Published online Sep 27, 2019. doi: 10.4254/wjh.v11.i9.678
Peer-review started: May 29, 2019
First decision: June 13, 2019
Revised: July 8, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 27, 2019
Processing time: 122 Days and 6.3 Hours
Despite being the world’s most widely used system for staging and therapeutic guidance in hepatocellular carcinoma (HCC) treatment, the Barcelona clinic liver cancer (BCLC) system has limitations, especially regarding intermediate-grade (BCLC-B) tumors. The recently proposed Hong Kong liver cancer (HKLC) staging system appears useful but requires validation in Western populations.
To evaluate the agreement between BCLC and HKLC staging on the management of HCC in a Western population, estimating the overall patient survival.
This was a retrospective study of HCC patients treated at a university hospital in southern Brazil between 2011 and 2016. Demographic, clinical, and laboratory data were collected. HCC staging was carried out according to the HKLC and BCLC systems to assess treatment agreement. Overall survival was estimated based on the treatment proposed in each system.
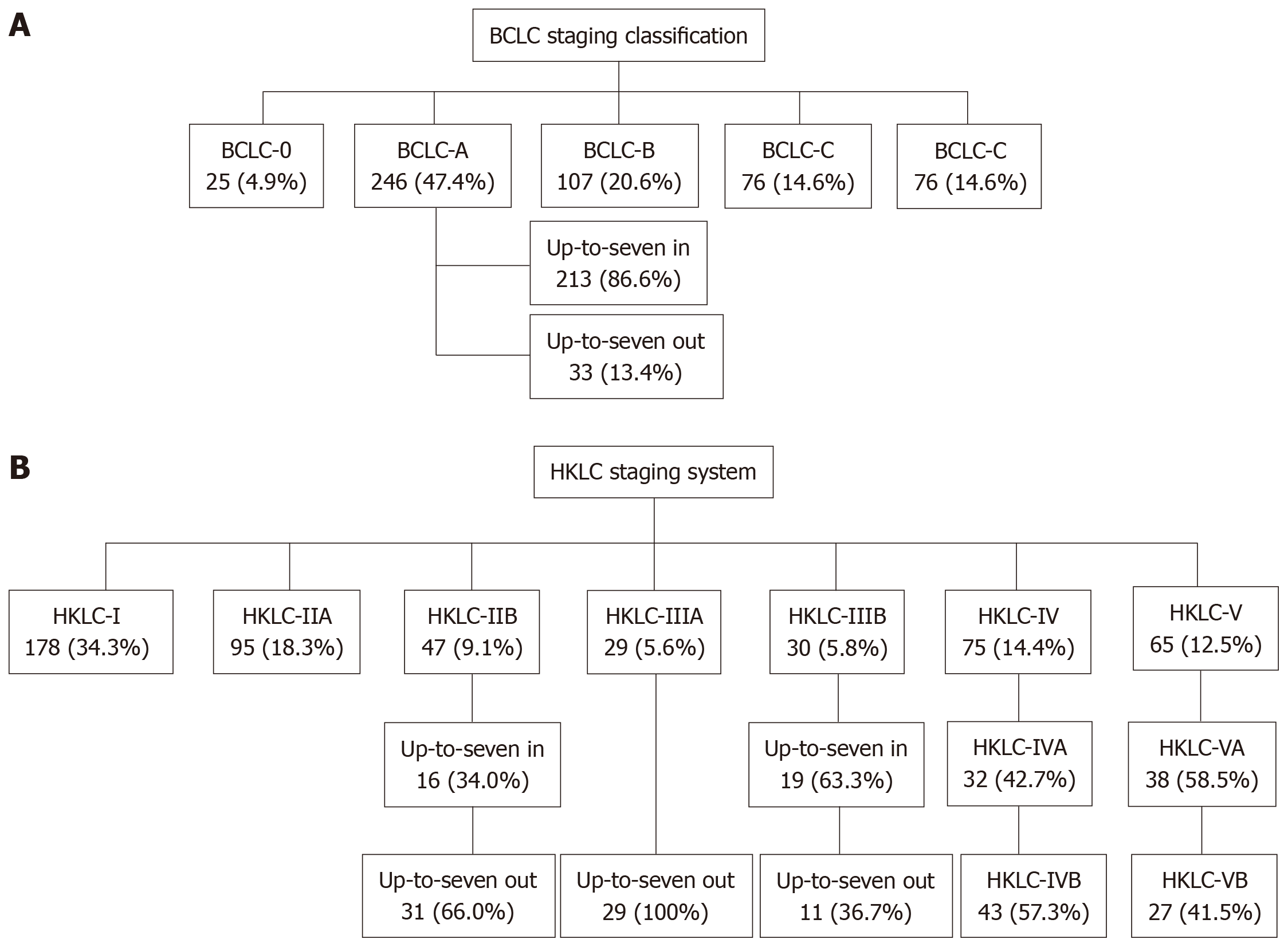
A total of 519 HCC patients were assessed. Of these, 178 (34.3%) were HKLC-I; 95 (18.3%) HKLC-IIA; 47 (9.1%) HKLC-IIB; 29 (5.6%) HKLC-IIIA; 30 (5.8%) HKLC-IIIB; 75 (14.4%) HKLC-IV; and 65 (12.5%) HKLC-V. According to the BCLC, 25 (4.9%) were BCLC-0; 246 (47.4%) BCLC-A; 107 (20.6%) BCLC-B; 76 (14.6%) BCLC-C; and 65 (12.5%) BCLC-D. The general agreement between the two systems was 80.0% - BCLC-0 and HKLC-I (100%); BCLC-A and HKLC-I/HKLC-II (96.7%); BCLC-B and HKLC-III (46.7%); BCLC-C and HKLC-IV (98.7%); BCLC-D and HKLC-V (41.5%). When sub-classifying BCLC-A, HKLC-IIB, HKLC-IIIA and HKLC-IIIB stages according to the up-to-7 in/out criterion, 13.4, 66.0, 100 and 36.7%, respectively, of the cases were classified as up-to-7 out.
