAFLD | CT | Atorvastatin | 80 | 71.0, 77.5 with NAFLD | 59 | Y | Y | 93% | 2% | 2% | 2% | 0.5% | 0% |
| 2011 | Van Wagner et al | RCT | NASH | Biopsy | Pentoxyfylline | 30 | 43.3 | 50.5 | Y | Y | 80% | 0% | 17% | 3% | 0% | 0% |
| 2011 | Zein et al | RCT | NASH | Biopsy | Pentoxyfylline | 55 | 69.1 | 50 | Y | N | 93% | NR | NR | NR | NR | 7%3 |
| 2011 | Torres et al | RCT | NASH | Biopsy | Rosiglitazone/ metformin | 108 | 50.4 | 49 | Y | Y | 65% | 4% | 22% | 4% | 5% | |
| 2012 | Le et al | RCT | NASH | MRI | Colesevelam | 50 | 46.0 | 47 | Y | Y | 38 | 0% | 28% | 22 | 8 | 0% |
| 2012 | Zein et al | RCT | NASH | Biopsy | Pentoxyfylline | 47 | 70.2 | 50 | Y | N | 92% | NR | NR | NR | NR | 8%3 |
| 2012 | Sullivan et al | RCT | NAFLD | Biopsy | Exercise | 18 | 27.8 | 48 | N | N | NR | NR | NR | NR | NR | NR |
| 2012 | Fealy et al | RCT | NAFLD | Imaging (MRI) | Exercise | 13 | NR | 58 | N | N | NR | NR | NR | NR | NR | NR |
| 2013 | Mudaliar et al | RCT | NAFLD | Biopsy | Obeticholic acid | 64 | 51.6 | 52 | Y | Y1 | 42% | 28% | 25% | 5% | 0% | 0% |
| 2013 | Beaton et al | Uncontrolled, open-label trial | NAFLD or NASH | Biopsy | Phlebotomy | 31 | 61.3 | 49 | N | N | NR | NR | NR | NR | NR | NR |
| 2014 | Sanyal et al | RCT | NASH | Biopsy | EPA-E | 243 | 39.1 | 48 | Y | N | 91% | 3% | 0% | 0% | 6% | 0% |
| 2015 | Dasarthy et al | RCT | NASH | Biopsy | Omega 3 fatty acids | 37 | 21.6 | 50 | Y | Y | 92% | 3% | 5% | 0% | 0% | 0% |
| 2015 | Argo et al | RCT | NASH | Biopsy | N-3 fish oil | 34 | 38.2 | 46 | Y | N | 97% | NR | NR | NR | NR | 3%3 |
| 2015 | Loomba et al | RCT | NASH | Biopsy | Ezetemibe | 50 | 38.0 | 49 | Y | Y | NR | NR | 34% | NR | NR | 66%4 |
| 2015 | Neuschwander et al | RCT | NAFLD | Biopsy | Obeticholic acid | 283 | 33.9 | 51 | Y | Y | 83% | 2% | 15% | 6% | 10% | 0% |
| 2015 | Vilar-Gomez et al | Prospective cohort | NASH | Biopsy | Bariatric surgery | 293 | 41.0 | 48 | Y | N | 98% | NR | NR | NR | NR | 2%3 |
| 2015 | Glass et al | Prospective cohort | NASH | Biopsy | Weight loss | 45 | 28.9 | 46 | N | N | NR | NR | NR | NR | NR | NR |
| 2016 | Harrison et al | RCT | NASH | Biopsy | GT020 (galectin 3 protein inhibitor) | 31 | 54.8 | 54 | N | NR | NR | NR | NR | NR | NR | NR |
| 2016 | Cusi et al | RCT | NASH | Biopsy | Pioglitazone | 101 | 70.3 | 51 | Y | Y | 25% | NR | 67% | NR | 8% | 0% |
