Published online Dec 27, 2020. doi: 10.4254/wjh.v12.i12.1358
Peer-review started: August 1, 2020
First decision: September 21, 2020
Revised: October 4, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: December 27, 2020
Processing time: 134 Days and 2.8 Hours
Hepatitis E virus (HEV) superinfection is a suspected promoting factor for hepatocellular carcinoma (HCC) in patients with chronic hepatitis and cirrhosis. However, to date, very few cases of HEV-related HCC have been reported. Nevertheless, the role of HEV re-infection in cirrhotic liver without other chronic hepatitis infections has rarely been explored.
A 53-year-old male farmer was diagnosed with liver cirrhosis and splenomegaly in August 2016, accompanied with negative HEV-IgM and positive HEV-IgG. No evidence of hepatitis B virus or hepatitis C virus infection was found. Since then the patient was evaluated for liver function and viral parameters every 3 mo. In June 2017, the patient presented severe fatigue with whole body itching and was diagnosed with HCC. Afterwards this patient experienced quick HCC development, progression, relapse, and metastasis in the following 8 mo, and presented persistent dual positivity of HEV-IgM and HEV-IgG. This patient had a long history of smoking and alcohol consumption.
This unique case invokes the importance of HEV surveillance and treatment among cirrhotic patients, HCC cases, and blood donors.
Core Tip: The role of chronic hepatitis E virus (HEV) superinfection in hepatocellular carcinoma (HCC) progression in cirrhotic patients with negative hepatitis B virus (HBV) infection has not been studied. We present herein a unique chronic HEV case with liver cirrhosis who experienced repeated HEV re-infection and rapid HCC development and relapse. This case highlights the importance to investigate the association between HEV re-infection and rapid development of HCC and progression in liver cirrhosis cases, even in the absence of HBV infection. Moreover, routinely detecting HEV infection in high risk occupational group and all blood donors is warranted. Additionally, the treatment for symptomatic and asymptomatic chronic HEV infection is highly suggested.
- Citation: Lin XN, Lin QX, Li SM, Xie KP, Hou J, Chen R. Hepatitis E virus re-infection accelerates hepatocellular carcinoma development and relapse in a patient with liver cirrhosis: A case report and review of literature. World J Hepatol 2020; 12(12): 1358-1366
- URL: https://www.wjgnet.com/1948-5182/full/v12/i12/1358.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i12.1358
To date, four pathogenic hepatitis E virus (HEV) genotypes have been identified. HEV genotypes 1 and 2 are transmitted mainly through the faecal-oral route while HEV genotypes 3 and 4 are likely to spread from infected animals to the human[1,2]. HEV infection has been indicated in certain populations, such as immunocompromised patients or patients with chronic hepatitis B virus (HBV) infection accompanied with or without hepatic decompensation, either as a promoting factor or a cause for the progression of cirrhosis and hepatocellular carcinoma (HCC)[3-6]. However, the impact of HEV infection on the risk of HCC development and progression in cirrhotic patients without HBV infection remains largely unknown. To date, only few chronic HEV-related HCCs have been reported[7], with most of these having existing cirrhosis prior to HEV infection. In addition, to our knowledge the cases with repeated HEV infection have not been reported up to now, which are defined by persistent dual positivity for HEV-IgG and HEV-IgM. Here, we descript a unique case of HEV reinfection in a patient with liver cirrhosis who had rapid HCC development, progression, relapse, and metastasis.
A 53-year-old male farmer presented himself to the clinic of general surgery in June 2017 because of severe fatigue with whole body itching for 10 mo.
In August 2016, the patient was admitted to the hospital due to hematemesis and melena. He had been diagnosed with liver cirrhosis, confirmed by ultrasonography and computed tomography (CT), complicated with esophagogastric varices by gastroscopy and splenomegaly by ultrasonography and CT. This patient had splenectomy in September 2016 and blood transfusion during surgery. The pathological result suggested a chronic congestive splenomegaly. At that moment, the serum test determined that he was anti-HEV IgM negative and IgG positive.
He had no hypertension, diabetes, or other chronic diseases.
The patient had a history of alcohol use with 200 mL daily intake and smoking 20 cigarettes a day for 20 years. Since June 2017 when HCC was diagnosed, the patient had quitted alcohol drinking and smoking. Moreover, he had no family history of cancer.