In a Western population, the general agreement between the two systems was 80.0%, although in BCLC-B cases the agreement was low, suggesting that some individuals could be candidates for the curative treatment recommended by the HKLC. The authors suggest that the BCLC system should be routinely employed, although for BCLC-B cases it should be associated with the HKLC system.
Core tip: Despite being the world’s most widely used system for staging and therapeutic guidance in hepatocellular carcinoma (HCC) treatment, the Barcelona clinic liver cancer (BCLC) system has limitation. Proposed Hong Kong liver cancer (HKLC) staging appears useful but requires validation in Western populations. This study showed that there is adequate agreement between the HKLC and BCLC systems regarding therapeutic management of HCC in Western populations, except in cases of intermediate HCC. Although staging systems should be further refined to cover the full diversity of HCC cases, these findings suggest that the BCLC system, which is more simple and intuitive, should be applied in all HCC cases, and that in BCLC-A and, especially, BCLC-B cases, the HKLC can contribute important information regarding patient management.
- Citation: Freitas LBR, Longo L, Santos D, Grivicich I, Álvares-da-Silva MR. Hepatocellular carcinoma staging systems: Hong Kong liver cancer vs Barcelona clinic liver cancer in a Western population. World J Hepatol 2019; 11(9): 678-688
- URL: https://www.wjgnet.com/1948-5182/full/v11/i9/678.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i9.678
Hepatocellular carcinoma (HCC) accounts for more than 90% of primary malignant neoplasms, being the sixth most prevalent type of cancer and the second most common cause of cancer-related mortality worldwide[1-3]. Although most cases occur in developing countries, their incidence in developed countries has increased in recent years due to the high prevalence of chronic hepatitis C, immigration from areas where hepatitis B and hepatitis C are common, and the increased prevalence of non-alcoholic fatty liver disease (NAFLD)[2-4]. Heterogeneous data on HCC incidence have been reported in Latin America[2,5-7]. In Brazil, the HCC incidence varies from 3.3%-6.0% per 100000 per year, and the mortality rates are similar due to high intrahepatic recurrence, distant metastasis and lack of effective treatment for cases diagnosed at an advanced stage[8,9]. The prognosis is generally somber and essentially depends on the tumor stage at diagnosis. In cirrhosis patients, the American Association for the Study of the Liver Diseases (AASLD) recommends screening for HCC by ultrasound, with or without an alpha-fetoprotein test, every six months[10,11]. Although HCC is commonly associated with cirrhosis, approximately one in five cases are unrelated to it. In such cases, the tumor is often detected in advanced stages, since non-cirrhotic patients are usually not screened. Chronic hepatitis B or NAFLD patients may also develop a tumor without associated cirrhosis[12,13]. In recent years, five-year HCC survival rates have improved considerably due to early detection and curative therapies[14]. However, despite efforts to detect the disease in early stages among patients with risk factors, a substantial number of cases are diagnosed at intermediate and late stages, when the survival rate is lower[4,15]. In Brazil, recent DATASUS figures indicate that upon diagnosis, palliative care is the only possible treatment in 62.2% of the cases[16].
Treating patients with HCC is not simple, since two serious diseases usually coincide: Cirrhosis and a malignant tumor. A number of staging systems have proposed treatment guidelines for HCC according to evolutionary stage[17-21]. The Barcelona Clinic Liver Cancer (BCLC) staging system, which considers tumor characteristics, liver function and performance status, is the most widely used and endorsed system in Western HCC management guidelines[4,11,17,22]. Published in 2014, the Hong Kong liver cancer (HKLC) staging system identifies subsets of patients with intermediate and advanced HCC and proposes more aggressive treatment to improve survival rates[18]. However, the HKLC system still requires validation in Western populations, since it was developed at a single Asian center that principally treats patients infected with the hepatitis B virus (HBV)[18,23]. Both systems suggest curative, supportive or palliative care according to the patient’s stage. The objective of this study was to assess the agreement of BCLC and HKLC therapeutic approaches according to HCC evolutionary stage in a Western population.
This retrospective cross-sectional study analyzed data from the medical records of individuals over 18 years of age diagnosed with HCC and treated at a referral service in a university hospital in southern Brazil between 2011 and 2016. Upon diagnosis, demographic and clinical data and laboratory results were collected, as well as performance status and Child-Pugh (CP) scores. Diagnosis was based on AASLD criteria[17]. Tumor characteristics (size, number of nodules, intra- and/or extrahepatic dissemination) were assessed with dynamic imaging (computed tomography or magnetic resonance imaging) prior to treatment and near the time of diagnosis. Tumors were considered multifocal when they involved more than three nodules, regardless of size. The management of each complication presented in decompensated patients was made accordingly the hospital Liver Unit protocol.
