Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1076
Peer-review started: June 2, 2020
First decision: June 15, 2020
Revised: June 23, 2020
Accepted: September 4, 2020
Article in press: September 4, 2020
Published online: November 27, 2020
Processing time: 174 Days and 20.9 Hours
Hepatitis B e antigen-negative chronic hepatitis B patients under nucleos(t)ids analogues (NAs) rarely achieve hepatitis B surface antigen (HBsAg) loss.
To evaluate if the addition of pegylated interferon (Peg-IFN) could decrease HBsAg and hepatitis B core-related antigen (HBcrAg) levels and increase HBsAg loss rate in patients under NAs therapy.
Prospective, non-randomized, open-label trial evaluating the combination of Peg-IFN 180 µg/week plus NAs during forty-eight weeks vs NAs in monotherapy. Hepatitis B e antigen-negative non-cirrhotic chronic hepatitis B patients of a tertiary hospital, under NAs therapy for at least 2 years and with undetectable viral load, were eligible. Patients with hepatitis C virus, hepatitis D virus or human immunodeficiency virus co-infection and liver transplanted patients were excluded. HBsAg and HBcrAg levels (log10 U/mL) were measured at baseline and during ninety-six weeks. HBsAg loss rate was evaluated in both groups. Adverse events were recorded in both groups. The kinetic of HBsAg for each treatment group was evaluated from baseline to weeks 24 and 48 by the slope of the HBsAg decline (log10 IU/mL/week) using a linear regression model.
Sixty-five patients were enrolled, 61% receiving tenofovir and 33% entecavir. Thirty-six (55%) were included in Peg-IFN-NA group and 29 (44%) in NA group. After matching by age and treatment duration, baseline HBsAg levels were comparable between groups (3.1 vs 3.2) (P = 0.25). HBsAg levels at weeks 24, 48 and 96 declined in Peg-IFN-NA group (-0.26, -0.40 and -0.44) and remained stable in NA group (-0.10, -0.10 and -0.10) (P < 0.05). The slope of HBsAg decline in Peg-IFN-NA group (-0.02) was higher than in NA group (-0.00) (P = 0.015). HBcrAg levels did not change. Eight (22%) patients discontinued Peg-IFN due to adverse events. The HBsAg loss was achieved in 3 (8.3%) patients of the Peg-IFN-NA group and 0 (0%) of the NA group.
The addition of Peg-IFN to NAs caused a greater and faster decrease of HBsAg levels compared to NA therapy. Side effects of Peg-IFN can limit its use in clinical practice.
Core Tip: The functional cure of chronic hepatitis B defined as the loss of the hepatitis B surface antigen is the optimal end-point with the currently available therapies. However, it is rarely achieved in hepatitis B e antigen-negative chronic hepatitis B patients under nucleos(t)ids analogues (NAs). In the present study, we report that the addition of pegylated interferon (Peg-IFN) to NAs during forty-eight weeks caused a greater and faster decrease of hepatitis B surface antigen levels compared to NA monotherapy. No changes in hepatitis B core-related antigen were observed. However, the low applicability and poor tolerance of Peg-IFN make difficult its use in clinical practice.
- Citation: Broquetas T, Garcia-Retortillo M, Micó M, Canillas L, Puigvehí M, Cañete N, Coll S, Viu A, Hernandez JJ, Bessa X, Carrión JA. Hepatitis B surface antigen and hepatitis B core-related antigen kinetics after adding pegylated-interferon to nucleos(t)ids analogues in hepatitis B e antigen-negative patients. World J Hepatol 2020; 12(11): 1076-1088
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1076.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1076
Chronic hepatitis B (CHB) affects around 240 million people worldwide[1]. Hepatitis B virus (HBV) cannot be completely eradicated with the available therapies due to the presence of covalently closed circular DNA (cccDNA) in the nuclei of infected hepatocytes[2]. Hepatitis B surface antigen (HBsAg) loss is the optimal treatment endpoint, representing a functional cure of CHB and improving long-term outcome[3].
Although liver biopsy for the quantification of intrahepatic cccDNA and intrahepatic HBV DNA remains the most accurate measurement for viral reservoir, it is limited by its invasive nature and the potential for sampling error. Therefore, noninvasive serological tests are necessary as surrogate markers of intrahepatic viral replicative activity. Serum HBsAg is the glycosylated envelope protein of the mature HBV, which is produced by transcription and translation of the surface genes[4]. On the other hand, the hepatitis B core-related antigen (HBcrAg) combines the antigenic reactivity resulting from denatured hepatitis B e antigen (HBeAg), HBV core antigen and a core-related protein (p22cr), all products of the precore/core gene share an identical 149 amino acid sequence[5].
Currently, there are two strategies to treat HBeAg-negative CHB patients, a finite course with pegylated interferon (Peg-IFN) or a long-term therapy with nucleos(t)ids analogues (NAs). Entecavir or tenofovir monotherapy have been shown to achieve the virological response in almost all adherent patients[6]. However, the reduction of HBsAg levels in HBeAg-negative CHB patients under NAs is very slow (-0.1 log IU/mL/yr)[7,8] with HBsAg loss rates < 1% after five years of NAs therapy[7,9] compared to 4% after 48 wk of Peg-IFN[10]. Moreover, it has been suggested that interleukin 28B (IL28B) rs12979860 polymorphism CC could confer a better probability of response to Peg-IFN in HBeAg-negative CHB patients infected by genotype D[11]. On the other hand, differences in Peg-IFN response rates have been demonstrated according to HBV genotype especially in HBeAg-positive patients[12]. Despite NAs are the most used therapy in HBeAg-negative CHB patients because of its safety, long term therapy is needed. In contrast, the addition of the immunomodulatory effect of Peg-IFN could improve HBsAg loss rates[10,13]. However, this strategy has been mostly evaluated in naïve treatment or HBeAg-positive patients being the information about pre-treatment predictors and the kinetics of serological markers (HBsAg and HBcrAg) scarce during the add-on strategy in HBeAg-negative patients.
In the present study, we have prospectively evaluated the levels of HBsAg and HBcrAg in HBeAg-negative non-cirrhotic CHB patients receiving NAs after the addition of Peg-IFN during forty-eight weeks. The primary aim was to compare the HBsAg and HBcrAg kinetics in both treatment strategies (NA group vs Peg-IFN-NA group). The secondary aim was to evaluate the proportion of HBsAg loss at week 96.
This is a single center, prospective, non-randomized, open-label trial including HBeAg-negative non-cirrhotic CHB patients, receiving NAs for at least 2 years. Recruitment period was from August 2014 to February 2016 in a tertiary center (Hospital del Mar, Barcelona, Spain). Patients were eligible if they received a stable NAs dose with virological response (undetectable HBV-DNA viral load during the last twelve months). Exclusion criteria were as follows: Patients with a previous Peg-IFN treatment, NA treatment for HBV reactivation prophylaxis, patients with human immunodeficiency virus, hepatitis D virus or hepatitis C virus co-infection, and liver transplanted patients. All patients provided written informed consent.