| 2016 | Cui et al | RCT | NAFLD | MRI | Sitagliptin | 84 | 41.7 | 53.5 | N | Y | 32% | NR | 36% | NR | NR | NR |
| 2016 | Ratziu et al | RCT | NASH | Biopsy | Elafibranor (PPAR agonist) | 274 | 55 | 52 | Y | N | 89% | NR | NR | NR | NR | NR |
| 2017 | Winn et al | RCT | NAFLD | MRI | Exercise | 21 | NR | 46 | N | N | NR | NR | NR | NR | NR | NR |
| 2017 | Joy et al | RCT | NASH | Biopsy | Sitagliptin | 12 | 41.7 | 56 | Y | N | 92% | NR | NR | NR | NR | 8.3%3 |
| 2017 | Loomba et al | RCT | NASH | Biopsy | Selonsertib (ASK1 inhibitor) | 72 | 31 | 54.2 | Y | N | 90% | NR | NR | NR | NR | 10%3 |
| 2017 | Lawitz et al | Prospective cohort | NAFLD | MRI | Acetyl-CoA carboxylase inhibitor (GS-0976) | 20 | 55 | 45 | Y | N | 100% | 0% | NR | 0% | 0% | NR |
| 2018 | Shiffman et al | RCT | NAFLD | Biopsy or MRI | Emricasan | 38 | 63.2 | NR | Y | N | 89% | NR | NR | NR | NR | 11%3 |
| 2018 | Schwenger et al | Prospective cohort | NAFLD | Biopsy | Bariatric surgery | 42 | 23.8 | 48 | N | N | NR | NR | NR | NR | NR | NR |
| 2018 | Chalasani et al | RCT | NAFLD | MRI | Leucine/metformin/sildenafil | 70 | 44.3 | 46 | Y | Y | 63% | 4.2% | 27% | 2.9% | 1.4% | 2.9% |
| 2019 | Harrison et al | Prospective cohort | NASH | Biopsy | FGF19 analog (NGM282) | 43 | 20.9 | 50 | N | Y | NR | NR | 76.7% | NR | NR | 23.34 |
Of the 38 identified trials, 25 (66%) included racial data with a total of 2531 total enrolled patients. Twenty-one (84%) trials were conducted in the United States, 2 (8%) trials were performed in Canada and 2 (8%) were multinational trials. The median age of enrolled patients was 49.5 years (range 41-58). NAFLD was diagnosed by biopsy in 80% (n = 20) of the trials, with 20% (n = 5) diagnosed by imaging. Interventions included medications (n = 23, 92%) or bariatric surgery (n = 2, 8%) (Table 1).
Among the 38 eligible trials, only 17 (44.7%) included information regarding patient ethnicity. Of the 2983 patients enrolled in all eligible trials, a total of only 346 (11.6%) Hispanic participants was reported. Among the 25 studies that included data on race, 14 included data on Hispanic participation. Of note, 3 studies that did not have racial data did provide data on Hispanic participation (Table 1).