Initial physical examination demonstrated pale skin with normal blood pressure (85/122 mmHg) and normal heart rate (80/min). No jaundice was observed in the skin and abdominal palpation elicited no pain.
Serum anti-HEV IgG and IgM detection performed by enzyme-linked immunosorbent assay revealed that this patient was HEV-IgM and HEV-IgG double positive at admission in June 2017, and no evidence of hepatitis A virus (HAV), HBV, or hepatitis C virus (HCV) infection was detected (Table 1).
| Time of admission (d) | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| Date of admission | 2016-08-19 | 2017-06-20 | 2017-09-26 | 2017-11-20 | 2018-02-07 | 2018-02-23 | 2018-04-16 |
| Diagnosis | Cirrhosis | HCC | HCC | HCC | HCC | HCC | HCC |
| Treatment | Splenectomy | TACE | RFA | Partial hepatectomy | MWA | TACE and MWA | |
| HEV IgM | Negative | Positive | Positive | Positive | Positive | ||
| HEV IgG | Positive | Positive | Positive | Positive | Positive | ||
| GLU (mmol/L) | 7.81 | 4.49 | 5.82 | 5.04 | 5.44 | 5.31 | 4.67 |
| BUN (mmol/L) | 13.1 | 3.1 | 3.54 | 4.35 | 4.3 | 4.2 | 3.7 |
| CO2_CP (mmol/L) | 23.7 | 21.2 | 22.3 | 24 | 25.4 | 25.9 | 23.5 |
| UA (umol/L) | 334 | 271 | 312 | 293 | 282 | 289 | 300 |
| CREA (umol/L) | 65.5 | 58.4 | 72.8 | 65.25 | 65.7 | 61.1 | 62 |
| ALT (U/L) | 27 | 24 | 40 | 45 | 20 | 20 | 43 |
| GGT (U/L) | 166 | 154 | 224 | 232 | 67 | 65 | 195 |
| ALP (U/L) | 59 | 121 | 119 | 132 | 114 | 104 | 151 |
| ChE (U/L) | 3405 | 3926 | 4545 | 4393 | 3834 | 3515 | 3966 |
| TP (g/L) | 56.5 | 63.2 | 63.8 | 63.8 | 67.6 | 63.2 | 70.7 |
| ALB (g/L) | 27.2 | 29.3 | 30.8 | 31.3 | 30.6 | 29.2 | 32.5 |
| TB (umol/L) | 30.9 | 33.7 | 26.6 | 36.1 | 29.8 | 33.5 | 30.8 |
| DB (umol/L) | 8 | 7.7 | 5.4 | 7.7 | 7.7 | 7 | 7.8 |