The patients were staged according to BCLC criteria[24,25]. For the purposes of this study, since the BCLC does not set a tumor size limit for BCLC-A cases, patients thus staged were classified according to the up-to-7 criterion as either in or out, i.e., when the sum of the nodules plus the diameter of the largest nodule is ≤ 7, these patients are most likely candidates for curative treatment, whereas when it is > 7, palliative treatment is usually recommended. The patients were also staged according to the HKLC system[18]. According to tumor size in relation to the proposed treatment, HKLC-IIB, -IIIA and -IIIB patients were subclassified as up-to-7 in/out. The approach of decompensated patients with BCLC, CP-B patients were managed with curative or palliative therapies depending on HCC characteristics and the presence of metastasis and/or vascular invasion, while CP-C ones were candidates for best supportive care. When applying HKLC scheme, CP-B patients were managed with curative or palliative therapies depending on HCC characteristics and the presence of metastasis and/or vascular invasion, while CP-C ones were either candidates for liver transplantation or best supportive care.
After the patients were staged according to both systems, the systems’ agreement regarding therapeutic approach for different stages was analyzed (Table 1). Overall patient survival was estimated from HCC diagnosis until the outcome, i.e., death, loss of follow-up, or the date of the last appointment at the referral hospital.
| BCLC-0 | BCLC-A | BCLC-B | BCLC-C | BCLC-D | |
| (ablation, LR or LT) | (ablation, LR or LT) | (TACE) | (SOR) | (ST) | |
| HKLC-I (ablation, LR or LT) | √ | √ | |||
| HKLC-IIA (ablation, LR or LT) | √ | √ | |||
| HKLC-IIB (LR) | √ | √ | |||
| HKLC-IIIA (TACE) | √ | ||||
| HKLC-IIIB (TACE) | √ | ||||
| HKLC-IVA (SOR) | √ | ||||
| HKLC-IVB (SOR or ST) | √ | ||||
| HKLC-VA (LT) | |||||
| HKLC-VB (ST) | √ |
This study was approved by the Hospital de Clínicas de Porto Alegre Ethics Committee (CAAE 57899016.8.0000.5327) and followed recommended guidelines for studies of human subjects.
Quantitative variables were expressed as mean ± SD or median and interquartile range (25th-75th). Categorical variables were described as frequencies and percentages. The Kaplan-Meier curve was applied to estimate survival time, and the log-rank test was used to calculate survival probability. A P ≤ 0.05 was considered statistically significant. Data were stored and processed using the Statistical Package for the Social Sciences 18.0 (SPSS Inc., Chicago, IL, United States).
A total of 568 patients were diagnosed with HCC according to AASLD criteria between 2011 and 2016. Of these, 49 (8.6%) were excluded because their medical records included no CP score report and/or no assessment of performance status. Thus, the final sample totaled 519 patients.
The patients’ demographic, laboratory and clinical data are described in Table 2. The median age at diagnosis was 60.9 (56.2-67.7) years; the sample was predominantly male (64.7%). The most common underlying etiology was hepatitis C virus infection (HCV – 78.4%), followed by alcohol abuse (37.4%). Most patients were staged as CP-A (52.6%), followed by CP-B (34.9%). Multifocal tumors were observed in 50.3% of the cases, and in 86.5% of the cases the size of the largest nodule was less than 10 cm.
| Variables1 | HCC (n = 519) |
| Race | |
| White | 462 (89.0) |
| Black | 35 (6.8) |
| Others | 22 (4.2) |
| Etiology | |
| HCV | 407 (78.4) |
| Alcohol abuse | 194 (37.4) |
| HBV | 31 (6.0) |
| NAFLD | 23 (4.4) |
| Cryptogenic cirrhosis | 9 (1.7) |
| Alpha-fetoprotein (ng/mL) | |
| < 400 | 361 (69.6) |
| ≥ 400 | 109 (21.0) |
| Data not available | 49 (9.4) |
| Child-Pugh score | |
| A | 273 (52.6) |
| B | 181 (34.9) |
| C | 65 (12.5) |
| Multifocal Tumor | |
| Yes | 261 (50.3) |
| No | 258 (49.7) |
| Tumor size, cm | |
| < 10 cm | 449 (86.5) |
| ≥ 10 cm | 30 (5.8) |
| Data not available | 40 (7.7) |
In the BCLC system, curative treatment is recommended for HCC stages BCLC-0 and A, palliative treatment with transarterial chemoembolization (TACE) is recommended for stage BCLC-B, systemic treatment is recommended for stage BCLC-C, and supportive treatment is the only alternative for BCLC-D. The cases, stratified according to the BCLC and HKLC systems, are shown in Figure 1.
According to the HKLC system, 178 (34.3%) of the 519 patients were HKLC-I and 95 (18.3%) were HKLC-IIA, stages in which curative therapy is recommended, including resection, liver transplantation or ablation. Another 47 cases (9.1%) were classified as HKLC-IIB, of which 16 (34.0%) were up-to-7 in and 31 (66.0%) were up-to-7 out. The HKLC system recommends resection in these cases. A total of 29 (5.6%) patients were classified as HKLC-IIIA, all of them up-to-7 out, and 30 (5.8%) were classified as HKLC-IIIB, for which the HKLC system indicates palliative care, with TACE as an optional procedure. However, of the 30 HKLC-IIIB cases, only 11 were up-to-7 out. In addition, 75 cases (14.4%) were classified as HKLC-IV, of which 32 (42.7%) were HKLC-IVA, for which systemic therapy is recommended. The 65 (12.5%) remaining cases were classified as HKLC-V, with 38 (58.5%) as HKLC-VA, i.e., candidates for liver transplant.