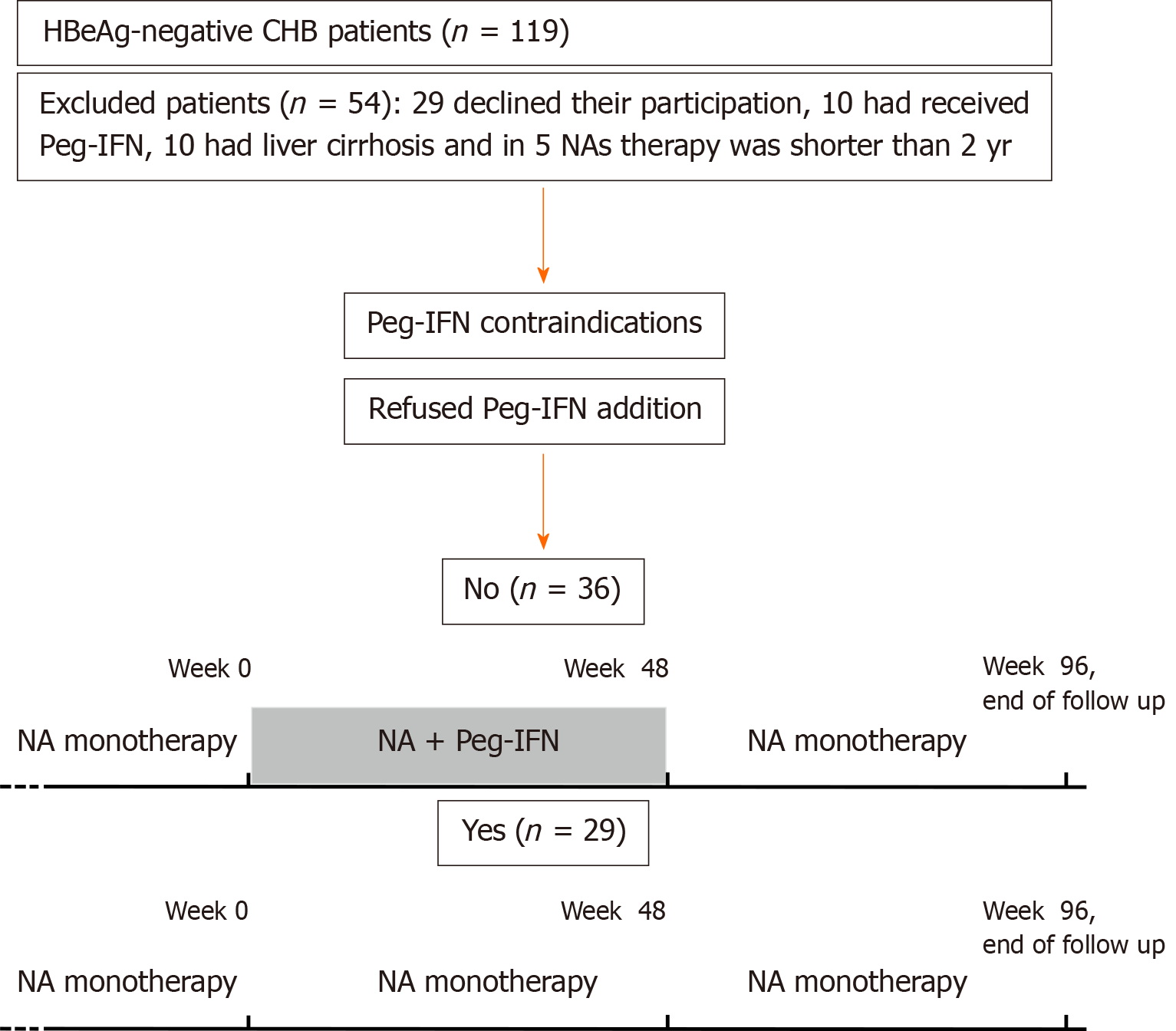
Patients with any malignancy in the last 5 years, those with psychiatric, thyroid or autoimmune disorders, and non-liver transplanted patients were only eligible for NAs monotherapy. Peg-IFN alpha-2a was offered to be added in all eligible patients. Those who accepted it, received 180 µg/week during forty-eight weeks (Peg-IFN-NA group) and all the other participants remained in NAs monotherapy (NA group). At week 48 all the patients continued with NAs in monotherapy and were followed up until week 96 or loss of follow-up. Protocol visits were at weeks 0, 12, 24, 48, 72 and 96. Figure 1 shows the flowchart of patients and study design.
The study protocol was approved by the Ethical Committee of our Institution “Comitè Ètic d’Investigació Clínica-Parc de Salut Mar”, study reference 2014/5787/I, in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Demographic data, liver stiffness measurement (LSM) and polymorphism rs12979860 of IL28B were assessed at baseline. HBV-genotype was collected from electronic data as it had been performed prior to the initiation of NAs therapy. The levels of HBV-DNA, HBsAg and HBcrAg were analyzed at weeks 0, 24, 48, 96. Adverse events were recorded at each protocol visit, following the Common Terminology Criteria for Adverse Events. All the data were collected and tabulated in a database with an access code to ensure patient confidentiality.
LSM was performed at baseline by a single experienced operator (> 5000 examinations), using the FibroScan® 502 Touch (FibroScan® EchosensTM, Paris, France) following the manufacturer’s recommendations as previously described[14]. Liver fibrosis was categorized according to previously published cut-offs for LSM considering significant fibrosis for LSM > 7.2 kPa. Patients with LSM > 12 kPa were considered as having cirrhosis and were excluded[15].
HBV DNA was measured by polymerase chain reaction with a limit of quantification of 10 IU/mL (ABBOTT RealTime HBV m2000®, Abbott Molecular Inc., IL, United States). Serum HBsAg was quantified by Electro-chemiluminescence immunoassay Elecsys® HBsAgII QuantII (Roche Diagnostic, Rotkreuz, Switzerland) according to the manufacturer’s instructions. The assay ranged from 0.05 to 117000 IU/mL. In highly concentrated samples above the upper limit, the value of manual dilution was multiplied by the dilution factor. Serum HBcrAg was measured using a quantitative fully automated chemiluminiscent enzyme immunoassay (LUMIPULSE®, Fujirebio Europe, Belgium).
The monoclonal antibodies used in this two-step immunoassay measure simultaneously denatured HBeAg, HBV core antigen and the precore protein p22cr (aa-28 to aa-150). Samples were processed according to the manufacturer’s instructions. The lower limit of detection was 2.0 log U/mL, and a linear range of 3.0 log U/mL-7.0 log U/mL (1 kU/mL was equal to 3 log U/mL).