| TBA (umol/L) | 2 | 27 | 46 | 22 | 76 | 41 | 32 |
| Na (mmol/L) | 141.6 | 141.2 | 140.7 | 141.9 | 139.3 | 141.4 | 139.9 |
| K (mmol/L) | 3.96 | 3.95 | 3.94 | 4.15 | 4.06 | 3.9 | 4.04 |
| Cl (mmol/L) | 109.6 | 109 | 107.1 | 108 | 109.9 | 109.3 | 107.7 |
| Ca (mmol/L) | 2 | 2 | 2.1 | 2.1 | 2.19 | 2.13 | 2.06 |
| PHOS (mmol/L) | 1.22 | 1.28 | 1.21 | 1.27 | 1.2 | 1.26 | 1.23 |
| Mg (mmol/L) | 0.81 | 0.7 | 0.81 | 0.82 | 0.83 | 0.81 | 0.84 |
| AST (U/L) | 38 | 39 | 49 | 67 | 38 | 37 | 55 |
| CІV (ng/mL) | 151.98 | 169.96 | 162.28 | ||||
| LN (ng/mL) | 32.25 | 82.65 | 60.27 | ||||
| PШP (ng/mL) | 10.9 | 17.33 | 15.82 | ||||
| HA (ng/mL) | 841.13 | 592.83 | 553.67 | ||||
| HBsAg (E) (COI) | 0.481 | 0.478 | 0.452 | 0.575 | 0.386 | ||
| Anti-HBs (E) (IU/L) | 494.2 | 309.7 | 308.1 | 353.5 | |||
| HBeAg (E) (COI) | 0.102 | 0.087 | 0.097 | 0.1 | |||
| Anti-HBe (E) (COI) | 1.27 | 1.12 | 1.15 | 1.23 | |||
| Anti-HBc (E) (COI) | 0.008 | 0.009 | 0.01 | 0.011 | |||
| HBV-DNA (FQ-PCR) (IU/mL) | 10 | 10 | 10 | < 500 | |||
| HAV IgM | Negative | Negative | Negative | Negative | Negative | ||
| Anti-HCV (COI) | 0.03 | 0.04 | 0.04 | ||||
| HIV COM (COI) | 0.21 | 0.22 | 0.24 | ||||
| Syphilis (COI) | 0.08 | 0.07 | 0.08 | ||||
| AFP (A) (ng/mL) | 4.12 | 3.6 | 4.57 | 8.59 | 2.7 | 3.79 | 3.58 |
| CEA (A) (ng/ml) | 4.74 | 4.86 | 4.32 | 5.47 | 4.71 | 5.89 | 5.65 |
| PSA (A) (ng/mL) | 0.1 |
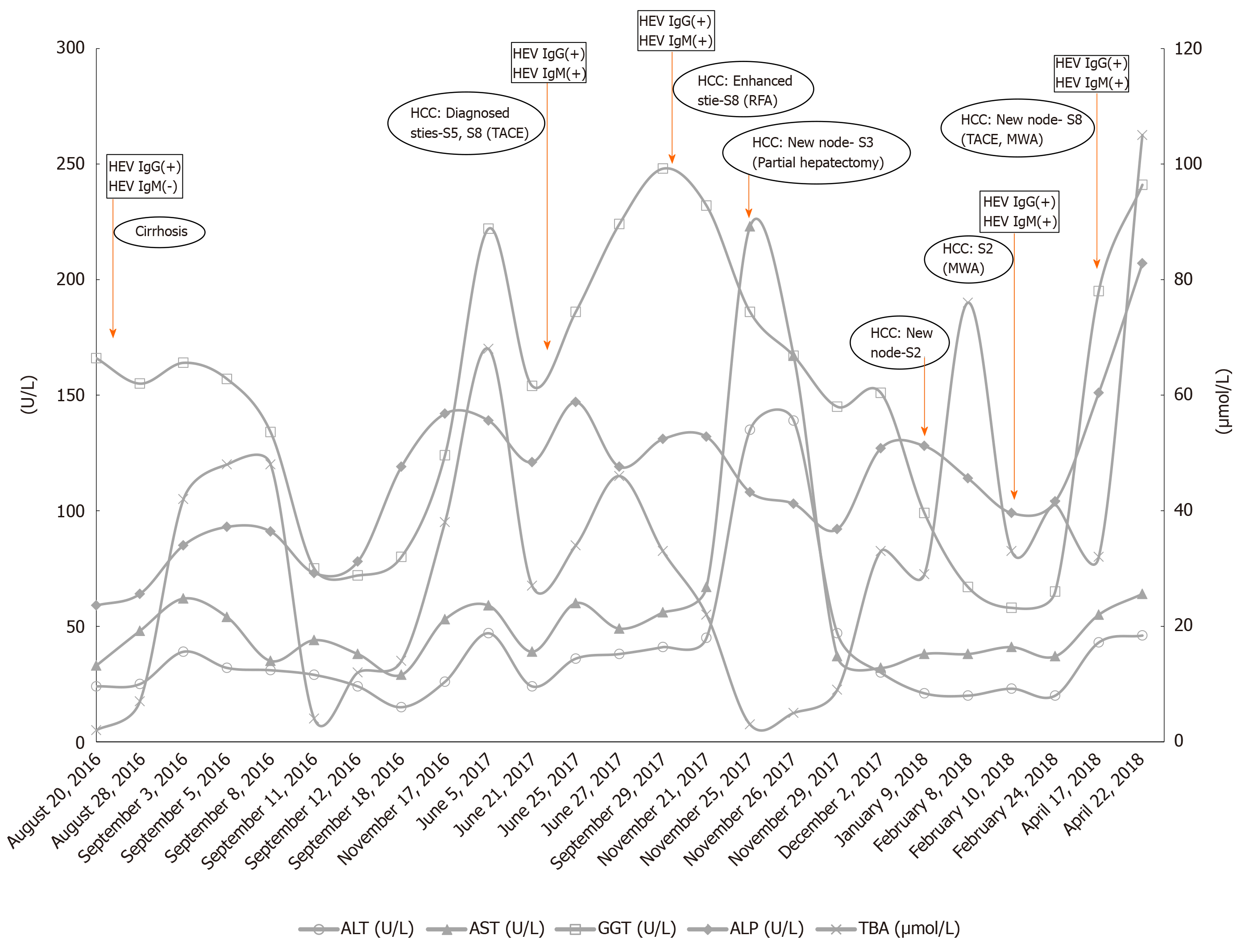
The biochemical test at admission showed normal alanine aminotransferase (ALT, < 40 U/L), slightly elevated aspartate aminotransferase (AST, 39-60 U/L) and alkaline phosphatase (ALP, 121-147 U/L), and highly elevated gamma glutamyl transferase (GGT, 154-186 U/L) and total bile acid (TBA, 27-34 µmol/L) (Figure 1, Table 1, and Supplementary Material).
