Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1055
Peer-review started: July 16, 2020
First decision: August 9, 2020
Revised: August 22, 2020
Accepted: October 9, 2020
Article in press: October 9, 2020
Published online: November 27, 2020
Processing time: 130 Days and 15.1 Hours
Portal hypertension is a major complication of cirrhosis that is associated with significant morbidity and mortality. The present gold-standard method to risk stratify and observe cirrhosis patients with portal hypertension is hepatic venous pressure gradient measurement or esophagogastroduodenoscopy. However, these methods are invasive, carry a risk of complications and are associated with significant patient discomfort. Therefore, non-invasive splenic parameters are of clinical interest as potential useful markers in determining the presence of portal hypertension. However, diagnostic accuracy and reproducibility remains unvalidated.
To assess the diagnostic accuracy of spleen stiffness, area and diameter in predicting the presence of portal hypertension.
Of 50 patients with varying liver disease pathologies were prospectively recruited from the St. Mary’s Hospital Liver Unit in London; 25 with evidence of portal hypertension and 25 with no evidence of portal hypertension. Liver stiffness, spleen stiffness, spleen diameter and spleen area were measured using the Philips Affiniti 70 elastography point quantification point shear wave elastography system. The aspartate aminotransferase-to-platelet-ratio-index (APRI) score was also calculated. Performance measures, univariate and multivariate logistic regression were used to evaluate demographic, clinical and elastography variables. Interclass correlation coefficient was used to determine the reproducibility of splenic area and diameter.
On univariate and individual performance, platelet count [area under the receiver operating characteristic (AUROC) 0.846, P value < 0.001], spleen area (AUROC 0.828, P value = 0.002) and APRI score (AUROC 0.827, P value < 0.001) were the most accurate variables in identifying the presence of portal hypertension. On multivariate logistic regression models constructed, the combination of spleen area greater than 57.90 cm2 and platelet count less than 126 × 109 had 63.2% sensitivity and 100% specificity, 100% positive predictive value and 100% negative predictive value. An alternative combination of spleen stiffness greater than 29.99 kPa and platelet count less than 126 × 109 had 88% sensitivity, 75% specificity, 78.6% positive predictive value and 85.7% negative predictive value. An interclass correlation coefficient value of 0.98 (95%CI: 0.94-0.99, P value < 0.001) and 0.96 (95%CI: 0.91-0.99, P value < 0.001) were determined for inter-operator variability for spleen area and diameter respectively.
Spleen area, spleen stiffness and platelet count may be useful markers to assess the presence of portal hypertension in patients of various etiologies.
Core Tip: Non-invasive splenic parameters are useful surrogate markers of portal hypertension (PH). A combination of spleen diameter, spleen area, liver stiffness and aspartate aminotransferase-to-platelet-ratio-index (APRI) score is able to predict the presence of PH. The APRI score has a similar diagnostic accuracy to combination index.
- Citation: Ahmad AK, Atzori S, Maurice J, Taylor-Robinson SD, Lim AKP. Non-invasive splenic parameters of portal hypertension: Assessment and utility. World J Hepatol 2020; 12(11): 1055-1066
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1055.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1055
Portal hypertension (PH) is a major complication of cirrhosis[1]. Esophageal varices (EV) are present in 40% of compensated advanced chronic liver disease (cACLD) patients and in 70% of decompensated cirrhosis patients. Strategies to identify individuals with clinically significant portal hypertension (CSPH) is vital to reduce morbidity and mortality[2].
The hepatic venous pressure gradient measurement (HVPG) and esophago-gastroduodenoscopy (EGD) form the backbone of diagnosis and surveillance of EV[3]. However, both methods are invasive and carry a risk of complications[1]. Therefore, non-invasive and safe methods of diagnosis and surveillance of PH are of great clinical interest.
Current guidelines propose that non-invasive methods of assessment of liver fibrosis can predict the incidence of cirrhosis-induced PH manifestations[4]. The Baveno VI guidelines suggest cirrhosis patients with a liver stiffness measurement < 20 kPa and a platelet count > 150000/μL can avoid screening endoscopy[3]. Nevertheless, while 20% of EGDs are spared, new algorithms are still required, as up to 40% of EGDs continue unnecessarily[5].
Ultrasound elastography techniques are based on the principle that tissue elastic properties can be distorted using shear waves to measure stiffness. Spleen stiffness measurements using ultrasound elastography have shown an association with CSPH as the spleen undergoes parenchymal remodelling and fibrogenesis, due to blood pooling in PH[5-7]. Interestingly, evidence on patients with chronic hepatitis C infection also suggests that spleen stiffness is dependent on inflammation present in the liver that directly contributes to the pathogenic mechanisms underlying PH[8,9].
Transient elastography (TE) is the most validated ultrasound elastography technique and shows a sensitivity ≥ 90% in detecting patients with CSPH[6,10]. Nevertheless, limitations exist due to its lack of 2D imaging guidance and attenuation of wave propagation in obesity and ascites[7].