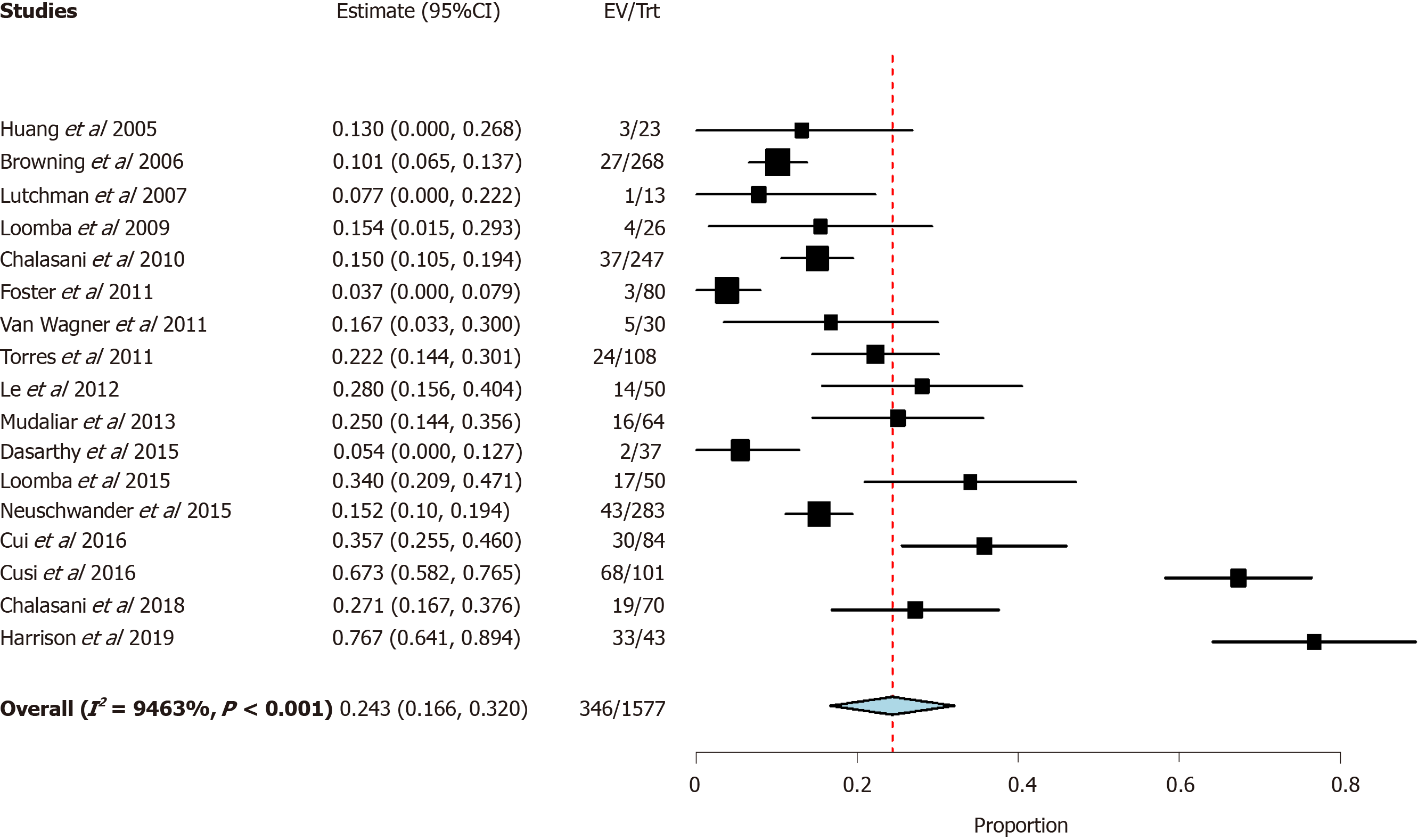
Among the 17 trials that reported Hispanic participation, there were 346 Hispanic patients out of 1577 total enrolled patients with a participation rate of 21.9% compared to 74.8% of Caucasian participants among those including data on Caucasian participation. The 21.9% unadjusted pooled prevalence of Hispanic trial participants was similar to the 22.8% unadjusted pooled NAFLD Hispanic prevalence (990/4332 total patients) in the recent systematic review by Rich et al[3] (P = 0.365).
A meta-analysis was then performed to estimate pooled prevalence while taking heterogeneity of included studies into consideration. The pooled prevalence was found to be 24.3% [95% confidence interval (CI) 16.6-32.0] with significant heterogeneity (I2 = 94.6%) (Figure 2).