At admission in June 2017, the CT and magnetic resonance imaging (MRI) examinations detected multi-site liver masses (S5, S8), cirrhosis, portal hypertension, and esophageal and fundus varices (Supplementary Material).
The patient was diagnosed with HCC based on CT and MRI results[8], accompanied with double positivity for HEV-IgM and HEV-IgG.
In July 2017, the patient was classified with Barcelona Clinical Liver Cancer (BCLC) stage B, and transarterial chemoembolization (TACE) was conducted at S5 and S8 according to the international guidelines[9,10].
The patient was then regularly followed every 3 mo upon discharge in August 2017. Since then, serum sample tests were persistently positive for HEV-IgM and HEV-IgG till April 2018, but HEV RNA was not detectable by quantitative real-time PCR.
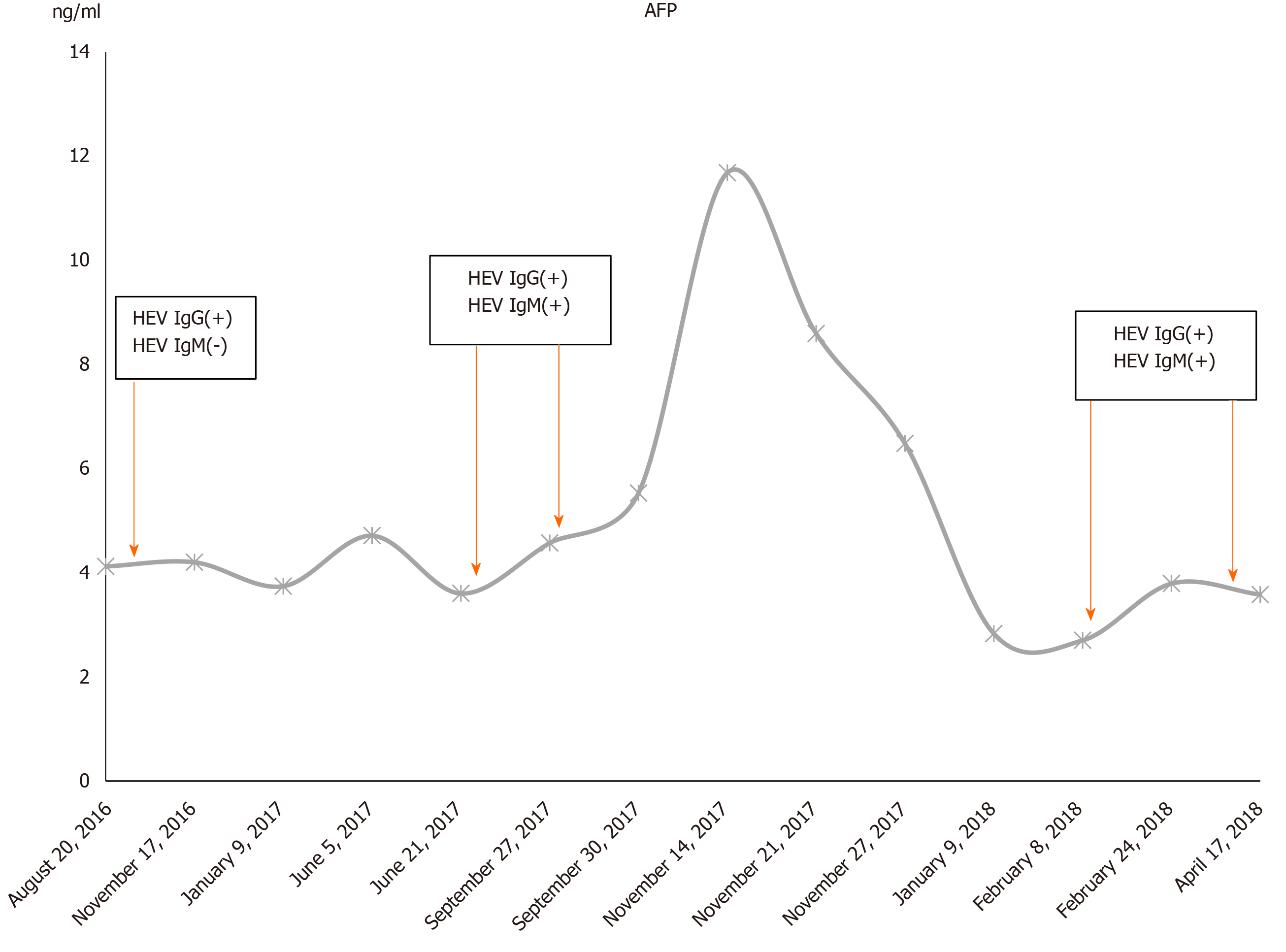
During the follow-up period till April 2018, the patient presented significantly increased blood levels of GGT, type IV pro-collagen, and hyaluronidase. There was an obvious peak of ALT (135-139) and AST (67-167) in November 2017 during hepatectomy. Except for this perioperative elevation, ALT and AST remained normal or slightly increased (Figure 1). A normal range of alpha fetoprotein (AFP) was also observed during the whole admission period (Figure 2). Serum HEV RNA result was negative by quantitative real-time PCR (Supplementary Material).
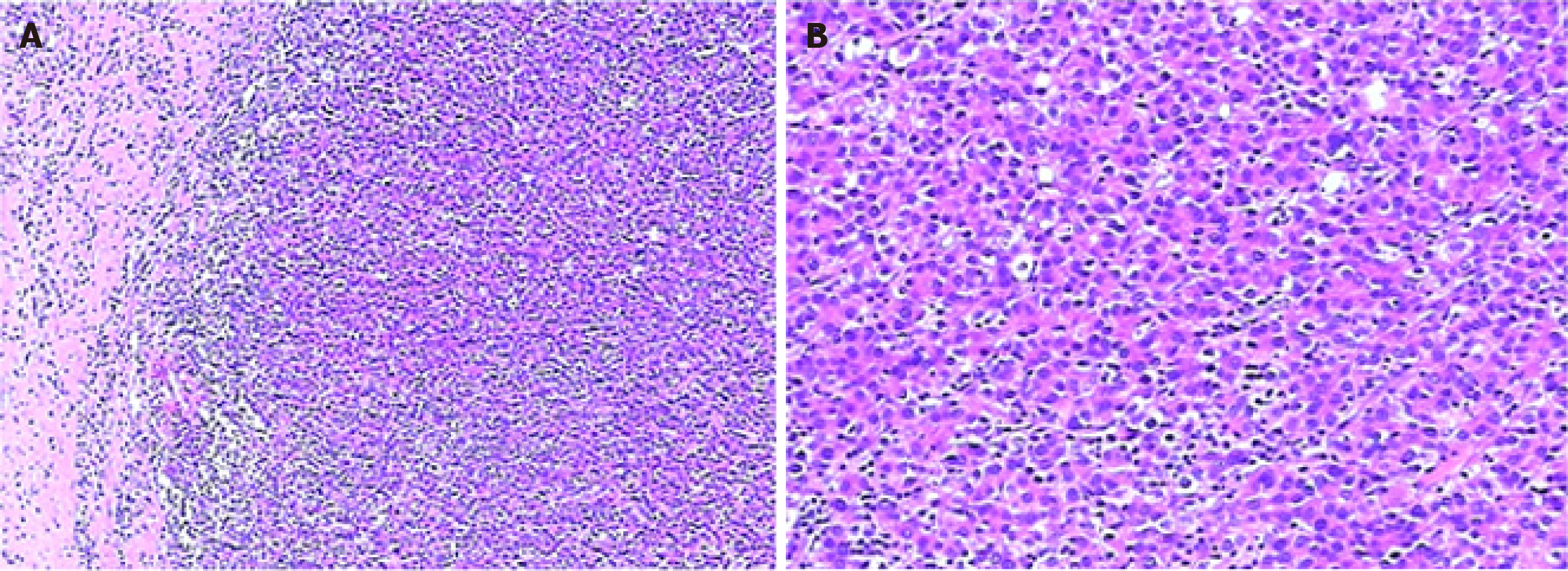
One month after the discharge, CT examination suggested enhanced areas in S8 again. Then the patient was treated with radiofrequency ablation (RFA), which was commonly applied among patients with old age and/or accompanied by other diseases such as liver cirrhosis[11], or used to shrink the tumor and reduce the surgical trauma probably caused by succeeding partial hepatectomy. Afterward, progressive HCC was evidenced by strengthened CT signal at the previously diagnosed site (S8) as well as a new site (S3) after 2 mo, in November 2017. A partial hepatectomy was conducted to remove the relapsed tumors at the previous treatment site (S8) and the new site (S3). The histological examination of surgically resected tissue confirmed cirrhosis and multi-site HCC grading from I to II, with sizes ranging from 0.6 cm to 0.9 cm. The histopathological examination revealed that tumors were CD10, CD34, CEA, and Glypican III positive (Figure 3, Supplementary Material).