Point shear wave elastography (p-SWE), often referred to as acoustic radiation force impulse, overcomes these issues by providing integrated 2D-ultrasound imaging which can be used in patients who are obese or have ascites[7]. Despite several meta-analyses on spleen stiffness measurements, it remains unclear whether TE or p-SWE has greater diagnostic accuracy[11,12]. Furthermore, although it is well established that spleen stiffness and combination variables such as liver stiffness-spleen diameter to platelet ratio (LSPS) score are superior to liver stiffness for detection of EV[13], little is known whether a combination of splenic parameters can improve diagnostic accuracy[14]. Finally, although two main p-SWE techniques exist–the elastography point quantification (ElastPQ®) and Virtual Touch Quantification (VTQ®)–fewer studies have looked at the performance of ElastPQ due to its novelty.
We aimed to assess whether spleen stiffness measurement, spleen area and spleen diameter can independently predict CSPH, or in combination with other biochemical or elastography parameters; and assess reproducibility of splenic area and diameter measurements.
Patients with varying liver disease etiology were prospectively recruited as part of an ongoing comparative imaging study (REC: 15/EE/0420). All subjects had evidence of chronic liver disease (CLD), were over the age of 18 and provided informed consent. Exclusion criteria included pregnancy, lack of liver disease pathology, transjugular portosystemic shunt (TIPSS) insertion or presence of hepatocellular carcinoma (HCC).
The primary analyses were conducted after all patients were recruited. The patients were divided into the following groups: Evidence of CSPH (group 1) and no evidence of CSPH (group 2). CSPH was defined either as presence of EV or portal hypertensive gastropathy (PHG) during an EGD or if patients had invasive procedures where the HVPG pressure ≥ 10 mmHg. Ultrasound elastography measurements must have been undertaken within a maximum of one year of EGD or HVPG measurements.
All patients had to be fasted for up to 6h prior to scans. Participants were placed supine with arms abducted away from the ultrasound probes. The Philips Affiniti 70 (ElastPQ) (Philips Medical Systems, Seattle, WA, United States) was used to record liver stiffness measurement and spleen stiffness measurement for each patient. Ten measurements were taken from the liver and ten measurements from the spleen. Liver elastography measurements were taken from the right lobe of the liver 2.4 cm (± 1 cm) from the liver capsule. Spleen elastography measurements were taken from the middle aspect of the spleen with homogeneous elasticity with the exclusion of big vessels. The median stiffness and IQR values were recorded. Spleen area and diameter were calculated from 2D images obtained.