Further sub-group meta-analyses were performed in patients with biopsy proven NASH and found Hispanic participation to be 24.7% (Table 2), considerably lower than the 45.4% prevalence of NASH in the Hispanic population found in the recent meta-analysis by Rich et al[3]. Caucasian and African American participation in studies using NASH as inclusion criteria, was slightly lower than those of NAFLD studies (67.3% vs 63.9% and 8.0% vs 2.7%, respectively).
| NASH | ||
| Prevalence (%) | 95%CI | |
| Hispanic | 24.7 | 9.1-40.4 |
| White persons | 63.9 | 42.4-85.5 |
| Black persons | 2.7 | 0.5-4.9 |
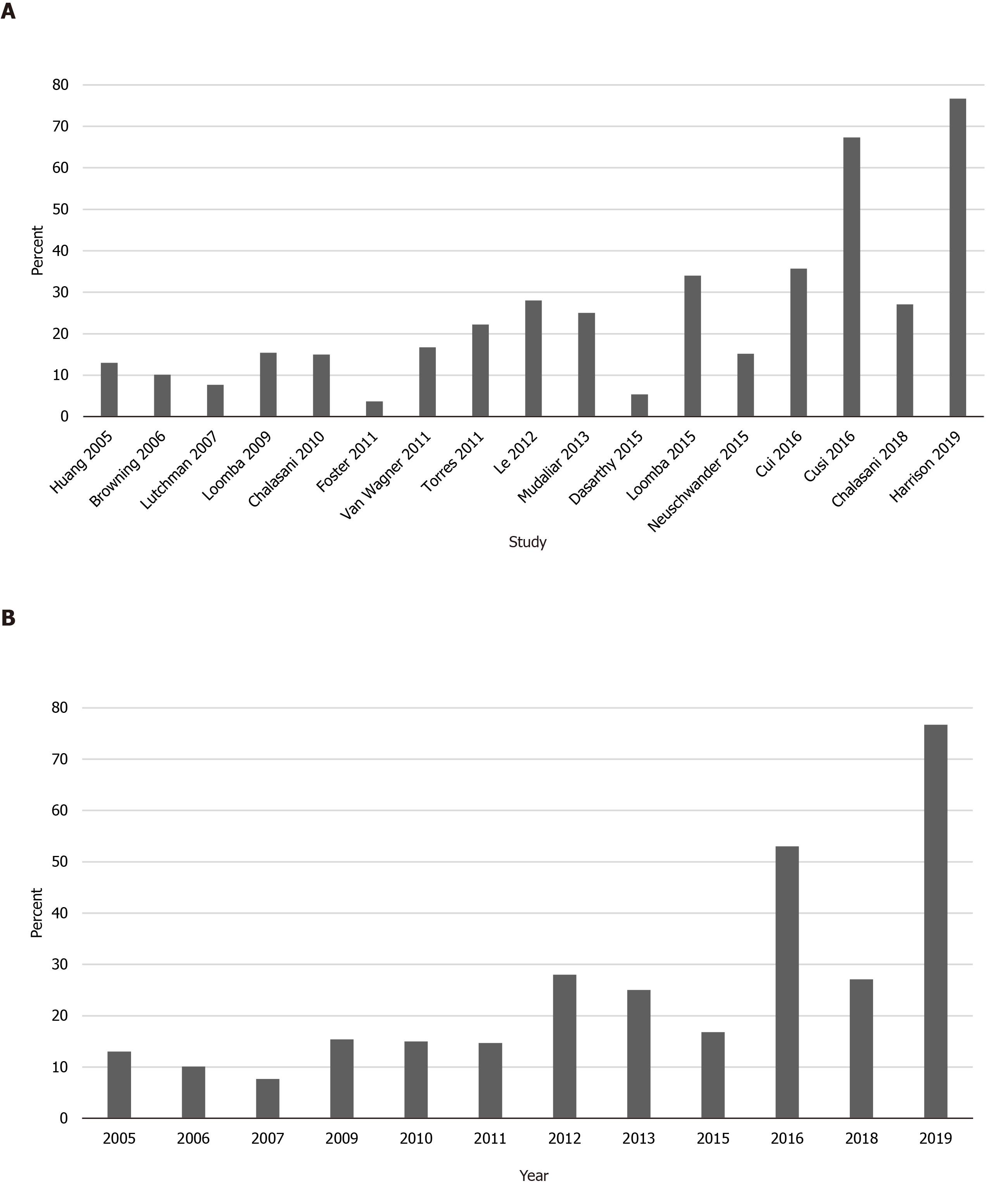
To determine if rates of Hispanic enrollment changed over time, studies conducted before and after 2015 were compared. The pooled prevalence of Hispanic patients in studies from 2005-2014 was 15%, compared to 37% for studies from 2015-2019. Trends in Hispanic study participation over time are displayed in Figure 3.