In the following 2 mo, a newly developed liver tumor was identified by CT at S2 and a suspected right upper zone lung metastasis was also indicated. Microwave ablation (MWA) treatment was applied at S2 for HCC relapse. In April 2018, suspected tumor nodes at S8 were detected by CT again, followed by TACE and MWA treatment at S8 (Supplementary Material).
This patient was treated with MWAs in July 2018 and December 2018 due to newly identified HCC relapses.
During the whole process, no anti-viral medications including interferon-based therapeutics were prescribed to the patient. And, no further HEV infection examination was performed after April 2018.
At the first presentation to the clinic, this patient suffered from chronic HEV infection, evidenced by positive HEV-IgG and negative HEV-IgM, and cirrhosis simultaneously, but no chronic HBV or HCV infection. In the following 20 mo, this patient was suspected with repeated HEV infection, supported by persistent dual positivity for HEV-IgM and HEV-IgG. More importantly, in the following 20 mo, the patient experienced rapid HCC development, progression, multiple times of relapse, and metastasis.
The patient is speculated to have initial infection of HEV in his farm where farm animals are a suspected source of HEV infection[12]. A previous study has defined farmers as a high risk group of HEV infection due to the potential dissemination of HEV infection in Chinese farms[13]. However, the presumed cause of HEV re-infection is blood transfusion during splenectomy. Previous studies have indicated that the likelihood of developing clinically relevant HEV infection after transfusion of a HEV positive blood product can be as high as approximately 50%[14,15]. And in immuno-suppressed patients, receiving HEV-RNA positive blood products might lead to or prompt the development of fatal acute-on-chronic liver failure[16,17]. Regarding to this, the blood authorities in Europe have advocated to implement HEV screening among