Quantitative variables were expressed as medians and ranges. Categorical variables were expressed as proportions. Continuous variables were compared by the Mann–Whitney U test or Kruskall-Wallis when appropriate and categorical by the Pearson chi-square test, Fisher test or the Mc Nemar test. Patients were categorized according to antiviral treatment (Peg-IFN-NA group vs NA group). Differences between NA and Peg-IFN-NA groups regarding age, sex, IL28B polymorphism, ethnicity, liver function, liver stiffness, treatment duration, viral genotype, HBsAg and HBcrAg levels and HBsAg loss rate were analyzed by univariate analysis. A two-sided P value < 0.05 was considered to indicate statistical significance. The kinetic of HBsAg for each treatment group was evaluated from baseline to weeks 24 and 48 by the slope of the HBsAg decline (log10 IU/mL per week) using a linear regression model (LRM). Statistical analyses were performed with the SPSS® 25.0 (SPSS Inc., Chicago, IL, United States) and LRM with the Prism 7.0 (© 1994-2016 GraphPad Software, Inc.).
From August 2014 to February 2016, 119 HBeAg-negative CHB patients were evaluated. Twenty-nine (24%) patients declined their participation, 10 (8.4%) had previously received Peg-IFN, 10 (8.4%) had liver cirrhosis and in 5 (4.2%) patients NAs therapy duration was shorter than 2 years. Among the 65 included patients, 5 were only eligible for the NA therapy due to Peg-IFN contraindications and 60 were eligible for both therapies: 36 accepted to receive Peg-IFN and 24 refused the addition of Peg-IFN. Therefore, 36 (55.4%) patients were included in the Peg-IFN-NA group and 29 (44.6%) in the NA group. Two patients in NA group were receiving low doses of corticosteroids (prednisone 2.5 to 5 mg/d) for rheumatoid arthritis and no kidney transplanted patients were included because none of them fulfilled the inclusion criteria.
Figure 1 shows the flowchart and Table 1 the main characteristics of the included patients. Patients in Peg-IFN-NA group compared to NA group were younger (age 45 vs 53, P = 0.01) and had a shorter previous NA treatment duration (259 vs 393 wk, P = 0.01), but were comparable in gender, IL28B polymorphism, ethnicity, liver function, liver stiffness, type of NA, HBV genotype and baseline HBcrAg and HBsAg levels. Due to the baseline differences, patients of both treatment groups were individually matched for age and treatment duration. Therefore, pre-treatment predictors and the kinetic of serological markers (HBsAg and HBcrAg) were performed in 48 patients. Table 2 shows the characteristics of matched patients.
| NA group (n = 29) | Peg-IFN-NA group (n = 36) | P value | |
| Age (yr) | 53 (36-70) | 45 (26-72) | 0.01 |
| Males, n (%) | 21 (72) | 29 (81) | 0.44 |
| IL28B polymorphism, n (%) | 0.16 | ||
| CC | 11 (37.9) | 20 (55.6) | |
| CT/TT | 14 (62.1) | 16 (44.4) | |
| Origin (ethnicity), n (%) | 0.70 | ||
| Europe | 20 (69) | 20 (56) | |
| Asia | 12 (33) | 12 (33) | |
| Africa | 3 (10) | 3 (8) | |
| AST (IU/mL) | 20 (15-59) | 22 (12-62) | 0.37 |
| ALT (IU/mL) | 19 (12-101) | 25 (12-91) | 0.20 |
| GGT (IU/mL) | 19 (9-197) | 22 (10-125) | 0.33 |
| LSM, n (%) | 0.91 | ||
| < 7.2 kPa | 28 (97) | 34 (97) | |
| 7.2-12 kPa | 1 (3) | 1 (3) | |
| NA treatment, n (%) | |||
| Tenofovir | 20 (69) | 22 (61) | 0.46 |
| Entecavir | 7 (24) | 11 (31) | |
| Others | 2 (7) | 3 (8) | |
| NA treatment duration (wk) | 393 (113-763) | 259 (118-496) | 0.01 |