The purpose of this systematic review and meta-analysis was to characterize the participation rate of Hispanic patients in clinical trials investigating therapies for NAFLD. Despite the importance of genetics and race in the prevalence of NAFLD, our results show that racial/ethnic demographic data are under-reported, with only 25 of 38 (66%) eligible clinical trials reporting race or ethnicity. Both the FDA and the National Institutes of Health (NIH) have published recommendations on how to report race and ethnicity data in clinical trials, however in practice these guidelines are not strictly followed or enforced[5,6]. Previous studies have demonstrated reporting of race/ethnicity to be similarly suboptimal in clinical trials across several specialties[7,8]. An analysis of clinical trial enrollment for several disease processes (spanning general medicine, oncology, cardiovascular disease, and infectious diseases) from 2009, found that 21% of studies failed to include racial or ethnic demographic data[7].
In this review, of the 38 trials that met eligibility criteria, 25 reported racial information. Among these only 17 (68%) provided data on ethnicity (participation of Hispanic patients). It is well established that the prevalence of NAFLD and NASH is higher among Hispanic patients than among either non-Hispanic whites or other minority groups, with a prevalence of 25%-40% and 25%, respectively[9-13]. Although Hispanic participation among trials that included information about Hispanic enrollment (24.3%) was close to that of the United States Hispanic population
Under-reporting of Hispanic trial participants could be in part due to heterogeneity in self-reported ethnicity among Hispanic patients and diversity of their country of origin and race[15]. Health sciences typically follow the United States Census practice of categorizing “Hispanic” as an ethnicity that is distinct from race. This practice can result in discordance between patients’ self-perceived race/ethnicity and their associated categorization in health systems in addition simplification of a diverse, heterogenous group, and inaccuracy in reporting[15,16].
Heterogeneity of enrollment practices for the included trials is also likely contributing significantly to Hispanic under-enrollment. Hispanic enrollment (among those reporting any Hispanic participants) ranged from 4%-67%. The I2 statistic of 94% highlights the significant heterogeneity of Hispanic enrollment among studies included in this meta-analysis. The finding that 10 of the 17 trials (59%) including data on Hispanic enrollment were conducted in states that shared a border with Mexico[17-26] highlights the opportunistic, rather than systematic, nature of trial recruitment and enrollment. The generalizability of such clinical trials is significantly compromised when they fail to include information about key demographics.
When comparing racial and ethnic enrollment between studies using NAFLD and NASH as inclusion criteria, we found that Hispanic enrollment in NASH trials increased relative to enrollment of Caucasian and African American participants. These findings are consistent with those of prior studies demonstrating that Hispanic NAFLD patients are more likely to progress to steatohepatitis than Caucasians or African Americans[3,27]. However, given that 45% of Hispanic NAFLD patients experience progression to steatohepatitis, compared to 32% and 20% of Caucasian and African American NAFLD patients, respectively[3], Hispanics are likely even more under-represented in studies of NASH relative to the disease burden in that population. The observed proportion of African American NASH participants in our study was particularly low (2.7%), but is likely a reflection of the low rate of African American enrollment in included studies in general.