blood donors[18]. Unfortunately, currently in China HEV is not tested on blood donations, which therefore lets the patients on the risk of HEV infection from blood transfusion. In this specific case, pre-existed liver cirrhosis predisposes the patient to HEV reinfection, as well as subsequent HCC development. Additionally, the persistent HEV re-infection might also be a result of the lack of anti-viral treatment for HEV chronic infection, especially after the patient has developed HCC and during the progression of HCC. In consequence, a small amount of virus in the liver is able to repeatedly reactivate or cause infection, and invoke weak immune response which is deficient to eliminate the viruses regardless of the production of a small amount of anti-HEV antibodies.
The patient had the history of alcohol drinking and smoking, which might be the underlying causes of cirrhosis[19-21], and the functional decompensated liver predisposes the patient to HEV re-infection and rapid HCC development, progression, relapse, and metastasis[22-24]. It was reported that long-term tobacco exposure would increase the levels of hepatic cancer stem cell-like markers and variate the expression of inflammatory factors IL-33[25]. Similarly, chronic and acute HEV infection would increase the levels of some inflammatory factors and compromise liver function. On the other hand, alcohol intake would lead to chromosomal loss, DNA methylation aberration, genetic susceptibility, oxidative stress, and retinoic acid level decrease in the liver[26]. Previous investigations revealed that excess alcohol consumption was associated with high seroprevalence of HEV in cirrhosis cases, indicating that the alcohol-decompensated liver would be more susceptible to HEV infection[22,27]. All abovementioned risk factors are exhibited in this patient. Thus, we have rationale to speculate a synergic effect evolved from proinflammatory state, genetic instability, and hepatic decompensation leading to accelerated malignant progression in an HEV-infected and re-infected cirrhotic liver. Nevertheless, the definite association between these factors and liver carcinogenesis remains to be further investigated.