| HBV genotype, n (%) | 0.99 | ||
| Non-D | 7 (24.1) | 16 (44.4) | |
| D | 12 (41.4) | 13 (36.1) | |
| Not available | 10 (34.5) | 7 (19.4) | |
| Baseline HBcrAg (log 10 U/mL) | 2.65 (< 2-4.9) | 2.30 (< 2-3.7) | 0.18 |
| Baseline HBsAg (log 10 IU/mL) | 2.96 (1.3-4.2) | 3.22 (1.6-4.6) | 0.07 |
| NA group (n = 24) | Peg-IFN-NA group (n = 24) | P value | |
| Age (yr) | 54 (36-60) | 45 (26-63) | 0.07 |
| Male sex, n (%) | 18 (75) | 22 (91) | 0.12 |
| IL28B polymorphism, n (%) | 0.25 | ||
| CC | 9 (38) | 13 (54) | |
| CT/CT | 15 (62) | 11 (46) | |
| Origin (ethnicity), n (%) | 0.20 | ||
| European | 17 (70) | 12 (50) | |
| Asia | 3 (12) | 9 (38) | |
| Africa | 2 (8) | 3 (12) | |
| AST (IU/mL) | 20 (15-59) | 22 (15-38) | 0.69 |
| ALT (IU/mL) | 20 (12-101) | 23 (15-50) | 0.41 |
| GGT (IU/mL) | 23 (9-197) | 22 (11-125) | 0.44 |
| LSM, n (%) | 0.32 | ||
| < 7.2 kPa | 23 (96) | 24 (100) | |
| 7.2-12 kPa | 1 (4) | 0 (0) | |
| NA treatment, n (%) | 0.32 | ||
| Tenofovir | 16 (67) | 12 (50) | |
| Entecavir | 6 (25) | 9 (38) | |
| Others | 2 (8) | 3 (12) | |
| NA treatment duration (wk) | 378 (113-763) | 272 (139-495) | 0.06 |
| HBV genotype, n (%) | 0.43 | ||
| A | 5 (21) | 4 (17) | |
| B | 1 (4) | 3 (12) | |
| C | 0 (0) | 2 (8) | |
| D | 10 (42) | 8 (33) | |
| E | 1 (4) | 2 (8) | |
| F | 0 (0) | 1 (4) | |
| Not available | 7 (29) | 4 (18) | |
| Baseline HBcrAg (log 10 U/mL) | 2.7 (< 2-4.9) | 2.3 (< 2-3.7) | 0.18 |
| Baseline HBcrAg (log10 U/mL), n (%) | 0.39 | ||
| < 2 | 6 (25) | 9 (38) | |
| 2-2.5 | 4 (17) | 6 (25) | |
| 2.5-3 | 6 (25) | 3 (12) | |
| 3-3.5 | 2 (8) | 3 (12) | |
| 3.5-4 | 3 (13) | 3 (12) | |
| > 4 | 3 (13) | 0 (0) | |
| Baseline HBsAg (log10 IU/mL) | 3.1 (1.3-4.2) | 3.2 (1.6-4.4) | 0.25 |
| Baseline HBsAg (IU/mL), n (%) | 0.22 | ||
| > 1000 | 12 (50) | 14 (48) | |
| 100-1000 | 7 (29) | 9 (38) | |
| < 100 | 5 (21) | 1 (4) | |
| HBcrAg decline (log10 U/mL) | |||
| Δ Week 24 | 0.00 (-1.10-1.21) | 0.00 (-0.71-0.30) | 0.96 |
| Δ Week 48 | 0.00 (-1.00-0.30) | 0.00 (-1.31-1.10) | 0.25 |
| Δ Week 96 | 0.00 (-1.00-0.10) | 0.00 (-0.71-0.71) | 0.12 |
| HBsAg decline (log10 IU/mL) | |||
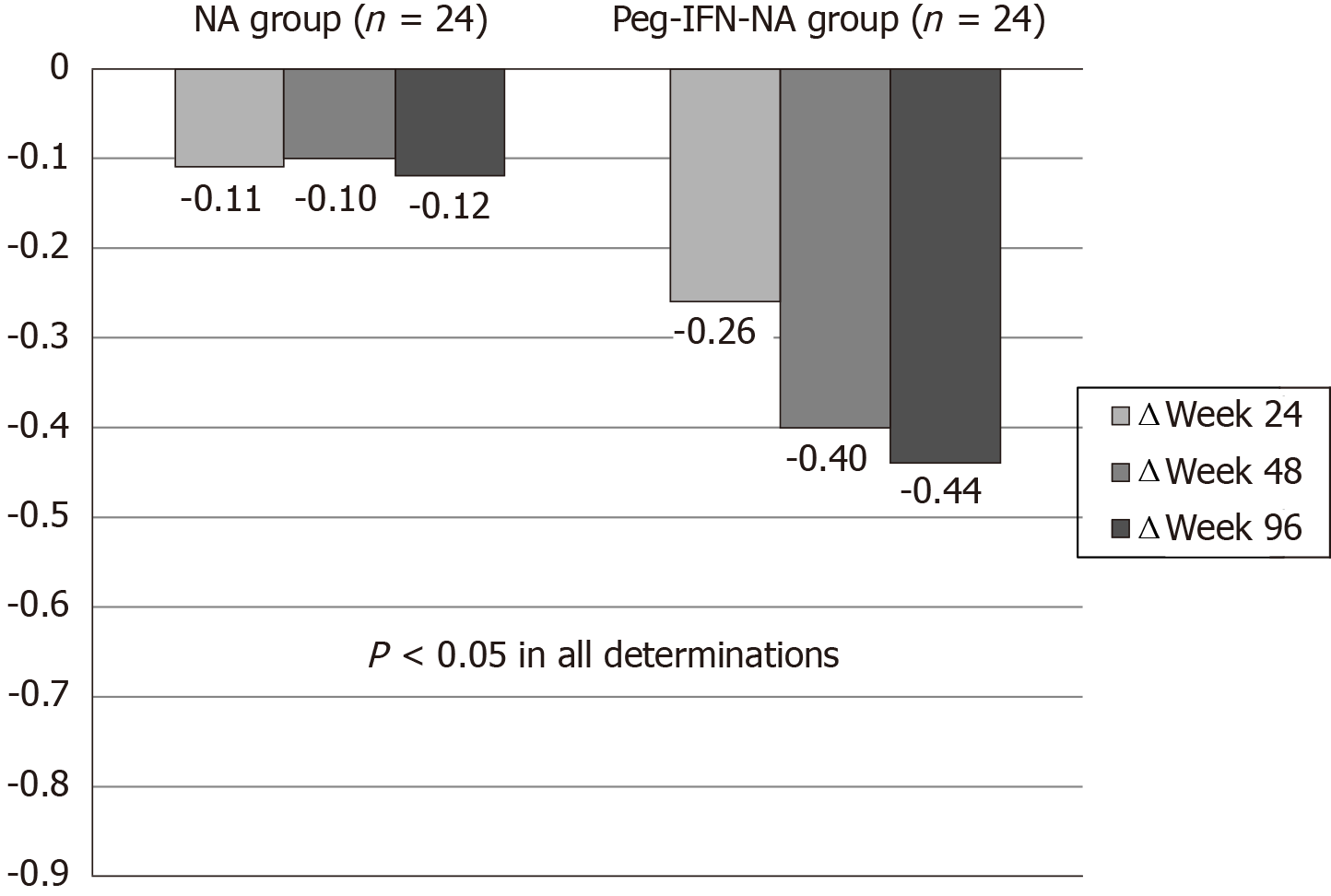
| Δ Week 24 | -0.11 (-0.04-0.00) | -0.26 (-3.8-0.1) | 0.01 |
| Δ Week 48 | -0.10 (-1.17-0.04) | -0.40 (-4-0.02) | 0.00 |
| Δ Week 96 | -0.12 (-1.39-0.96) | -0.44 (-4-0.01) | 0.00 |
| HBsAg Loss; n (%) | 0 (0) | 3 (12.5) | 0.07 |
The median (range) HBcrAg values (log 10 U/mL) was 2.7 (< 2-4.9) in NA group and 2.3 (< 2-3.7) in Peg-IFN-NA group (P = 0.18) at baseline. The rate of patients with HBcrAg values below the limit of detection (HBcrAg < 2 log10 U/mL) was 25% and 38%, respectively (P = 0.39). The HBcrAg kinetics was described as the delta (Δ) of its levels at weeks 24, 48 and 96. The HBcrAg levels remained stable at weeks 24, 48 and 96 (Table 2). We did not detect differences on HBcrAg levels between both treatment strategies according to the treatment group, the IL28B polymorphism or the HBV genotype. We did not find any correlation between HBcrAg and HBsAg levels nor HBsAg loss rate (data not shown).
The baseline levels of HBsAg (log 10 IU/mL) were similar in NA and Peg-IFN-NA groups (3.1 vs 3.2) (P = 0.25). The HBsAg kinetics was described as the delta (Δ) of their levels at weeks 24, 48 and 96. The decline of the HBsAg level was greater in Peg-IFN-NA group (-0.26, -0.40, -0.44) compared to NA group (-0.11, -0.10, -0.12) (P < 0.05 in all determinations) (Figure 2).