Clinical and biological parameters including body mass index (BMI), skin to liver capsule distance, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), platelet count, prothrombin time, albumin, bilirubin and international normalized ratio (INR) were obtained for all patients at time of recruitment. APRI score was calculated as: AST (IU/L)/PLT (× 109/L)[15]. Parameters determining presence of PH such as HVPG measurements or EGD findings were recorded. Cirrhosis was defined either by histological findings at biopsy or if decompensation had occurred.
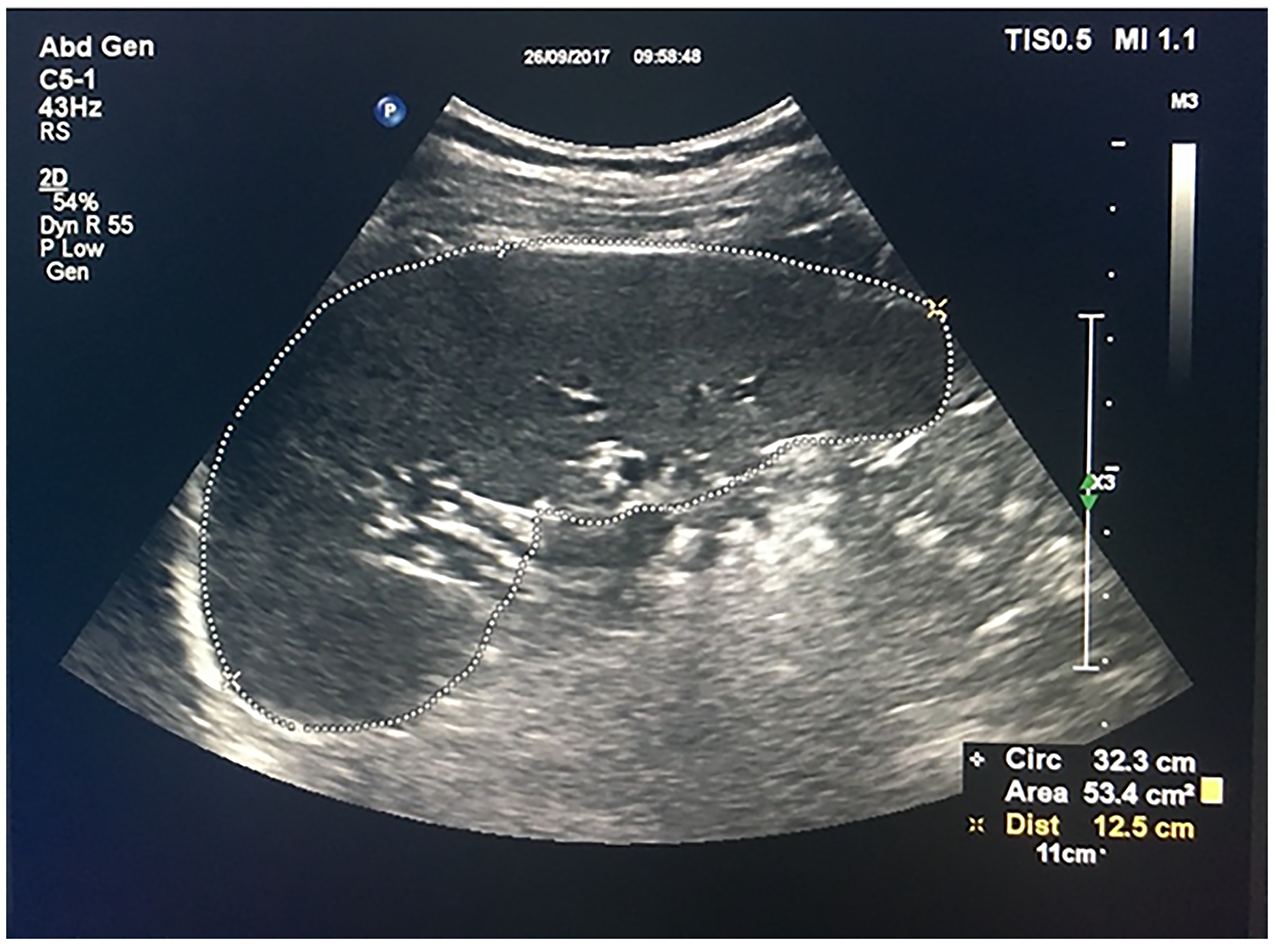
Spleen area and diameter measurements were calculated using maximum spleen diameter and borders that included the splenic hilum in the transverse plane with the area (Figure 1). Measurements were repeated 4 mo later by two authors independently using a random sample of 19 study patients to calculate inter-operator variabilities.
Descriptive statistics were carried out to compare groups 1 and 2. Ultrasound measurements, BMI and laboratory results were analysed by univariate and multivariate analyses. Correlations between variables were examined using Pearson’s correlation coefficient and P values determined using ANOVA. A multivariate logistic regression model was built using a stepwise selection to determine the association of spleen area and platelet count and spleen stiffness and platelet count with the presence of CSPH. It was ensured that the data fulfilled all necessary criteria prior to application of the logistic regression analysis. Diagnostic accuracy was assessed using receiver operating characteristic (ROC) curves. Youden’s index was used to determine the cut-off values for each parameter. P values for ROC curves were identified based on Wilcoxon’s test. As subjects were random patients and operators were fixed, a one-way random interclass correlation coefficient (ICC) model on absolute agreement to determine inter-operator variability for spleen area and diameter was carried out. P values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 24.0.
This study was performed in accordance with the 1975 Declaration of Helsinki. Written and informed consent was obtained from all patients.
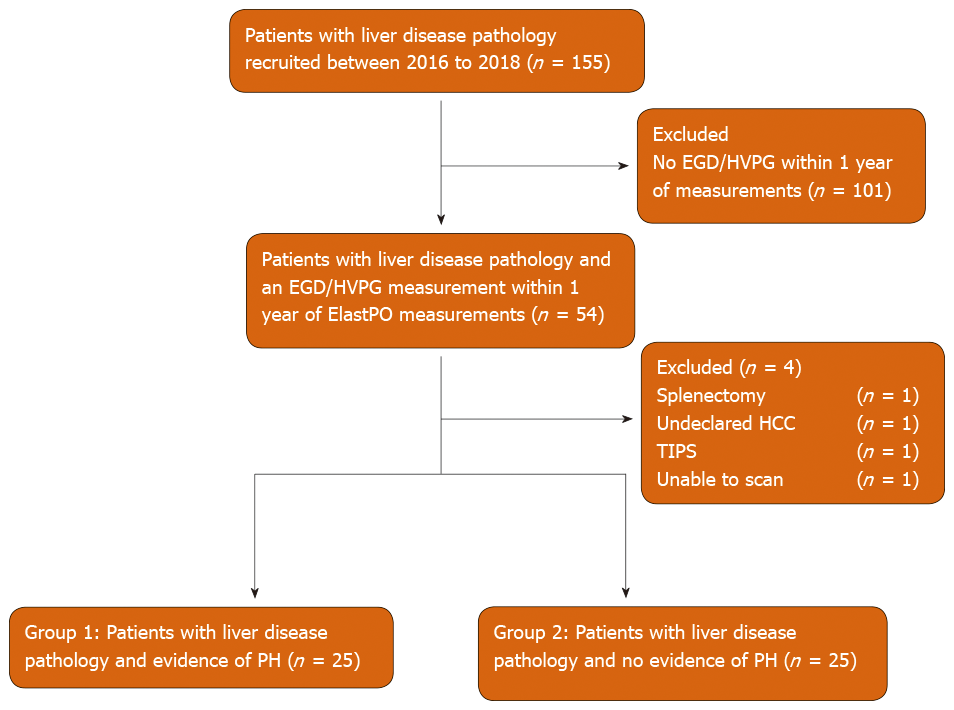
Fifty four of 155 patients recruited had an EGD/HVPG measurement taken within one year of ElastPQ measurements. Four patients were excluded as summarized in Figure 2. A total of 50 patients (mean age 57.86, 62.0% male) were included in final analysis: 25 with evidence of CSPH (group 1) (mean age 60.44, 60.0% male) and 25 with no evidence of CSPH (group 2) (mean age 55.28, 64.0% male). The median time difference between ultrasound elastography measurements and EGD/HVPG was 4 mo.