Acknowledging the importance of diverse trial participation to generalizability of findings in the development of new therapies, the NIH has stipulated that all sponsored clinical trials include women and minority patients since 1993[7]. Aside from ethical issues related to equity and justice, racial differences in response to pharmacologic therapy identified in diverse trials have been used to guide current clinical practice[28,29]. Recent work suggesting differences in the underlying genetic contributions to NAFLD in patients of different racial/ethnic backgrounds[30,31] highlight the importance of diversity in clinical trial participation. Genetic variants on PNPLA3, TM6SF2, and neurcan (NCAN) can increase the heritability of NAFLD by up to 27% within families. A missense mutation of PNPLA3 has a strong association with hepatic fat accumulation and with a higher susceptibility to develop more severe histologic liver damage, irrespective of the degree of obesity or presence of diabetes[32-34]. This variant in PNPLA3 gene has been observed in highest frequency in Hispanics[4].
Despite the benefits of diversity in trial enrollment, minority patients have historically been underrepresented in clinical trials. Barriers to minority participation in clinical trials include mistrust of providers/research, reduced access to healthcare, financial and time constraints, lack of education about clinical trials, and cultural or language differences impairing communication with trial recruiters or providers[35]. Hispanic patients, in particular, have been underrepresented in clinical trials for multiple conditions. In a 2004 analysis of colorectal, lung and prostate cancer studies, Murthy et al[36] found that Hispanic patients only constituted 3.1% trial participants. Compared to African American patients, Hispanic patients are less likely to be aware of or recruited to participate in clinical research[37-39]. Although lack of access to healthcare resources and lower socioeconomic status are shared among multiple minority groups, language barriers create a burden for Hispanic patients in particular. Interventions such as provision of Spanish-speaking recruitment materials or personnel have been shown to improve enrollment of Hispanic patients in clinical trials[40,41] and serve as potential targets for increasing diversity of study populations for NAFLD. In spite of these historic barriers to Hispanic participation in clinical trials, a trend toward increasing Hispanic enrollment over time was observed in our study, with Hispanic enrollment in studies conducted after 2015 nearly triple that of studies from 2005-2014. While these results are encouraging, future efforts are needed to standardize reporting of race/ethnicity in clinical trials and encourage diverse, representative enrollment.
A major limitation of this study is the low rate of reporting demographic data on Hispanic participation among the trials analyzed. Although many trials did not include any racial/ethnic demographic data, the rate of inclusion of data on Hispanic participation was particularly poor (44% of eligible trials). From the information available, it is not known if these trials did not actually recruit any Hispanic participants or if they simply failed to collect or report data on their inclusion.
In conclusion, North American clinical trials of NAFLD from 2015-2019 did not consistently include data on Hispanic participation. Among trials that did include racial/ethnic demographic data, Hispanic patients may be underrepresented relative to the burden of NAFLD and NASH among this population. Future efforts aimed at improving or standardizing reporting of race in clinical trials and at increasing enrollment of diverse and representative study populations are needed to address this disparity.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States and has a heterogeneous distribution across racial and ethnic groups, with a disproportionate burden among Hispanics. Although it remains unclear why Hispanics are at a higher risk of developing NAFLD and nonalcoholic steatohepatitis (NASH), there is likely an interplay of multifactorial causes including genetics, culture, socioeconomic status and environment. Despite this high burden of disease, there are currently no approved therapies for the treatment of NAFLD. Several promising therapies are currently being investigated in clinical trials but it is unknown if Hispanics are appropriately represented in these clinical trials.
Identifying possible racial disparities is the first step in improving targeted interventions for patient subgroups. The purpose of this systematic review and meta-analysis was to characterize the participation rate of different races and ethnicities in clinical trials investigating therapies for NAFLD.
The aim of this study was to evaluate the enrollment of Hispanics in NAFLD trials conducted in the United States and Canada. We hypothesized that the expected rate of Hispanics in NAFLD therapy trials should be proportionate to the burden of disease among Hispanics within the NAFLD population.
The literature search was performed using the PubMed (US National Institutes of Health, Bethesda, MD, United States) database from January 1, 2005 to March 31, 2019 using the following search terms: Nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and fatty liver. Randomized controlled trials (RCTs) or prospective cohort studies conducted in the United States and Canada with human subjects aged 18 years or older were included. Descriptive statistics were performed with frequencies and proportions reported. Two-tailed z-test was performed to compare differences in proportions. All meta-analyses were performed using random effects models and results were pooled using the maximum likelihood estimation.