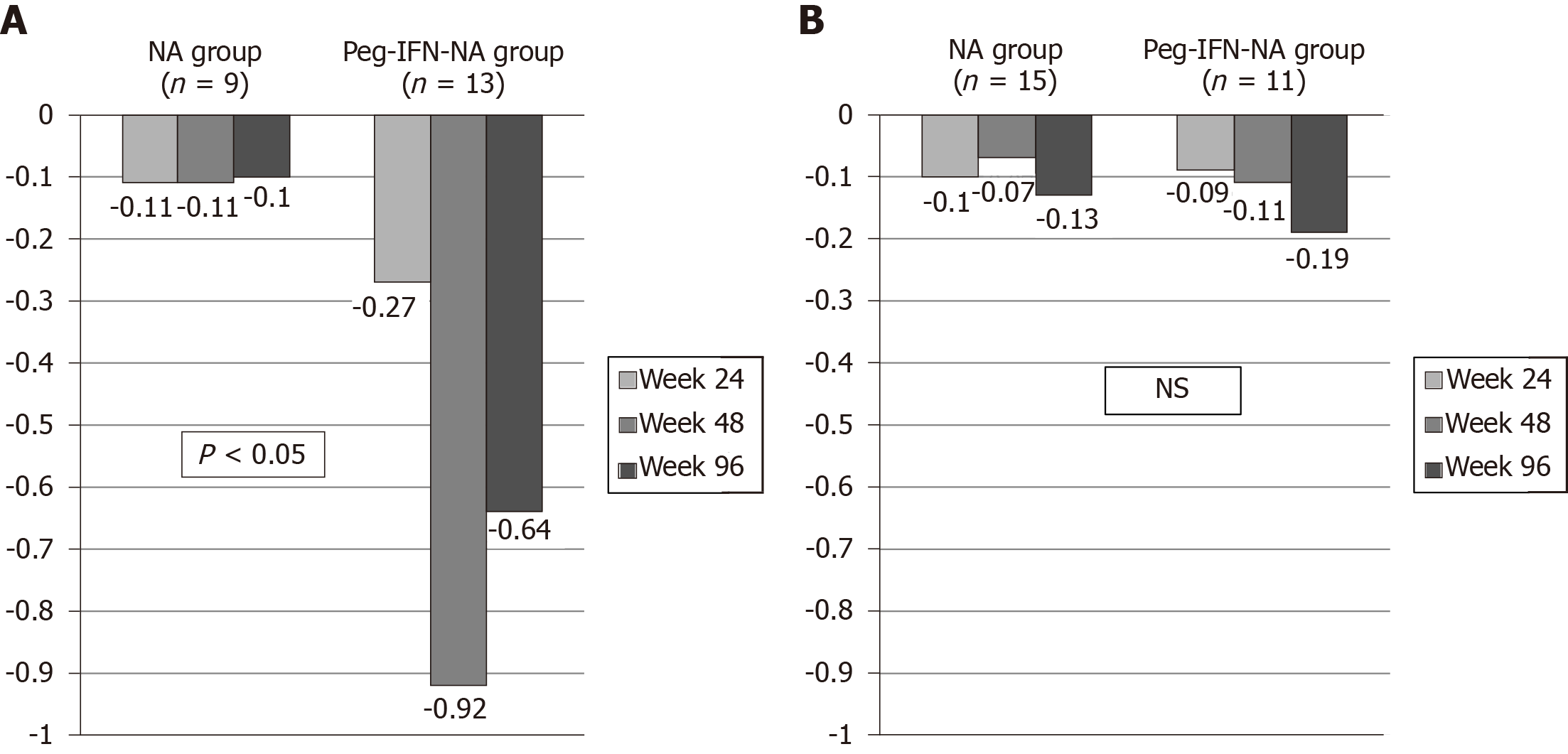
The HBsAg kinetics was different between treatment arms according to IL28B polymorphism and HBV genotype. In patients with IL28B CC polymorphism (n = 22) the decline of HBsAg at weeks 24, 48 and 96 was greater in Peg-IFN-NA group (-0.27, -0.92 and -0.64) than in NA group (-0.11, -0.11 and -0.10) (P < 0.05 in all cases) (Figure 3A). In contrast, in patients with IL28B CT/TT (n = 26) we did not find differences on HBsAg kinetics at weeks 24, 48 and 96 between Peg-IFN-NA group (-0.09, -0.11 and -0.19) and NA group (-0.10, -0.07 and 0.13) (not significant in all determinations) (Figure 3B). Moreover, the decline of HBsAg were different between NA and Peg-IFN-NA group at weeks 48 and 96 in patients infected by HBV genotype A (-0.07 vs -1.05 and -0.08 vs -0.53) and genotype D (-0.08 vs -0.42 and -0.51 vs -0.80) (P < 0.05 in all cases) (data not shown).
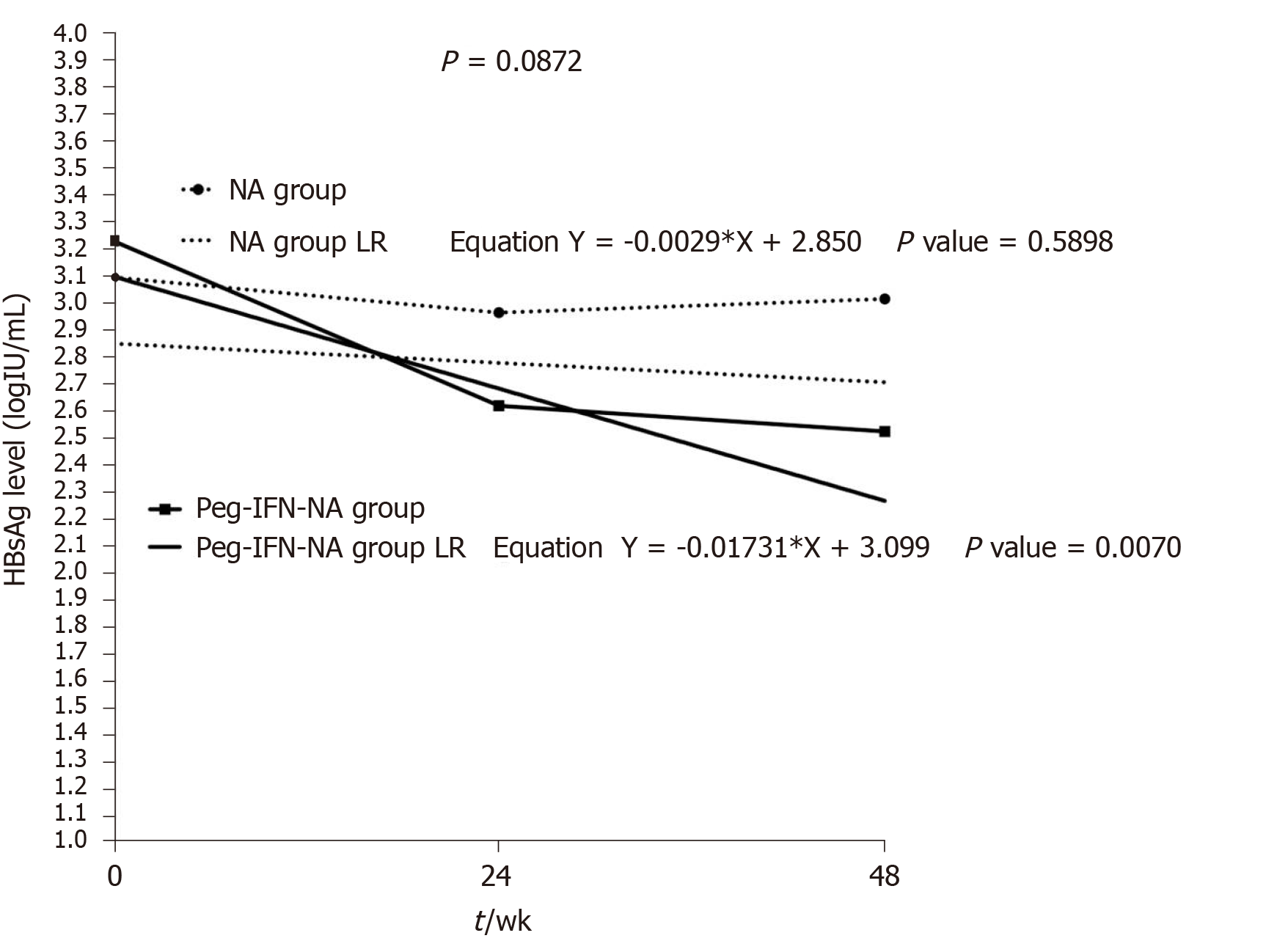
In order to demonstrate the existence of different HBsAg kinetics for each treatment strategy, we evaluated the slope of the HBsAg decline (log10 IU/mL per week) from baseline to weeks 24 and 48 using a LRM (Figure 4). In patients receiving NA monotherapy, HBsAg levels did not decrease during the forty-eight weeks. The slope of HBsAg kinetics in NA group (-0.00) was similar to zero (P = 0.6). On the contrary, in patients receiving Peg-IFN-NA, HBsAg levels significantly decreased during the forty-eight weeks and the slope of HBsAg kinetic (-0.02) was different to zero (P < 0.001) and greater than that found in NA group (P = 0.015).