Our study has several limitations. First, HEV genotype was not determined because this test is not routinely performed for patients infected with HEV in clinical practice in China. It has been well documented that in China genotype 4 is the most dominant type in human chronic HEV infection, and the cases infected with other genotypes have been reported but remain sporadic in China[28-30]. Nevertheless, in future a routine diagnosis of HEV genotype should be implemented in both clinical and research settings to acquire a deep insight into the association between chronic HEV infection and development of HCC. Second, other potential HCC serum biomarkers such as PIVKA were not screened. This case presented a normal range of AFP during the whole admission period, which warned us the importance of utilizing other biomarkers to assist in early detection of HCC, especially for cases with an uncommon etiology. Further studies should set effort to develop a set of multiple biomarkers, complementary with AFP, for clinical diagnosis of HCC.
Recent studies have suggested that immunocompromised patients are predisposed to HEV infection[31,32], and HEV might promote the progression of HCC in patients with chronic HBV infection and/or cirrhosis[33,34]. However, the role of HEV re-infection in patients with hepatic decompensation with or without chronic HBV infection has not yet been explored, to our knowledge. Our observation in this unique case has indicated that, regardless of chronic HBV infection, in patients with liver cirrhosis HEV superinfection might promote not only HCC development and progression, but also relapse and metastasis. Our report provides new knowledge to HEV-related carcinogenesis and clinical management of HEV-associated liver pathologies. Future studies should emphasize on the mechanisms underlying HEV re-infection accelerated malignant transformation of cirrhotic liver in the presence or absence of chronic HBV infection. The potentially distinct effect on HCC progression exposed by unique sequential acquirement of HEV infection and cirrhosis is an important question to address in future study as well.
This unique case highlights an urgent need to investigate the effect of HEV re-infection on rapid HCC development and progression in cirrhotic liver, despite the presence of chronic HBV infection. Our report also reveals the importance of routine screening HEV in blood donations. Further, antiviral treatment for symptomatic and asymptomatic HEV infection to cirrhosis and HCC patients is highly suggested.
| 1. | Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 2. | Colson P, Raoult D. Autochthonous hepatitis E: a common and fatal but neglected emerging disease in France. Clin Microbiol Infect. 2017;23:898-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Amougou Atsama M, Atangana PJA, Noah Noah D, Moundipa PF, Pineau P, Njouom R. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: Preliminary Observations. Int J Infect Dis. 2017;64:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Bai MJ, Zhou N, Dong W, Li GX, Cong W, Zhu XQ. Seroprevalence and risk factors of hepatitis E virus infection in cancer patients in eastern China. Int J Infect Dis. 2018;71:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Tseng TC, Liu CJ, Chang CT, Su TH, Yang WT, Tsai CH, Chen CL, Yang HC, Liu CH, Chen PJ, Chen DS, Kao JH. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol. 2020;72:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, Kumar Panda S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Borentain P, Colson P, Bolon E, Gauchez P, Coso D, Gérolami R. Hepatocellular carcinoma complicating hepatitis E virus-related cirrhosis. Hepatology. 2018;67:446-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 462] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 9. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3144] [Article Influence: 393.0] [Reference Citation Analysis (3)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6423] [Article Influence: 802.9] [Reference Citation Analysis (9)] |