Baseline clinical and biochemical characteristics for all patients included in statistical analysis are summarized in Table 1. Patients with diagnosis of cirrhosis were found in both groups n = 25 in group 1; n = 11 in group 2) with majority classified as Child-Pugh A (n = 18, 36.0%). The most common primary etiology in group 1 was alcoholic liver disease n = 7, 28.0%), while non-alcoholic fatty liver disease (n = 8, 32.0%) was more common in group 2.
| Parameter | Mean (standard deviation) or n | ||
| Groups 1 and 2 (n = 50) | Group 1 (n = 25) | Group 2 (n = 25) | |
| Age | 57.86 (12.22) | 60.44 (9.89) | 55.28 (13.90) |
| Sex (M:F) | 31:19 | 15:10 | 16:9 |
| BMI | 29.42 (7.61) | 28.59 (5.76) | 30.31 (9.26) |
| Skin to liver capsule Distance (cm) | 2.43 (0.99) | 2.46 (1.01) | 2.40 (0.99) |
| METAVIR score | 3.13 (1.276) | 3.74 (0.752) | 2.52 (1.410) |
| ALD | 12 | 7 | 5 |
| NAFLD | 12 | 4 | 8 |
| ALD and NAFLD | 4 | 3 | 1 |
| HCV | 2 | 2 | 0 |
| AIH | 3 | 0 | 3 |
| Miscellaneous | 17 | 9 | 9 |
| Total bilirubin (μmol/L) | 28.00 (44.728) | 39.92 (60.139) | 16.08 (13.108) |
| ALP (IU/L) | 127.33 (66.902) | 144.56 (75.900) | 109.38 (51.679) |
| GGT (IU/L) | 147.68 (192.683) | 160.96 (166.671) | 134.96 (217.570) |
| ALT (IU/L) | 60.84 (12.219) | 48.52 (29.427) | 73.16 (83.490) |
| AST (IU/L) | 62.50 (49.043) | 64.88 (32.720) | 60.12 (61.872) |
| Albumin (g/L) | 34.10 (6.129) | 30.88 (5.761) | 37.32 (4.679) |
| Platelet count (× 109) | 151.42 (73.016) | 112.16 (60.023) | 190.68 (63.804) |
| Prothrombin time (sec) | 12.572 (1.9886) | 13.470 (2.2077) | 11.540 (1.0007) |
| INR | 1.184 (0.1742) | 1.260 (0.1871) | 1.108 (0.1222) |
| EGD (PH present) | 25 | 25 | 0 |
| HVPG (PH present) | 5 | 5 | 0 |
We hypothesized that clinical parameters associated with CLD may predict the presence of CSPH. The clinical parameters tested were BMI, ALT, AST, GGT, ALP, bilirubin, platelet count, albumin, prothrombin time, APRI score, liver stiffness, spleen stiffness, spleen area and spleen diameter. The univariate analysis showed that bilirubin, platelet count, albumin, prothrombin time, APRI score, liver stiffness, spleen area and diameter correlated with the presence of CSPH (Table 2). The best individual predictor of CSPH was platelet count (AUROC 0.846, P value < 0.001), followed by spleen area (AUROC 0.828, P value = 0.002) and APRI score (AUROC 0.827, P value < 0.001). No statistically significant discrimination was found between liver stiffness measured by the ElastPQ and CSPH (AUROC 0.657, P value = 0.061).