Of the 38 trials that met eligibility criteria, twenty-five reported racial information. Among these only 17 (68%) provided data on ethnicity (participation of Hispanic patients). Among the 2983 patients enrolled in all eligible trials, a total of only 346 (11.6%) Hispanic participants was reported. Among the 17 trials that reported Hispanic participation, there were 346 Hispanic patients out of 1577 total enrolled patients with a participation rate of 21.9% compared to 74.8% of Caucasian participants among those including data on Caucasian participation. A meta-analysis was then performed to estimate pooled prevalence while taking heterogeneity of included studies into consideration. The pooled prevalence was found to be 24.3% (95%CI: 16.6-32.0) with significant heterogeneity (I2 = 94.6%). To determine if rates of Hispanic enrollment changed over time, studies conducted before and after 2015 were compared. The pooled prevalence of Hispanic patients in studies from 2005-2014 was 15%, compared to 37% for studies from 2015-2019.
North American clinical trials of NAFLD from 2015-2019 did not consistently include data on Hispanic participation. Among trials that did include racial/ethnic demographic data, Hispanic patients may be underrepresented relative to the burden of NAFLD and NASH among this population.
Future efforts aimed at improving or standardizing reporting of race in clinical trials and at increasing enrollment of diverse and representative study populations are needed to address this disparity. It is not known whether the low rate of Hispanic participation in these trials is due to lack of collection of ethnic demographic data on behalf of the investigators, failure to report ethnicity by subjects, or true under-enrollment. Despite the benefits of diversity in trial enrollment, minority patients have historically been underrepresented in clinical trials. Barriers to minority participation in clinical trials include mistrust of providers/research, reduced access to healthcare, financial and time constraints, lack of education about clinical trials, and cultural or language differences impairing communication with trial recruiters or providers. Interventions such as provision of Spanish-speaking recruitment materials or personnel have been shown to improve enrollment of Hispanic patients in clinical trials and serve as potential targets for increasing diversity of study populations for NAFLD.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7936] [Article Influence: 793.6] [Reference Citation Analysis (8)] |
| 2. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 3. | Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:198-210.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 379] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 4. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2701] [Article Influence: 150.1] [Reference Citation Analysis (2)] |
| 5. | US Dept. of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiologic Health. Collection of Race and Ethnicity Data in Clinical Trials. [cited 2018 December 1]. In Food and Drug Administration 2005. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials. |
| 6. | National Institute of Health. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. [cited 2018 December 1]. In NIH Central Resource for Grand and Funding Information. Available from: http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm 2017. |
| 7. | Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt). 2011;20:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Chen MS, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120 Suppl 7:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 366] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 9. | Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Kallwitz ER, Guzman G, TenCate V, Vitello J, Layden-Almer J, Berkes J, Patel R, Layden TJ, Cotler SJ. The histologic spectrum of liver disease in African-American, non-Hispanic white, and Hispanic obesity surgery patients. Am J Gastroenterol. 2009;104:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1-16, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Sherif ZA, Saeed A, Ghavimi S, Nouraie SM, Laiyemo AO, Brim H, Ashktorab H. Global Epidemiology of Nonalcoholic Fatty Liver Disease and Perspectives on US Minority Populations. Dig Dis Sci. 2016;61:1214-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | United States Census Bureau. QuickFacts. [cited 2018 December 1]. Available from: http://www.census.gov/quickfacts/table/PST045215/00. |
| 15. | Caballero AE. Understanding the Hispanic/Latino patient. Am J Med. 2011;124:S10-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2550] [Article Influence: 159.4] [Reference Citation Analysis (18)] |