| 11. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Ganzang Bing Zazhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 12. | Capai L, Falchi A, Charrel R. Meta-Analysis of Human IgG anti-HEV Seroprevalence in Industrialized Countries and a Review of Literature. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Kang YH, Cong W, Zhang XY, Wang CF, Shan XF, Qian AD. Hepatitis E virus seroprevalence among farmers, veterinarians and control subjects in Jilin province, Shandong province and Inner Mongolia Autonomous Region, China. J Med Virol. 2017;89:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, Russell K, Tettmar KI, Tossell J, Ushiro-Lumb I, Tedder RS. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 411] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 15. | Satake M, Matsubayashi K, Hoshi Y, Taira R, Furui Y, Kokudo N, Akamatsu N, Yoshizumi T, Ohkohchi N, Okamoto H, Miyoshi M, Tamura A, Fuse K, Tadokoro K. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion. 2017;57:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Westhölter D, Hiller J, Denzer U, Polywka S, Ayuk F, Rybczynski M, Horvatits T, Gundlach S, Blöcker J, Schulze Zur Wiesch J, Fischer N, Addo MM, Peine S, Göke B, Lohse AW, Lütgehetmann M, Pischke S. HEV-positive blood donations represent a relevant infection risk for immunosuppressed recipients. J Hepatol. 2018;69:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Müllhaupt B, Niederhauser C. Hepatitis E blood donor screening - More than a mere drop in the ocean? J Hepatol. 2018;69:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O'Riordan J, Boland F, Harritshøj L, Nascimento MSJ, Ciccaglione AR, Politis C, Adlhoch C, Flan B, Oualikene-Gonin W, Rautmann G, Strengers P, Hewitt P. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Simpson RF, Hermon C, Liu B, Green J, Reeves GK, Beral V, Floud S; Million Women Study Collaborators. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health. 2019;4:e41-e48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Goh GB, Chow WC, Wang R, Yuan JM, Koh WP. Coffee, alcohol and other beverages in relation to cirrhosis mortality: the Singapore Chinese Health Study. Hepatology. 2014;60:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 171] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Fantilli AC, Trinks J, Marciano S, Zárate F, Balderramo DC, Wassaf MGM, Haddad L, Gadano A, Debes JD, Pisano MB, Ré VE. Unexpected high seroprevalence of hepatitis E virus in patients with alcohol-related cirrhosis. PLoS One. 2019;14:e0224404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 24. | Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Xie C, Zhu J, Wang X, Chen J, Geng S, Wu J, Zhong C, Li X. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J Exp Clin Cancer Res. 2019;38:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 402] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 27. | Said B, Ijaz S, Kafatos G, Booth L, Thomas HL, Walsh A, Ramsay M, Morgan D; Hepatitis E Incident Investigation Team. Hepatitis E outbreak on cruise ship. Emerg Infect Dis. 2009;15:1738-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Sridhar S, Lo SK, Xing F, Yang J, Ye H, Chan JF, Teng JL, Huang C, Yip CC, Lau SK, Woo PC. Clinical characteristics and molecular epidemiology of hepatitis E in Shenzhen, China: a shift toward foodborne transmission of hepatitis E virus infection. Emerg Microbes Infect. 2017;6:e115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Geng Y, Zhao C, Fan J, Harrison TJ, Zhang H, Lian H, Geng K, Wang Y. Genotype analysis of hepatitis E virus from sporadic hepatitis E cases in northern China. Infect Genet Evol. 2013;20:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Zhang W, He Y, Wang H, Shen Q, Cui L, Wang X, Shao S, Hua X. Hepatitis E virus genotype diversity in eastern China. Emerg Infect Dis. 2010;16:1630-1632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Versluis J, Pas SD, Agteresch HJ, de Man RA, Maaskant J, Schipper ME, Osterhaus AD, Cornelissen JJ, van der Eijk AA. Hepatitis E virus: an underestimated opportunistic pathogen in recipients of allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Grewal P, Kamili S, Motamed D. Chronic hepatitis E in an immunocompetent patient: a case report. Hepatology. 2014;59:347-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Chen C, Zhang SY, Zhang DD, Li XY, Zhang YL, Li WX, Yan JJ, Wang M, Xun JN, Lu C, Ling Y, Huang YX, Chen L. Clinical features of acute hepatitis E super-infections on chronic hepatitis B. World J Gastroenterol. 2016;22:10388-10397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Hoan NX, Tong HV, Hecht N, Sy BT, Marcinek P, Meyer CG, Song le H, Toan NL, Kurreck J, Kremsner PG, Bock CT, Velavan TP. Hepatitis E Virus Superinfection and Clinical Progression in Hepatitis B Patients. EBioMedicine. 2015;2:2080-2086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bock CT, Hu J S-Editor: Huang P L-Editor: Wang TQ P-Editor: Li JH