| Variable | Odds ratio | Cut-off | P value | Estimated AUROC curve | P value of AUROC |
| BMI | 0.969 | > 26.42 | 0.425 | 0.506 | 0.951 |
| ALT (IU/L) | 0.993 | > 94.00 | 0.147 | 0.514 | 0.869 |
| AST (IU/L) | 1.002 | > 64.00 | 0.728 | 0.652 | 0.065 |
| GGT (IU/L) | 1.001 | > 85.00 | 0.639 | 0.643 | 0.094 |
| ALP (IU/L) | 1.001 | > 96.00 | 0.050 | 0.678 | 0.033 |
| Bilirubin | 1.037 | > 11.00 | 0.013a | 0.722 | 0.007b |
| Platelet count (× 109) | 0.979 | < 126.00 | < 0.001c | 0.846 | < 0.001 |
| Albumin (g/L) | 0.786 | < 33.00 | < 0.001c | 0.820 | < 0.001c |
| Prothrombin time (sec) | 2.721 | > 12.20 | < 0.001c | 0.800 | < 0.001c |
| APRI score | 2.030 | > 0.81 | 0.008b | 0.827 | < 0.001c |
| ElastPQ median liver stiffness (kPa) | 1.048 | > 10.16 | 0.021a | 0.657 | 0.061 |
| ElastPQ median spleen stiffness (kPa) | 1.007 | > 29.99 | 0.368 | 0.712 | 0.010b |
| ElastPQ spleen area (cm2) | 1.075 | > 57.90 | < 0.001c | 0.828 | 0.002c |
| ElastPQ spleen diameter (cm) | 1.651 | > 13.90 | 0.002c | 0.804 | 0.007b |
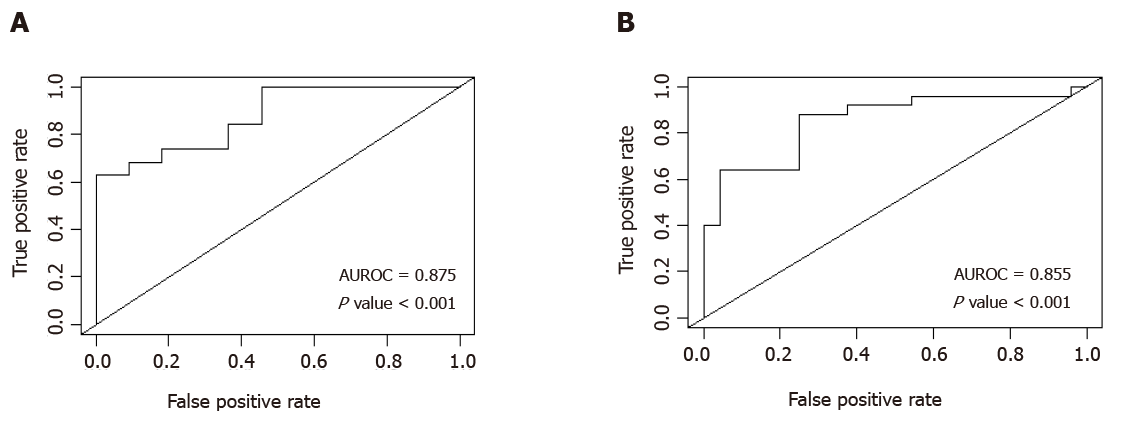
A multiple logistic regression model showed that two combinations independently predict CSPH (Table 3). The combination with the greatest diagnostic accuracy revealed that patients with a combination of spleen area > 57.9 cm2 and platelet count < 126 × 109 produced an estimated area under receiver operating characteristic (AUROC) curve of 0.876 (P value < 0.001), with sensitivity of 63.2%, specificity of 100%, positive predictive value (PPV) of 100% and a negative predictive value (NPV) of 61.1%. An alternative combination of spleen stiffness > 29.99 kPa and platelet count < 126 × 109 displayed a similar diagnostic accuracy with an estimated AUROC of 0.855 (P value < 0.001) and sensitivity of 88%, specificity of 75%, PPV of 78.6% and NPV of 85.7%. AUROC curves are displayed in Figure 3.
| Variables | AUROC curve | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| ElastPQ median spleen area (> 57.90 cm2) and Platelet count (< 126 × 109) | 0.875 | 63.2 | 100.0 | 100.0 | 61.1 |
| ElastPQ median spleen stiffness (> 29.99 kPa) and platelet count (< 126 × 109) | 0.855 | 88.0 | 75.00 | 78.6 | 85.7 |
An estimated single measures one-way random ICC for inter-operator variability for splenic area generated a value of 0.98 (95%CI: 0.94-0.99, P value < 0.001). Similarly, splenic diameter generated a value of 0.96 (95%CI: 0.91-0.99, P value < 0.001). Table 4 outlines the inter-operator ICC values for spleen area and diameter.
| Spleen area | Spleen diameter | |
| Number of operators | 2 | 2 |
| Number of measurements | 19 | 19 |
| Mean | 0.98 | 0.96 |
| 95% confidence interval | 0.94-0.99 | 0.91-0.99 |
The present study aimed to assess the performance of non-invasive splenic parameters using a new generation p-SWE machine–the ElastPQ–in identifying the presence of CSPH. We demonstrated that spleen stiffness (AUROC 0.712), spleen area (AUROC 0.828) and splenic diameter (AUROC 0.804) may predict the presence of CSPH in patients with mixed underlying etiologies. Adding platelet count to either spleen area (AUROC 0.875) or spleen stiffness (AUROC 0.855) increased diagnostic accuracy. Splenic area and diameter showed little inter-operator variability.
Our findings that spleen stiffness measured by p-SWE has a good diagnostic accuracy (cut-off > 29.99, AUROC 0.712) in identifying patients with CSPH is supported by other studies in the literature[16-18]. However, these studies report varying diagnostic threshold values and performance (AUROC 0.970-0.688)[16-18]. Differences between studies may be explained by varying methodologies employed, as well as use of different p-SWE techniques. Nevertheless a recent study, which adopted a similar methodology to our own, identified a cut off of < 31 kPa to rule out the presence of EV of any grade which resonates with our findings[19].
Interestingly, our study did not identify liver stiffness as a predictor of CSPH, which differs from findings of recent studies[16,18,20] and suggestions made by the Baveno VI Guidelines[3]. But, contrasting findings are not uncommon, as differences between studies within the literature are also seen. A possible explanation for this may lie in the heterogeneity of populations in our study and between studies in the literature. Furthermore, studies comparing the ElastPQ technique to VTQ have shown significantly lower liver stiffness values, which may provide an added explanation for discrepancies seen[21]. Given the novelty of the ElastPQ, research focus has remained on its ability to detect fibrosis in comparison to other elastography techniques such as TE and VTQ[21-23]. As a result, there is limited data on the ability of ElastPQ to predict the presence of CSPH.