| 18. | Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, Brenner D, Sirlin C, Loomba R; San Diego Integrated NAFLD Research Consortium (SINC). Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 20. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-82.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 751] [Article Influence: 57.8] [Reference Citation Analysis (4)] |
| 21. | Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, Hooker J, Kono Y, Bhatt A, Hernandez L, Nguyen P, Noureddin M, Haufe W, Hooker C, Yin M, Ehman R, Lin GY, Valasek MA, Brenner DA, Richards L; San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015;61:1239-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 22. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1841] [Article Influence: 167.4] [Reference Citation Analysis (3)] |
| 23. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 772] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 24. | Chalasani N, Vuppalanchi R, Rinella M, Middleton MS, Siddiqui MS, Barritt AS 4th, Kolterman O, Flores O, Alonso C, Iruarrizaga-Lejarreta M, Gil-Redondo R, Sirlin CB, Zemel MB. Randomised clinical trial: a leucine-metformin-sildenafil combination (NS-0200) vs placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:1639-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, Banerjee R, Jaros MJ, Owers S, Baxter BA, Ling L, DePaoli AM. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. Hepatology. 2020;71:1198-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 26. | Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 27. | Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (5)] |
| 28. | Moser M, Lunn J. Responses to captopril and hydrochlorothiazide in black patients with hypertension. Clin Pharmacol Ther. 1982;32:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Yancy CW, Fowler MB, Colucci WS, Gilbert EM, Bristow MR, Cohn JN, Lukas MA, Young ST, Packer M; U. S. Carvedilol Heart Failure Study Group. Race and the response to adrenergic blockade with carvedilol in patients with chronic heart failure. N Engl J Med. 2001;344:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB; Genetics of Obesity-Related Liver Disease (GOLD) Consortium, Nguyen T, Kamel IR, Bonekamp S, Eberhardt MS, Clark JM, Kao WH, Speliotes EK. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2013;11:1183-1190.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Martínez LA, Larrieta E, Kershenobich D, Torre A. The Expression of PNPLA3 Polymorphism could be the Key for Severe Liver Disease in NAFLD in Hispanic Population. Ann Hepatol. 2017;16:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, NASH CRN. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 33. | Speliotes EK, Butler JL, Palmer CD, Voight BF; GIANT Consortium; MIGen Consortium; NASH CRN, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 34. | Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E, Bugianesi E, Maggioni M, Fracanzani AL, Fargion S, Day CP. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 35. | Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF, Powe NR, Bass EB. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 823] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 36. | Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 1728] [Article Influence: 78.5] [Reference Citation Analysis (1)] |
| 37. | Garza MA, Quinn SC, Li Y, Assini-Meytin L, Casper ET, Fryer CS, Butler J, Brown NA, Kim KH, Thomas SB. The Influence of Race and Ethnicity on Becoming a Human Subject: Factors Associated with Participation in Research. Contemp Clin Trials Commun. 2017;7:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Langford A, Resnicow K, An L. Clinical trial awareness among racial/ethnic minorities in HINTS 2007: sociodemographic, attitudinal, and knowledge correlates. J Health Commun. 2010;15 Suppl 3:92-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: Changes over time and sociodemographic disparities. Clin Trials. 2015;12:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Fischer SM, Kline DM, Min SJ, Okuyama S, Fink RM. Apoyo con Cariño: Strategies to Promote Recruiting, Enrolling, and Retaining Latinos in a Cancer Clinical Trial. J Natl Compr Canc Netw. 2017;15:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Sanossian N, Rosenberg L, Liebeskind DS, Starkman S, Eckstein M, Stratton S, Pratt FD, Hamilton S, Kim-Tenser M, Sharma LK, Restrepo L, Valdes-Suieras M, Conwit R, Saver JL; FAST-MAG Investigators and Coordinators. A Dedicated Spanish Language Line Increases Enrollment of Hispanics Into Prehospital Clinical Research. Stroke. 2017;48:1389-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership(s) in Professional Societies: American Gastroenterological Association, No. 1016895; American Association for the Study of Liver Diseases, No. 120137; American College of Gastroenterology, No. 37262.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen HW S-Editor: Liu M L-Editor: A P-Editor: Wang LL