According to BCLC staging, 25 (4.9%) of the 519 patients were BCLC-0, 246 (47.4%) were BCLC-A, 213 (86.6%) of which were up-to-7 in and 33 (13.4%) up-to-7 out, 107 (20.6%) were BCLC-B (intermediate HCC), 76 (14.6%) were BCLC-C (advanced HCC), and 65 (12.5%) were BCLC-D (terminal HCC).
The treatment agreement between the BCLC and HKLC staging systems is shown in Table 3. The overall agreement for the two curative and palliative classifications was 80.0%. The treatment for all BCLC-0 cases was in agreement with that of HKLC-I. The agreement between BCLC-A cases and HKLC-I, HKLC-IIA and HKLC-IIB stages was 96.7%. However, the agreement between treatments for BCLC-B and parallel HKLC stages was only 46.7%. Agreement between BCLC-C and HKLC was 98.7%, including stages HKLC-IVA and HKLC-IVB. The agreement between BCLC-D and HKLC-V was also low (41.5%).
| Variables1 | BCLC-0 (ablation, LR or LT) | BCLC-A (ablation, LR or LT) | BCLC-B (TACE) | BCLC-C (SOR) | BCLC-D (ST) |
| HKLC-I (ablation, LR or LT) | 25 (100%) | 129 (52.4%) | 24 (22.4%) | ||
| HKLC-IIA (ablation, LR or LT) | 84 (34.1%) | 11 (10.3%) | |||
| HKLC-IIB (LR) | 25 (10.2%) | 22 (20.6%) | |||
| HKLC-IIIA (TACE) | 8 (3.3%) | 20 (18.7%) | 1 (1.3%) | ||
| HKLC-IIIB (TACE) | 30 (28.0%) | ||||
| HKLC-IVA (SOR) | 32 (42.1%) | ||||
| HKLC-IVB (SOR or ST) | 43 (56.6%) | ||||
| HKLC-VA (LT) | 38 (58.5%) | ||||
| HKLC-VB (ST) | 27 (41.5%) |
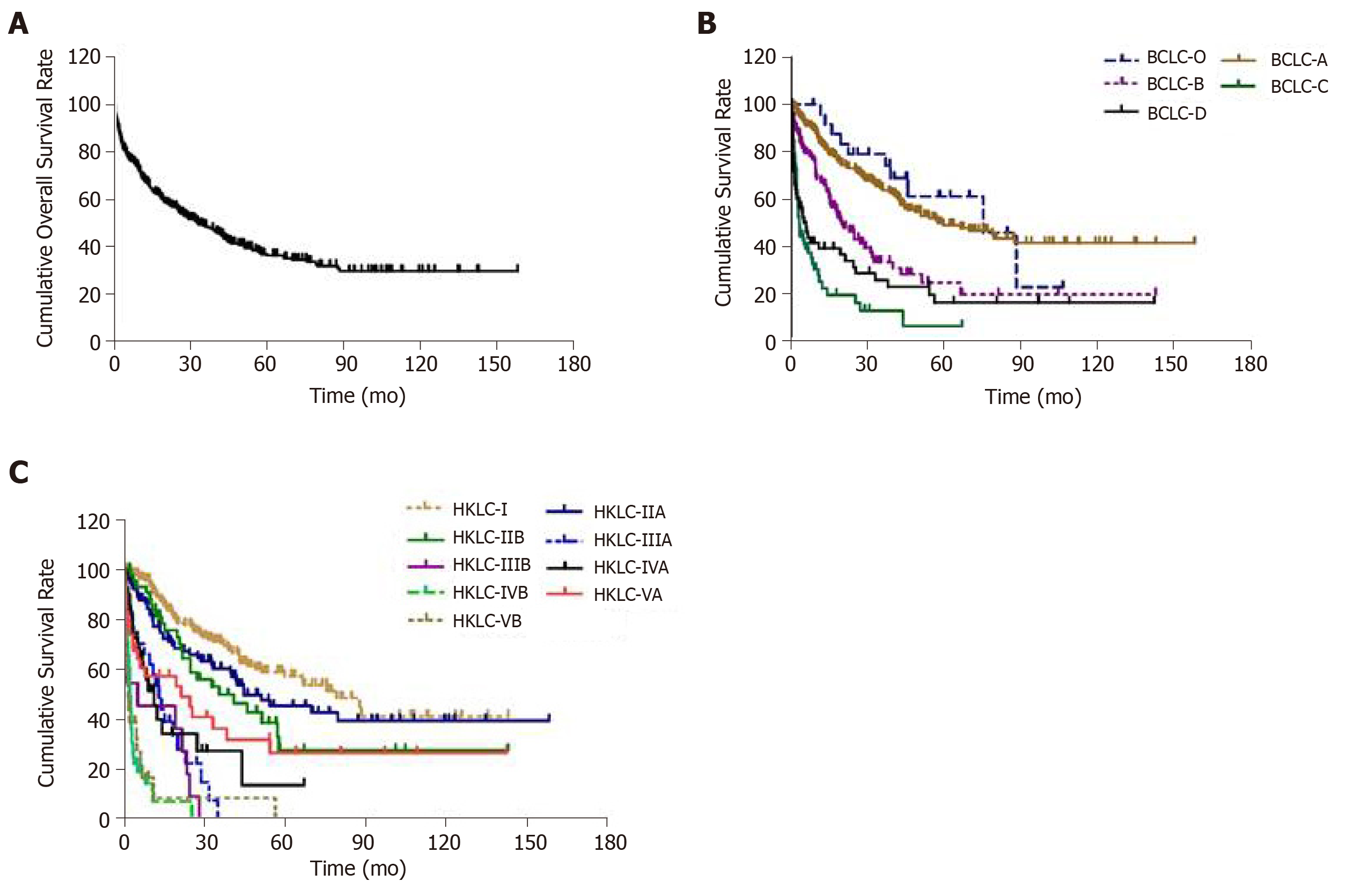
The median overall survival was 32.7 mo (95%CI: 25.1-40.3). A total of 265 patients (51.1%) had died by the time of data collection. The overall survival probability one year after diagnosis was 67.6%, which decreased to 35.9% after five years (Figure 2A).