Splenic area and diameter demonstrated a modest ability to diagnose the presence of CSPH. Previous studies have explored spleen size by consideration of splenic diameter[22], which has shown to have acceptable reproducibility in the context of platelet count/spleen diameter ratio. However, to our knowledge, there has only been one other study which has considered spleen area as a potential non-invasive diagnostic parameter. In this study, Giuffrè et al[19] reported similar findings with a median splenic area of 59.2 cm2 and diameter of 13.1 cm in its cohort of 210 patients[19]. Given the excellent reproducibility seen in our study and confirmation of similar findings in one other study, spleen area may be a useful adjunct in predicting CSPH. Further research with an external cohort is needed to validate our findings.
Perhaps one of the most striking results from our study is the diagnostic accuracy of the APRI score. A cut-off value of > 0.81 generated an AUROC of 0.827 in predicting the presence of CSPH. This was comparable to diagnostic performance of spleen stiffness and spleen area. Nevertheless, these findings differ to those in the literature, which has described lower sensitivities in higher cut-off values[24-26]. Only one study by Salzl et al[16] demonstrated a similar diagnostic performance (AUROC 0.805), but the cut-off value (1.90) remained higher than seen in our cohort[16]. Differences between studies may be reflected by the smaller sample size and varied etiology within our study population. Of note, the study by Giuffrè et al[19], which had a similar study population, demonstrated APRI to be a statistically significant determinant of CSPH with a similar median of 0.70[19]. Although highly applicable due to its non-invasive nature, the APRI score is affected by inflammatory processes such as acute hepatitis, which can generate false positive results that are not seen with p-SWE[4]. We propose that the APRI score may be a useful tool in the follow-up of CLD patients in primary care while p-SWE may fare better in secondary practice.
Since the introduction of liver stiffness measurements by TE, combinations of liver stiffness with spleen size have been carried out. The most common of these is the LSPS score[27-29]. However, despite spleen stiffness being increasingly recognized as a better predictor of CSPH[13], few studies have been carried out combining spleen stiffness measurements to other markers of CLD. Our study showed that the combination of spleen stiffness measurements and platelet counts has a high diagnostic index (AUROC 0.855). Although an exact model has not been replicated, a similar model applying spleen stiffness measurements measured by TE and the Baveno guidelines VI has shown promising results[30]. Colecchia et al[30] utilised a combined model where a cut-off of ≤ 46 kPa for spleen stiffness and < 20 kPa for liver stiffness measurements by TE, and a platelet count > 150000/mm3 could effectively rule out CSPH in cACLD patients[30]. A different study by Bota et al[31] used a different combination index of liver stiffness and spleen stiffness measured by VTQ, and presence of ascites which generated an AUROC of 0.721[31]. Finally, Giuffrè et al[19] was perhaps the most comparable of all the studies mentioned as his team used the ElastPQ model to develop the spleen stiffness probability index[19]. All of the findings above support the premise that a combination of non-invasive parameters may be a better diagnostic indicator than a single parameter alone. No study in the literature has considered addition of spleen area to combination variables. Further studies are needed to validate our proposed spleen area and platelet count combination and determine which set of non-invasive parameters generate the best accuracy.
Both the use of a novel p-SWE machine (ElastPQ) and investigation of spleen area describe a unique approach in our study compared to others carried out in the field. To our knowledge, this is one of the first studies to assess the role of spleen stiffness, spleen area and splenic diameter measurements in predicting CSPH using the ElastPQ. As a result, our study took into consideration inter-operator variability of splenic area and diameter, which supported its potential use in clinical practice. The prospective recruitment of patients with mixed etiologies described a population representative in clinical practice, but in view of the novelty of p-SWE it would be worth determining the effect of specific etiologies on splenic measurements.
This study has some limitations, the most pertinent of which being that we only assessed for presence or absence of PH, rather than degree of PH. Furthermore, an interval gap of one year between spleen stiffness measurements and EGD/HVPG readings may represent a consistent bias within our study due to the considerable length of time between readings. However, it could be argued that the correlation between CSPH and spleen stiffness may be better if there were a shorter time interval proposed. We did not exclude patients taking pharmacological treatment for PH from the original protocol as it was suspected that non-selective beta blockers and banding of varices would be unlikely to affect splenic measurements[32]. However, the most recent data on cirrhotic patients with high risk varices suggests that taking non-selective beta blockers can affect splenic stiffness[33,34]. Nevertheless these studies were undertaken using Fibroscan® and VTQ (Siemens Acuson S2000TM) ultrasound systems and so, further information is still needed in order to confirm that similar findings are present with the ElastPQ.
