©The Author(s) 2017.
World J Hepatol. Feb 8, 2017; 9(4): 217-223
Published online Feb 8, 2017. doi: 10.4254/wjh.v9.i4.217
Published online Feb 8, 2017. doi: 10.4254/wjh.v9.i4.217
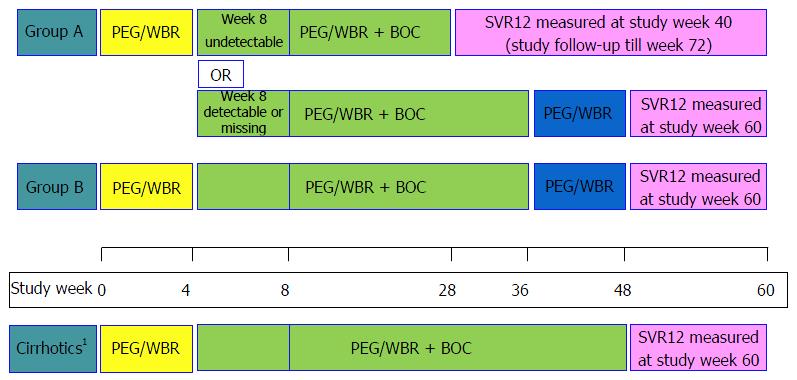
Figure 1 Overall study design.
Group A refers to treatment naïve participants while Group B refers to treatment experienced participants. PEG/WBR treatment is pegylated-interferon alfa 2b (PEG-IFN) and weight-based ribavirin (WBR). 1Cirrhotic participants received 44 wk of triple therapy. SVR12: HCV RNA < LLOQ, target not detected at 12 wk post treatment discontinuation; BOC: Boceprevir; SVR: Sustained viral response; HCV: Hepatitis C virus; LLOQ: Lower limit of quantification.
- Citation: Sherman KE, Kang M, Sterling R, Umbleja T, Marks K, Kiser JJ, Alston-Smith B, Greaves W, Butt AA, the ACTG 5294 BIRTH Study Team. Phase 3 trial of first generation protease inhibitor therapy for hepatitis C virus/human immunodeficiency virus coinfection. World J Hepatol 2017; 9(4): 217-223
- URL: https://www.wjgnet.com/1948-5182/full/v9/i4/217.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i4.217