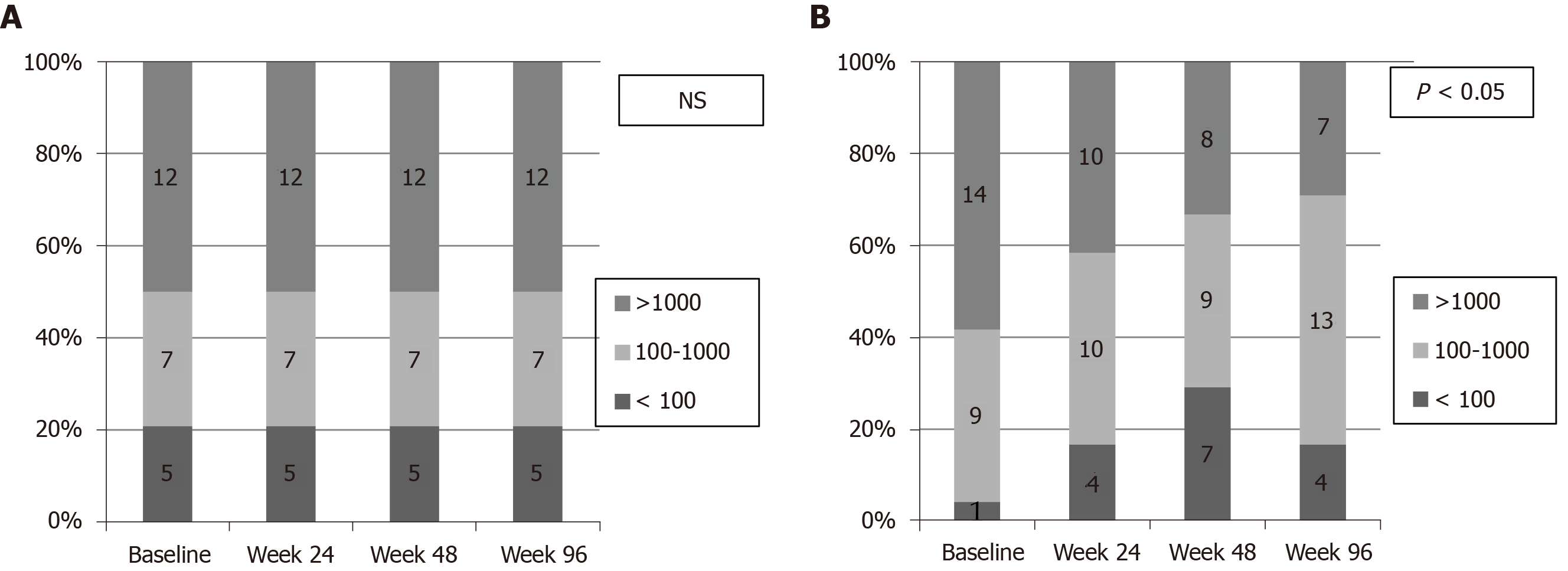
The proportion of patients reaching low levels of HBsAg (HBsAg < 100 IU/mL) at baseline and at weeks 24, 48 and 96 are depicted in Figure 5. In the NA group the rate of patients with low HBsAg levels was 21% at baseline, but did not change at weeks 24, 48 and 96 (not significant) (Figure 5A). On the contrary, rate of patients with low HBsAg levels in Peg-IFN-NA group was 4.2% at baseline and increased at weeks 24 (16.7%), 48 (29.6%) and 96 (16.7%) (P = 0.001) (Figure 5B). The proportion of patients achieving HBsAg loss in the Peg-IFN-NA group (n = 3, 12.5%) was higher compared to NA group (n = 0, 0%), but the difference did not reach the statistical significance (P = 0.07).
Patients with HBsAg loss were male, with low fibrosis stage (F0-F1), and infected by HBV-genotype A (n = 1) or B (n = 2). Two patients had an IL28B CC polymorphism and the other a CT polymorphism. All of them had been on NAs therapy for more than 5 years before the addition of Peg-IFN. The NAs treatment was entecavir (n = 1), tenofovir (n = 1) and telbivudine (n = 1). Baseline levels of HBsAg (log10 IU/mL) were 4.0, 2.1 and 1.6, and baseline levels of HBcrAg (log10 U/mL) were 2.7, < 2 and 3.4, respectively. All of them received Peg-IFN during forty-eight weeks. Two patients lost HBsAg during therapy (week 24 and 36) and one at week 24 after Peg-IFN discontinuation (week 72).
No serious adverse events were observed during treatment and follow-up. However, 8 (22%) patients did not complete Peg-IFN treatment. The reasons for Peg-IFN discontinuation were flu-like symptoms and asthenia (n = 3), DNA flare (n = 3), polyarthritis (n = 1) and Graves’ thyroiditis (n = 1). No patients discontinued antiviral treatment in NA group.
In this controlled trial of HBeAg-negative CHB non-cirrhotic patients under NAs treatment and with undetectable DNA, the addition of 48 wk of Peg-IFN alfa-2a reduced HBsAg levels further and faster than continuing with NAs monotherapy. However, the proportion of patients with HBsAg loss during the first ninety-six weeks did not reach the statistical significance with this add-on strategy.
HBsAg kinetics has been shown as one of the best predictors of treatment response[8,16,17]. However, patients of our Peg-IFN-NA group were younger and had a shorter previous NA treatment duration compared to NA group. According to previously published studies showing a decrease of HBsAg levels with NA therapy[18] and a higher probability to HBsAg clearance in aged populations[19] we decided to match the included patients for age and treatment duration.
The present study prospectively confirms our previously published results[7] regarding the slow decline of HBsAg levels in HBeAg-negative CHB patients receiving NAs therapy. The current study has demonstrated a very low decline (-0.12 log10 IU/mL at week 96) and very slow change (-0.00 log10 IU/mL per week) of HBsAg levels in patients receiving NAs. As a consequence, the rate of patients with low HBsAg levels (< 100 IU/mL) did not change at weeks 24, 48 and 96, and no patient achieved HBsAg loss. On the contrary, the addition of Peg-IFN clearly increased the decline (-0.44 log10 IU/mL at week 96) and accelerate the decrease (-0.02 log10 IU/mL per week) of HBsAg levels compared to NA group. Therefore, in the Peg-IFN-NA group the rate of patients with low HBsAg levels was higher at weeks 24 (16.7%) and 48 (29.6%) and the rate of HBsAg loss increased (n = 3, 12.5%) compared to NA group (n = 0, 0%).
We also analyzed the HBcrAg levels during the study in both treatment strategies. However, levels of HBcrAg remained stable during the 96 wk without differences between both treatment strategies and without correlation with HBsAg levels or HBsAg loss rate. This could be explained by the fact that baseline levels of HBcrAg in our cohort of HBeAg negative patients, receiving NAs during a long time period before inclusion, were already low. As described before, the rate of patients with a baseline HBcrAg value below the limit of detection (< 2 log10 U/mL) was high in both treatment groups (25% and 38%). Recent studies have shown that HBcrAg can reflect cccDNA transcriptional activity in the different phases of HBV infection[20,21]. However as HBeAg is included in HBcrAg, this could explain the low baseline HBcrAg levels in our cohort of HBeAg-negative patients. Moreover, recent studies, have described that HBcrAg levels can decline over the time in patients undergoing NAs therapy, especially in HBeAg-negative patients[22,23]. Thus, according to our results, we have not found that HBcrAg determination could be a useful serum marker in clinical practice for monitoring treatment response in HBeAg-negative patients receiving NAs or Peg-IFN-NAs.
It has been suggested that low levels of HBsAg are related to higher rates of HBsAg loss after NA discontinuation, being advisable to achieve low levels of HBsAg before stopping NA therapy[24,25]. Our study showed that the rate of patients with HBsAg < 100 IU/mL increased in the Peg-IFN-NA group from 4.2% at baseline to 29.6% at 48 wk (P = 0.001). The NAs have shown to restore partly adaptive immunity, whereas Peg-IFN boosts innate immunity and depletes the ccc-DNA, which leads to a major HBsAg loss[26-29]. The analysis performed in matched patients by age and treatment duration showed that the proportion of HBsAg loss during the first 96 wk was higher in the Peg-IFN-NA group compared to the NA group. However, this difference did not reach the statistical significance probably due to the limited number of included patients and the short follow-up time of our study. Nevertheless, our results are in accordance with smaller studies previously published[30,31] and in line with the results published by Bourlière et al[32] during the execution of the current study.
Previous studies have linked the presence of IL28B CC polymorphisms with the HBsAg loss in HBeAg-negative CHB patients receiving Peg-IFN. It has been shown that CC polymorphism could confer a better response profile to Peg-IFN therapy than CT/TT polymorphisms, especially in patients infected by HBV genotype D[11,33]. We analyzed the HBsAg kinetics according to IL28B polymorphism, and we found that patients with CC polymorphism showed a higher HBsAg decline in Peg-IFN-NA group compared to NA group. On the contrary, HBsAg kinetics was similar in both treatment strategies in CT/TT patients. Therefore, the add-on strategy should not be recommended in patients with IL28B CT or TT polymorphism.
Our study has several limitations. First, the treatment assignment was not randomized. However, patients on both treatment strategies were individually matched for age and treatment duration to make the cohort comparable. Second, the acceptance of the add-on strategy was low and only 40% of eligible patients with a previous (well-tolerated) NA therapy accepted the addition of Peg-IFN due to its potential toxicity. Third, the frequent adverse events of Peg-IFN (22% of discontinuations) caused a low number of patients completing 48 wk of therapy making this therapeutic strategy difficult to be introduced in clinical practice. However, this applicability and tolerability are in line with previous published data[32]. Fourth, the treatment duration of Peg-IFN was limited to 48 wk and the follow-up period to 96 wk. Therefore, patients with a rapid HBsAg decline could have taken advantage of a longer therapy or longer follow-up. Finally, the low rate of HBsAg loss did not allow to identify predictors associated with HBsAg loss. However, the LRM demonstrated different HBsAg kinetics after adding Peg-IFN.
In conclusion, our prospective, non-randomized, open-label clinical trial has demonstrated that the addition of Peg-IFN to NAs decreased HBsAg levels further and faster compared to NA monotherapy. The HBcrAg levels remained stable. Despite the low applicability and poor tolerance of Peg-IFN making difficult its use in clinical practice, it could be considered in selected patients with favorable HBV genotype and IL28B polymorphism.
Functional cure of chronic hepatitis B (CHB), defined as the loss of hepatitis B surface antigen (HBsAg), is very unusual with current antiviral treatments in hepatitis B e antigen (HBeAg)-negative patients. HBsAg levels decline very slow in patients receiving nucleos(t)ids analogues (NAs). Therefore, they need long-term antiviral treatment.
The hypothesis that we wanted to answer with our study was that the addition of pegylated-interferon (Peg-IFN) could accelerate the decline of HBsAg levels in patients that were receiving NAs and that this therapeutic strategy could increase the HBsAg loss rate.
In our study we wanted to evaluate in patients under NAs therapy if the addition of Peg-IFN could decrease HBsAg and hepatitis B core-related antigen (HBcrAg) levels, and increase HBsAg loss rate. If HBeAg-negative patients could achieve low levels of HBsAg it could be a good strategy to shorten the antiviral treatment.
We have performed a prospective, non-randomized, open-label trial evaluating the combination of Peg-IFN 180 µg/wk plus NAs during forty-eight weeks vs NAs in monotherapy, in HBeAg-negative non-cirrhotic CHB patients after a minimum of two years of NA therapy and with virological response.
We have shown that the addition of Peg-IFN 180 µg/wk during forty-eight weeks to NAs caused a greater and faster decrease of HBsAg levels compared to NA therapy alone, especially in those patients with interleukin 28B polymorphism CC. However, the HBcrAg levels remained stable after adding Peg-IFN to NAs. We have also shown that, the low acceptance by the patients of this therapeutic strategy and the side effects of Peg-IFN can limit its use in clinical practice.