Although IQR measurements were taken, the validity of spleen stiffness and liver stiffness could not be determined as quality criteria has not yet been established for this technique[4]. However, Pawluś et al[35] conducted a small study in which he measured the spleen stiffness of 59 healthy volunteers using p-SWE, which has provided a reference point of 16.6 ± 2.5 kPa as the normal range with good reproducibility of measurement results[35]. Given the similar methodologies, this has supported our findings despite the small sample size in this study. Ultimately, further studies are needed to validate our findings, but our multivariate models suggest that findings are likely to correlate with CSPH in larger cohorts.
p-SWE is a non-invasive, rapid tool that carries minimal complications. It is painless, better tolerated than current gold-standard techniques and is more applicable than TE. Furthermore, this technique can be implemented on regular ultrasound machines and performed during routine screening for HCC in cirrhosis, which is likely to be cost-effective and less time-consuming.
Combinations of spleen area and platelet count, or spleen stiffness and platelet count as measured by the ElastPQ may be safe and effective methods to diagnose CSPH. Currently, this non-invasive technique cannot replace gold-standard as further studies are needed to create validation criteria and assess the diagnostic accuracy of non-invasive parameters in patients with differing degrees of PH.
Portal hypertension is a major complication of cirrhosis with a significant morbidity and mortality associated with it. Many of those with advanced chronic liver disease have esophageal varices and so, many patients undergo the gold-standard invasive procedures of performing an esophago-gastroduodenoscopy (EGD) or having the hepatic venous pressure gradient measurement taken through interventional radiology. However, both of these methods are invasive and carry a risk of complications.
Current guidelines propose that non-invasive methods can predict the incidence of clinically significant portal hypertension (CSPH). The latest guidelines suggest cirrhosis patients with a liver stiffness measurement < 20 kPa and a platelet count > 150000/μL can avoid screening endoscopy. Nevertheless, new algorithms are still required, as up to 40% of EGDs continue unnecessarily.
The aim of this study was to assess whether spleen stiffness measurement, spleen area and spleen diameter can independently predict CSPH, or in combination with other biochemical or elastography parameters. We also aimed to assess reproducibility of splenic area and diameter measurements.
This was a single-centre prospective cohort study where a total of 50 patients were split into two groups and included in a retrospective analysis: 25 with evidence of CSPH (group 1) and 25 with no evidence of CSPH (group 2). The Philips EPIQ7 [elastography point quantification (ElastPQ)] (Philips Medical Systems, Seattle, United States) was used to record liver stiffness, spleen stiffness, spleen area and spleen diameter measurements for each patient. Univariate, multivariate and one-way random interclass correlation coefficient analyses were performed to assess the diagnostic accuracy of splenic parameters.
Body mass index, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, bilirubin, platelet count, albumin, prothrombin time, aspartate aminotransferase-to-platelet-ratio-index (APRI) score, liver stiffness, spleen stiffness, spleen area and spleen diameter were assessed in their ability to predict the presence of CSPH. A univariate analysis showed the best individual predictor of CSPH was platelet count [area under the receiver operating characteristic (AUROC) 0.846, P value < 0.001], followed by spleen area (AUROC 0.828, P value = 0.002) and APRI score (AUROC 0.827, P value < 0.001). A multiple logistic regression model revealed that two combinations independently predict CSPH. The combination with the greatest diagnostic accuracy included a combination of spleen area > 57.9 cm2 and platelet count < 126 × 109 which had 63.2% sensitivity, 100% specificity, 100% positive predictive value (PPV), 61.1% negative predictive value (NPV) (AUROC 0.876, P value < 0.001). An alternative combination of spleen stiffness >29.99 kPa and platelet count < 126 × 109 displayed a similar diagnostic accuracy with 88% sensitivity, 75% specificity, 78.6% PPV, 85.7% NPV (AUROC 0.855, P value < 0.001). Spleen area and spleen diameter demonstrated little inter-operator variability as measured by a one-way random interclass correlation coefficient (spleen area: 0.98, P value < 0.001; spleen diameter: 0.96, P value < 0.001).
Combinations of spleen area and platelet count, or spleen stiffness and platelet count as measured by the ElastPQ may be safe and effective methods to diagnose CSPH. At present this cannot replace the gold standard.
Performing large scale prospective studies with long-term follow-up and are needed to validate our findings.
We recognise Hoogenboom T and Patel N for their help and advice.