The median overall survival was 75.7 mo (95%CI: 41.2–110.1) for BCLC-0 cases, 60.0 mo (95%CI: 38.0–81.9) for BCLC-A, 19.6 mo (95%CI: 11.5–27.6) for BCLC-B, 3.5 mo (95%CI: 2.6–4.3) for BCLC-C, and 5.2 mo (95%CI: 2.2–8.3) for BCLC-D (P < 0.001). The median overall survival rate was 79.2 mo (95%CI: 56.9–101.6) for HKLC-I; 44.7 mo (95%CI: 18.8–70.7) for HKLC-IIA; 35.5 mo (95%CI: 12.6–58.4) for HKLC-IIB; 13.2 months (95%CI: 8.6–17.7) for HKLC-IIIA, 4.7 mo (95%CI: 1.6–7.9) for HKLC-IIIB, 11.2 mo (95%CI: 2.5–6.2) for HKLC-IVA, 2.3 mo (95%CI: 1.7–3.0) for HKLC-IVB, 21.5 mo (95%CI: 1.9–41.0) for HKLC-VA, and 1.5 months (95%CI: 0.4–2.5) for HKLC-VB (P < 0.001).
Median overall survival of BCLC-0 and BCLC-A patients was significantly higher than BCLC-B (P = 0.001 and P < 0.001, respectively), BCLC-C (P < 0.001 and P < 0.001, respectively), and BCLC-D patients (P < 0.001 and P < 0.001, respectively). Median overall survival was significantly higher for BCLC-B patients than BCLC-C (P < 0.001) and BCLC-D (P = 0.011) patients. The overall survival probability of BCLC-0 and BCLC-A patients 7 years after diagnosis was similar: 46.0% and 44.0%, respectively. The survival probability of BCLC-B cases two years after diagnosis was 45.6%, which was significantly higher than BCLC-C (19.4%) and BCLC-D (30.5%) (Figure 2B).
The median overall survival for HKLC-I was significantly higher than HKLC-IIB (P = 0.015), HKLC-IIIA (P < 0.001), HKLC-IIIB (P < 0.001), HKLC-IVA (P < 0.001), HKLC-IVB (P < 0.001), HKLC-VA (P < 0.001), and HKLC-VB (P < 0.001). This significant increase in overall survival was also observed for HKLC-IIA and HKLC-IIB cases compared to HKLC-IIIA (P < 0.001), HKLC-IIIB (P < 0.001), HKLC-IVA (P < 0.001), HKLC-IVB (P < 0.001 and P = 0.002, respectively) and HKLC-VB (P < 0.001). Median overall survival for HKLC-IIIA, HKLC-IIIB and HKLC-IVA was significantly higher than HKLC-IVB (P < 0.001, P = 0.003 and P < 0.001, respectively) or HKLC-VB (P = 0.004, P = 0.023 and P < 0.001, respectively). Median overall survival for HKLC-VA was higher than HKLC-VB (P <0.001). The overall survival probability of HKLC-I patients 7 years after diagnosis was 48.7%. The overall survival probability for HKLC-IIA and HKLC-IIB cases 2 years after diagnosis was 67.3% and 64.5% respectively. This probability was lower for HKLC-IIIA (22.3%), HKLC-IIIB (30.7%) and HKLC-IVA (34.2%) patients. The overall survival probability of HKLC-VA patients 1 year after diagnosis was 57.3%, which was higher than that of HKLC-IVB (7.0%) or HKLC-VB (7.7%) patients (Figure 2C).
Over the last 30 years, a number of staging systems have been developed to address the interrelationship of prognostic factors in HCC patients and to propose an adequate course of therapy according to disease stage. However, due to the clinical, biological and etiological heterogeneity of different populations, there is no flawless staging system. Despite the fact that the BCLC is the most predominant system worldwide and is mandatory in HCC management, it involves controversial points, such as the maximum tumor diameter in BCLC-A and recommending TACE for all patients with intermediate tumors (BCLC-B). Moreover, especially in the latter case, the BCLC does not consider moving from palliative to curative therapy in TACE responders or escalating to systemic therapy for TACE non-responders or those who have multifocal tumors without metastases.
The objective of this study was to evaluate the agreement between BCLC and HKLC staging systems regarding therapeutic management of HCC in Western populations, and the results showed high general agreement between the two systems. However, agreement was low in intermediate HCC cases, indicating, as the HKLC suggests, that TACE is not mandatory for all BCLC-B cases. It was not surprising that agreement was also low for BCLC-D cases, since the BCLC suggests supportive treatment for CP-C patients, while according to HKLC this population could, depending on tumor mass, benefit from liver transplant. Because this discrepancy can be easily dealt with, BCLC-D patients will not be further discussed.
Tumor etiology is among the most significant variables in determining therapy type, and it involves important regional differences[2,3,26]. HCV infection and NAFLD, the main causes of HCC in Western populations, are usually associated with cirrhosis[10,26], while HBV infection is the leading cause of HCC in Asian and African populations. Many of these patients are not cirrhotic and have preserved liver function, which facilitates the success of curative treatments[26]. Thus, Asians with HCC could particularly benefit from HKLC, since it indicates more aggressive curative treatments than the BCLC. However, like many previous studies, the present study involved a Western sample[8,23,27,28]. Only 6% of the cases were HBV related, and most patients had chronic HCV infection. Cirrhosis was present in all cases, especially CP-A patients, characterizing a population with controlled liver disease, which facilitates more aggressive HCC treatments.
Although treatment selection is crucial for patient survival, determining the most appropriate therapy has been controversial[29,30]. A previous study showed that the HKLC system has greater discriminatory and prognostic power than the BCLC[18]. However, no external validation has been performed. Similar studies to this one have been conducted in different countries, but with disparate results[23,30-32]. A Korean study found that the overall survival of intermediate-stage HCC patients (BCLC-B) was higher for liver resection (which is proposed by HKLC) than TACE[33]. On the other hand, a multicenter study in France found that the HKLC system is not associated with better prognostic or therapeutic power than the BCLC [31]. This divergence is probably due to etiological and pathophysiological differences between Asian and European populations. The present study, conducted with Latin American patients, found high agreement between the staging systems, except for certain niches, especially patients with intermediate HCC. Most of the patients were candidates for curative treatments, which agrees with other published studies[23,26,31,34]. The fact that these patients were diagnosed with early-stage HCC can probably be attributed to screening, since these cirrhosis cases were diagnosed and followed up at a university institution, which contributed to greater overall survival. In addition, studies show that tumor diagnosis tends to occur at earlier stages in populations in which HCV and alcohol are the most frequent etiologies[23,31].
The median overall survival in this study was 32.7 mo, which was higher than the 12.7 mo observed by Yau et al[18]. Overall survival was significantly higher in BCLC-0 and BCLC-A than the other stages, as expected. In the HKLC system, the highest survival rates were in the HKLC-I, HKLC-IIA and HKLC-IIB stages.
Although the BCLC staging system is currently the main tool for determining the prognosis and treatment of HCC[17], it has been criticized for being too conservative, especially in therapeutic management of the BCLC-B stage[35-37]. This point was highlighted in the results of the present study since, according to the HKLC, more than 50% of these patients could have been candidates for curative treatment rather than the palliative treatment recommended by BCLC. These findings agree with prior publications in Asia and Europe[26,30,31,34].
Although the HKLC and BCLC staging systems are comprehensive, some patients do not fit neatly into the pre-specified categories. This could lead to different therapeutic recommendations for the same patient[37],which reinforces the need to further explore the issue. In this study, the highest agreement was found between stages HKLC-I and BCLC-0 (100%) and HKLC-IV and BCLC-C (98.7%). These results can be explained by the tumor characteristics and liver function common to both systems. Cases staged as BCLC-A, as well as HKLC-IIB, HKLC-IIIA and HKLC-IIIB, were subdivided according to the up-to-7 in/out criterion, which demonstrates the limitations of both systems, since they cannot clearly discriminate between patients who need curative or palliative care. Although the up-to-7 criterion is an anatomopathological, rather than radiological, classification, it has been used in a similar fashion in clinical practice. However, rather than indicating a limitation in these systems, it shows that therapy should be individualized[38]. We found BCLC-A and HKLC-IIB, HKLC-IIIA and HKLC-IIIB up-to-7 out patients who would not qualify as liver transplant candidates due to their tumor volume[39,40], as well as HKLC-IIIA and HKLC-IIIB patients who would not qualify as TACE candidates because they have nodules larger than 10 cm or have decompensated cirrhosis (CP-B and CP-C)[41].
This study presents some limitations that should be addressed. First, the differences in overall survival are not exactly real because they were estimated according to the different therapeutic options suggested by the systems. Some other should be considered, such as the fact that it is a single center, observational and retrospective analysis.
In conclusion, this study showed that there is adequate agreement between the HKLC and BCLC staging systems regarding therapeutic management of HCC in Western populations, except in cases of intermediate HCC (BCLC-B). However, it is clear that both systems have limitations, as demonstrated by the need to apply the up-to-7 criterion in BCLC-A, HKLC-IIB, HKLC-IIIA and HKLC-IIIB to determine when curative treatment should be recommended. Although staging systems should be further refined to cover the full diversity of HCC cases, these findings suggest that the BCLC system, which is more simple and intuitive, should be applied in all HCC cases, and that in BCLC-A and, especially, BCLC-B cases, the HKLC can contribute important information regarding patient management.
Treating patients with hepatocellular carcinoma (HCC) is not simple, since two serious diseases usually coincide: Cirrhosis and a malignant tumor. The Barcelona clinic liver cancer (BCLC) staging system, is the most widely used and endorsed system in Western HCC management guidelines. The Hong Kong liver cancer (HKLC) staging system identifies subsets of patients with intermediate and advanced HCC and proposes more aggressive treatment; however, this system still requires validation in Western populations. This study to assess the agreement of BCLC and HKLC therapeutic approaches according to HCC evolutionary stage in this population.
Evaluating the agreement of the treatments proposed by the BCLC and HKLC system according to HCC evolutionary stage in a Western population, can optimize the therapeutic approaches, promoting an increase in patient survival time.
This study aimed first to evaluate the agreement between BCLC and HKLC staging on the management of HCC in a Western population. Secondary aim was estimating the overall patient survival with HCC.
Retrospective cross-sectional study analyzed data from the medical records of individuals over 18 years of age diagnosed with HCC and treated at a referral service in a university hospital in southern Brazil between 2011 and 2016. Upon diagnosis, demographic and clinical data and laboratory results were collected, as well as performance status and Child-Pugh (CP) scores. Diagnosis was based on the American Association for the Study of the Liver Diseases criteria. The patients were staged according to BCLC criteria and HKLC system. After, the agreement of the treatment proposed by both systems was performed. Overall patient survival was estimated from HCC diagnosis until the outcome, i.e., death, loss of follow-up, or the date of the last appointment at the referral hospital.
A total of 519 HCC patients were assessed. Of these, 178 (34.3%) were HKLC-I; 95 (18.3%) HKLC-IIA; 47 (9.1%) HKLC-IIB; 29 (5.6%) HKLC-IIIA; 30 (5.8%) HKLC-IIIB; 75 (14.4%) HKLC-IV; and 65 (12.5%) HKLC-V. According to the BCLC, 25 (4.9%) were BCLC-0; 246 (47.4%) BCLC-A; 107 (20.6%) BCLC-B; 76 (14.6%) BCLC-C; and 65 (12.5%) BCLC-D. The general agreement between the two systems was 80.0% - BCLC-0 and HKLC-I (100%); BCLC-A and HKLC-I/HKLC-II (96.7%); BCLC-B and HKLC-III (46.7%); BCLC-C and HKLC-IV (98.7%); BCLC-D and HKLC-V (41.5%). When sub-classifying BCLC-A, HKLC-IIB, HKLC-IIIA and HKLC-IIIB stages according to the up-to-7 in/out criterion, 13.4, 66.0, 100 and 36.7%, respectively, of the cases were classified as up-to-7 out.
This study showed that there is adequate agreement between the BCLC and HKLC staging systems (80.0%) regarding therapeutic management of HCC in Western populations, although in BCLC-B cases the agreement was low, suggesting that some individuals could be candidates for the curative treatment recommended by the HKLC. However, it is clear that both systems have limitations to determine when curative treatment should be recommended. Although staging systems should be further refined to cover the full diversity of HCC cases, these findings suggest that the BCLC system should be routinely employed in Western populations; although for BCLC-B cases it should be associated with the HKLC system.
Demonstrated adequate agreement between the BCLC and HKLC systems in relation to the therapeutic management of HCC in Western population evaluated. However, new multicenter and prospective studies are needed to assess this issue in the Western population.
The authors would like to thank the Research Incentive Fund of the Hospital de Clínicas de Porto Alegre, CNPq (National Counsel of Technological and Scientific Development) and CAPES (Coordination for the Improvement of Higher Education Personnel) for financial support.
| 1. | Horvat N, Monti S, Oliveira BC, Rocha CCT, Giancipoli RG, Mannelli L. State of the art in magnetic resonance imaging of hepatocellular carcinoma. Radiol Oncol. 2018;52:353-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Piñero F, Poniachik J, Ridruejo E, Silva M. Hepatocellular carcinoma in Latin America: Diagnosis and treatment challenges. World J Gastroenterol. 2018;24:4224-4229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (12)] |
| 4. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1315] [Article Influence: 131.5] [Reference Citation Analysis (3)] |
| 5. | Paranaguá-Vezozzo DC, Ono SK, Alvarado-Mora MV, Farias AQ, Cunha-Silva M, França JI, Alves VA, Sherman M, Carrilho FJ. Epidemiology of HCC in Brazil: incidence and risk factors in a ten-year cohort. Ann Hepatol. 2014;13:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Appel-da-Silva MC, Miozzo SA, Dossin IA, Tovo CV, Branco F, de Mattos AA. Incidence of hepatocellular carcinoma in outpatients with cirrhosis in Brazil: A 10-year retrospective cohort study. World J Gastroenterol. 2016;22:10219-10225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Longo L, de Freitas LBR, Santos D, Grivicich I, Álvares-da-Silva MR. Sorafenib for Advanced Hepatocellular Carcinoma: A Real-Life Experience. Dig Dis. 2018;36:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Almeida-Carvalho SR, Gomes-Ferraz ML, Loureiro-Matos CA, Benedito-Silva AT, Carvalho-Filho RJ, Renato-Perez R, Miziara-Gonzalez A, Salzedas-Netto AA, Szejnfeld D, D'Ippolito G, Pereira-Lanzoni V, Souza-Silva I. Practical Considerations of Real Life of Hepatocellular Carcinoma in a Tertiary Center of Brazil. Ann Hepatol. 2017;16:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 835] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 11. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3143] [Article Influence: 392.9] [Reference Citation Analysis (3)] |
| 12. | Kikuchi L, Oliveira CP, Alvares-da-Silva MR, Tani CM, Diniz MA, Stefano JT, Chagas AL, Alencar RS, Vezozzo DC, Santos GR, Campos PB, Alves VA, Ratziu V, Carrilho FJ. Hepatocellular Carcinoma Management in Nonalcoholic Fatty Liver Disease Patients: Applicability of the BCLC Staging System. Am J Clin Oncol. 2016;39:428-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Cotrim HP, Oliveira CP, Coelho HS, Alvares-da-Silva MR, Nabuco L, Parise ER, Ivantes C, Martinelli AL, Galizzi-Filho J, Carrilho FJ. Nonalcoholic steatohepatitis and hepatocellular carcinoma: Brazilian survey. Clinics (Sao Paulo). 2016;71:281-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 314] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 15. | Han KH, Kim DY, Park JY, Ahn SH, Kim J, Kim SU, Kim JK, Lee KS, Chon CY. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months: a 15-year prospective study. J Clin Gastroenterol. 2013;47:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Chagas A, Neto BHF, Varaldo C. Carcinoma hepatocelular: barreiras ao acesso ao diagnóstico e tratamento no cenário brasileiro atual. White Paper: CHC. DATASUS 12, 2017.. |
| 17. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6629] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 18. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 19. | Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 361] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 967] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 21. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 22. | European Association For The Study Of The Liver. ; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (4)] |
| 23. | Wallace MC, Huang Y, Preen DB, Garas G, Adams LA, MacQuillan G, Tibballs J, Ferguson J, Samuelson S, Jeffrey GP. HKLC Triages More Hepatocellular Carcinoma Patients to Curative Therapies Compared to BCLC and Is Associated with Better Survival. Dig Dis Sci. 2017;62:2182-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 24. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4359] [Article Influence: 544.9] [Reference Citation Analysis (6)] |
| 25. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3364] [Article Influence: 480.6] [Reference Citation Analysis (45)] |
| 26. | Lee YS, Seo YS, Kim JH, Lee J, Kim HR, Yoo YJ, Kim TS, Kang SH, Suh SJ, Joo MK, Jung YK, Lee BJ, Yim HJ, Yeon JE, Kim JS, Park JJ, Um SH, Bak YT, Byun KS. Can More Aggressive Treatment Improve Prognosis in Patients with Hepatocellular Carcinoma? A Direct Comparison of the Hong Kong Liver Cancer and Barcelona Clinic Liver Cancer Algorithms. Gut Liver. 2018;12:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Parikh ND, Scaglione S, Li Y, Powell C, Yerokun OA, Devlin P, Mumtaz S, Mittal S, Singal AG. A Comparison of Staging Systems for Hepatocellular Carcinoma in a Multicenter US Cohort. Clin Gastroenterol Hepatol. 2018;16:781-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Scaffaro LA, Stella SF, Alvares-Da-Silva MR, Kruel CD. Survival rates according to barcelona clinic liver cancer sub-staging system after transarterial embolization for intermediate hepatocellular carcinoma. World J Hepatol. 2015;7:628-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Tellapuri S, Sutphin PD, Beg MS, Singal AG, Kalva SP. Staging systems of hepatocellular carcinoma: A review. Indian J Gastroenterol. 2018;37:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Li JW, Goh BG, Chang PE, Tan CK. Barcelona Clinic Liver Cancer outperforms Hong Kong Liver Cancer staging of hepatocellular carcinoma in multiethnic Asians: Real-world perspective. World J Gastroenterol. 2017;23:4054-4063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Adhoute X, Penaranda G, Bronowicki JP, Raoul JL. Usefulness of the HKLC vs. the BCLC staging system in a European HCC cohort. J Hepatol. 2015;62:492-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Yan X, Fu X, Cai C, Zi X, Yao H, Qiu Y. Validation of models in patients with hepatocellular carcinoma: comparison of Hong Kong Liver Cancer with Barcelona Clinic Liver Cancer staging system in a Chinese cohort. Eur J Gastroenterol Hepatol. 2015;27:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh AM, Joh JW, Cho SK, Shin SW, Carriere KC, Ahn JH, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:250-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Liu PH, Hsu CY, Lee YH, Su CW, Hsia CY, Huang YH, Chiou YY, Lin HC, Huo TI. Hong Kong Liver Cancer Staging System Is Associated With Better Performance for Hepatocellular Carcinoma: Special Emphasis on Viral Etiology. Medicine (Baltimore). 2015;94:e1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 36. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10523] [Article Influence: 584.6] [Reference Citation Analysis (9)] |
| 37. | Kim KM, Sinn DH, Jung SH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. The recommended treatment algorithms of the BCLC and HKLC staging systems: does following these always improve survival rates for HCC patients? Liver Int. 2016;36:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Longo L, Rodrigues de Freitas LB, Santos D, Grivicich I, Álvares-da-Silva MR. BCLC-B Subclassification and the Hong Kong Liver Cancer System in Intermediate Hepatocellular Carcinoma: Identifying Candidates for Curative Therapy. Am J Clin Oncol. 2019;42:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 40. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1953] [Article Influence: 195.3] [Reference Citation Analysis (4)] |
| 41. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
Conflict of interest statement: The authors have nothing to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gatselis NK, Nah YW, Streba LAM, Tchilikidi KY S-Editor: Ma YJ L-Editor: A E-Editor: Qi LL