This study shows that the addition of Peg-IFN to NA therapy accelerates the decline of HBsAg, especially in patients with interleukin 28B polymorphism CC. Therefore, even Peg-IFN has several side effects, this treatment strategy could be offered to some selected patients in order to achieve the functional cure of CHB. On the other hand, our study shows that HBcrAg levels do not seem useful to monitor this kind of treatment, neither as a predictor of HBsAg loss.
It is well known that patients with HBeAg-negative CHB usually need a long-term therapy with NAs, even lifelong, to achieve HBsAg loss. However, it has been suggested that low levels of HBsAg are related to higher rates of HBsAg loss after NA discontinuation, being advisable to achieve low levels of HBsAg before stopping NA therapy.
The authors thank Duran X in Hospital del Mar Medical Research Institute for assistance with the statistical analysis.
| 1. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2052] [Article Influence: 186.5] [Reference Citation Analysis (4)] |
| 2. | Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely "occult"? Hepatology. 2001;34:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 423] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 4. | Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Wong DK, Tanaka Y, Lai CL, Mizokami M, Fung J, Yuen MF. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol. 2007;45:3942-3947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 446] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 7. | Broquetas T, Garcia-Retortillo M, Hernandez JJ, Puigvehí M, Cañete N, Coll S, Cabrero B, Giménez MD, Solà R, Carrión JA. Quantification of HBsAg to predict low levels and seroclearance in HBeAg-negative patients receiving nucleos(t)ide analogues. PLoS One. 2017;12:e0188303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Seto WK, Wong DK, Fung J, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology. 2013;58:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1410] [Article Influence: 108.5] [Reference Citation Analysis (1)] |
| 10. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, Lai MY, Button P, Pluck N; Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 862] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 11. | Lampertico P, Viganò M, Cheroni C, Facchetti F, Invernizzi F, Valveri V, Soffredini R, Abrignani S, De Francesco R, Colombo M. IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis B. Hepatology. 2013;57:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL, Janssen HL. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 13. | Marcellin P, Bonino F, Yurdaydin C, Hadziyannis S, Moucari R, Kapprell HP, Rothe V, Popescu M, Brunetto MR. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013;7:88-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Puigvehí M, Broquetas T, Coll S, Garcia-Retortillo M, Cañete N, Fernández R, Gimeno J, Sanchez J, Bory F, Pedro-Botet J, Solà R, Carrión JA. Impact of anthropometric features on the applicability and accuracy of FibroScan® (M and XL) in overweight/obese patients. J Gastroenterol Hepatol. 2017;32:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Zoutendijk R, Hansen BE, van Vuuren AJ, Boucher CA, Janssen HL. Serum HBsAg decline during long-term potent nucleos(t)ide analogue therapy for chronic hepatitis B and prediction of HBsAg loss. J Infect Dis. 2011;204:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Marcellin P, Buti M, Krastev Z, de Man RA, Zeuzem S, Lou L, Gaggar A, Flaherty JF, Massetto B, Lin L, Dinh P, Subramanian GM, McHutchison JG, Flisiak R, Gurel S, Dusheiko GM, Heathcote EJ. Kinetics of hepatitis B surface antigen loss in patients with HBeAg-positive chronic hepatitis B treated with tenofovir disoproxil fumarate. J Hepatol. 2014;61:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Papatheodoridis G, Goulis J, Manolakopoulos S, Margariti A, Exarchos X, Kokkonis G, Hadziyiannis E, Papaioannou C, Manesis E, Pectasides D, Akriviadis E. Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis B patients under 4-year entecavir therapy. J Hepatol. 2014;60:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Tsai PS, Chang CJ, Chen KT, Chang KC, Hung SF, Wang JH, Hung CH, Chen CH, Tseng PL, Kee KM, Yen YH, Tsai CC, Lu SN. Acquirement and disappearance of HBsAg and anti-HCV in an aged population: a follow-up study in an endemic township. Liver Int. 2011;31:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 21. | Chen EQ, Feng S, Wang ML, Liang LB, Zhou LY, Du LY, Yan LB, Tao CM, Tang H. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B. Sci Rep. 2017;7:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | van Bömmel F, Deichsel D, Loglio A, Facchetti F, Pfeifferkorn M, Brehm M, Berg T, Lampertico P. HBV RNA can be detected more frequently than HBcrAg but decreases during long term treatment with nucleos (t)ide analogues up to 14 years in patients with HBeAg negative chronic hepatitis B. J Hepatol. 2019;70:e487. [DOI] [Full Text] |
| 23. | Carey I, Gersch J, Bruce M, Moigboi C, Wang B, Kuhns M, Cloherty G, Dusheiko G, Agarwal K. The markers of HBV transcriptional activity-HBcrAg and pre-genomic HBV DNA during antiviral therapy with nucleos (t)ide analogue help to predict optimal timing of therapy withdrawal. J Hepatol. 2019;70:e33-e34. [DOI] [Full Text] |
| 24. | Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 2012; 143: 629-636. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 26. | Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler F, Zankel M, Fischer C, Currie G, Brosgart C, Petersen J. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 27. | Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 28. | Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, Colombo M, Missale G, Ferrari C. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012; 143: 963-73. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 29. | Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou L, Martins EB, Dinh P, Lin L, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HL; Study 149 Investigators. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology 2016; 150: 134-144. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 30. | Kittner JM, Sprinzl MF, Grambihler A, Weinmann A, Schattenberg JM, Galle PR, Schuchmann M. Adding pegylated interferon to a current nucleos(t)ide therapy leads to HBsAg seroconversion in a subgroup of patients with chronic hepatitis B. J Clin Virol. 2012;54:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Ouzan D, Pénaranda G, Joly H, Khiri H, Pironti A, Halfon P. Add-on peg-interferon leads to loss of HBsAg in patients with HBeAg-negative chronic hepatitis and HBV DNA fully suppressed by long-term nucleotide analogs. J Clin Virol. 2013;58:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Bourlière M, Rabiega P, Ganne-Carrie N, Serfaty L, Marcellin P, Barthe Y, Thabut D, Guyader D, Hezode C, Picon M, Causse X, Leroy V, Bronowicki JP, Carrieri P, Riachi G, Rosa I, Attali P, Molina JM, Bacq Y, Tran A, Grangé JD, Zoulim F, Fontaine H, Alric L, Bertucci I, Bouvier-Alias M, Carrat F; ANRS HB06 PEGAN Study Group. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 33. | Boglione L, Cusato J, Allegra S, Esposito I, Patti F, Cariti G, Di Perri G, D'Avolio A. Role of IL28-B polymorphisms in the treatment of chronic hepatitis B HBeAg-negative patients with peginterferon. Antiviral Res. 2014;102:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Asociación Española para el Estudio del Hígado.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barone M, Kasirga E, Tuna N S-Editor: Gao CC L-Editor: A P-Editor: Li JH