| 1. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 2. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 649] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2358] [Article Influence: 214.4] [Reference Citation Analysis (4)] |
| 4. | European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1381] [Article Influence: 125.5] [Reference Citation Analysis (1)] |
| 5. | Augustin S, Pons M, Genesca J. Validating the Baveno VI recommendations for screening varices. J Hepatol. 2017;66:459-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 7. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 793] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 8. | Ravaioli F, Colecchia A, Dajti E, Marasco G, Alemanni LV, Tamè M, Azzaroli F, Brillanti S, Mazzella G, Festi D. Spleen stiffness mirrors changes in portal hypertension after successful interferon-free therapy in chronic-hepatitis C virus patients. World J Hepatol. 2018;10:731-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Giuffrè M, Fouraki S, Campigotto M, Colombo A, Visintin A, Buonocore MR, Aversano A, Budel M, Tinè F, Abazia C, Masutti F, Crocè LS. Alanine aminotransferase and spleno-portal dynamics affect spleen stiffness measured by point shear-wave elastography in patients with chronic hepatitis C in the absence of significant liver fibrosis. J Ultrasound. 2020;:. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J, Zeuzem S, Sarrazin C. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 13. | Manatsathit W, Samant H, Kapur S, Ingviya T, Esmadi M, Wijarnpreecha K, McCashland T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol. 2018;33:1696-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Abraldes JG, Reverter E, Berzigotti A. Spleen stiffness: toward a noninvasive portal sphygmomanometer? Hepatology. 2013;57:1278-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3344] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 16. | Salzl P, Reiberger T, Ferlitsch M, Payer BA, Schwengerer B, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Evaluation of portal hypertension and varices by acoustic radiation force impulse imaging of the liver compared to transient elastography and AST to platelet ratio index. Ultraschall Med. 2014;35:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Takuma Y, Morimoto Y, Takabatake H, Toshikuni N, Tomokuni J, Sahara A, Matsueda K, Yamamoto H. Measurement of Spleen Stiffness With Acoustic Radiation Force Impulse Imaging Predicts Mortality and Hepatic Decompensation in Patients With Liver Cirrhosis. Clin Gastroenterol Hepatol 2017; 15: 1782-1790. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Attia D, Schoenemeier B, Rodt T, Negm AA, Lenzen H, Lankisch TO, Manns M, Gebel M, Potthoff A. Evaluation of Liver and Spleen Stiffness with Acoustic Radiation Force Impulse Quantification Elastography for Diagnosing Clinically Significant Portal Hypertension. Ultraschall Med. 2015;36:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Giuffrè M, Macor D, Masutti F, Abazia C, Tinè F, Bedogni G, Tiribelli C, Crocè LS. Spleen Stiffness Probability Index (SSPI): A simple and accurate method to detect esophageal varices in patients with compensated liver cirrhosis. Ann Hepatol. 2020;19:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Sporea I, Bota S, Grădinaru-Taşcău O, Şirli R, Popescu A. Comparative study between two point Shear Wave Elastographic techniques: Acoustic Radiation Force Impulse (ARFI) elastography and ElastPQ. Med Ultrason. 2014;16:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lee JE, Shin KS, Cho JS, You SK, Min JH, Kim KH, Song IS, Cheon KS. Non-invasive Assessment of Liver Fibrosis with ElastPQ: Comparison with Transient Elastography and Serologic Fibrosis Marker Tests, and Correlation with Liver Pathology Results. Ultrasound Med Biol. 2017;43:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Ma JJ, Ding H, Mao F, Sun HC, Xu C, Wang WP. Assessment of liver fibrosis with elastography point quantification technique in chronic hepatitis B virus patients: a comparison with liver pathological results. J Gastroenterol Hepatol. 2014;29:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Berzigotti A, Gilabert R, Abraldes JG, Nicolau C, Bru C, Bosch J, García-Pagan JC. Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis. Am J Gastroenterol. 2008;103:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Tafarel JR, Tolentino LH, Correa LM, Bonilha DR, Piauilino P, Martins FP, Rodrigues RA, Nakao FS, Libera ED, Ferrari AP, da Silveira Röhr MR. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. Eur J Gastroenterol Hepatol. 2011;23:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Mattos AZ, Alves de Mattos A, Daros LF, Musskopf MI. Aspartate aminotransferase-to-platelet ratio index (APRI) for the non-invasive prediction of esophageal varices. Ann Hepatol. 2013;12:810-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013; 144: 102-111. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 28. | Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim DY. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I, Matsueda K, Yamamoto H. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 2013; 144: 92-101. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Colecchia A, Ravaioli F, Marasco G, Colli A, Dajti E, Di Biase AR, Bacchi Reggiani ML, Berzigotti A, Pinzani M, Festi D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol. 2018;69:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 31. | Bota S, Sporea I, Sirli R, Focsa M, Popescu A, Danila M, Strain M. Can ARFI elastography predict the presence of significant esophageal varices in newly diagnosed cirrhotic patients? Ann Hepatol. 2012;11:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Piscaglia F, Donati G, Cecilioni L, Celli N, Stagni B, Pini P, Gaiani S, Gherlinzoni F, Bolondi L. Influence of the spleen on portal haemodynamics: a non-invasive study with Doppler ultrasound in chronic liver disease and haematological disorders. Scand J Gastroenterol. 2002;37:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Marasco G, Dajti E, Ravaioli F, Alemanni LV, Capuano F, Gjini K, Colecchia L, Puppini G, Cusumano C, Renzulli M, Golfieri R, Festi D, Colecchia A. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol Int. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Kim HY, So YH, Kim W, Ahn DW, Jung YJ, Woo H, Kim D, Kim MY, Baik SK. Non-invasive response prediction in prophylactic carvedilol therapy for cirrhotic patients with esophageal varices. J Hepatol. 2019;70:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Pawluś A, Inglot MS, Szymańska K, Kaczorowski K, Markiewicz BD, Kaczorowska A, Gąsiorowski J, Szymczak A, Inglot M, Bladowska J, Zaleska-Dorobisz U. Shear wave elastography of the spleen: evaluation of spleen stiffness in healthy volunteers. Abdom Radiol (NY). 2016;41:2169-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giuffrè M S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT