©The Author(s) 2016.
World J Hepatol. Dec 8, 2016; 8(34): 1511-1520
Published online Dec 8, 2016. doi: 10.4254/wjh.v8.i34.1511
Published online Dec 8, 2016. doi: 10.4254/wjh.v8.i34.1511
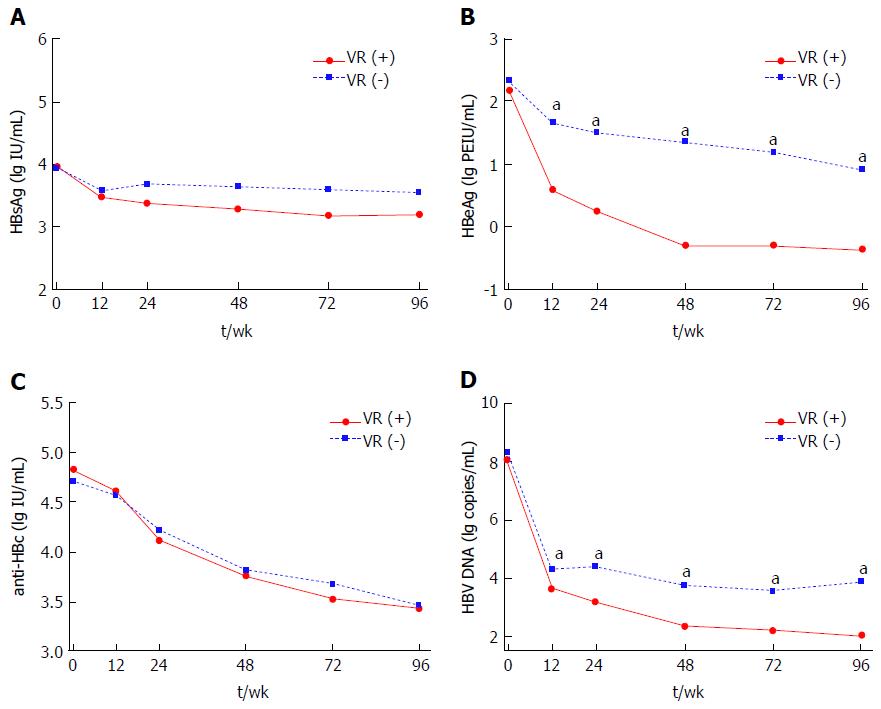
Figure 1 Dynamic changes of hepatitis B surface antigen (A), hepatitis B e antigen (B), hepatitis B core antibody (C) and hepatitis B virus DNA (D) levels from baseline to 96-wk in chronic hepatitis B patients received nucleos(t)ide analogues therapy stratified by virological response at 96-wk.
aP < 0.05; VR (+): Virological response, HBV DNA ≤ 300 copies/mL; VR (-): Without virological response; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; anti-HBc: hepatitis B core antibody; HBV: Hepatitis B virus; VR: Virological response.
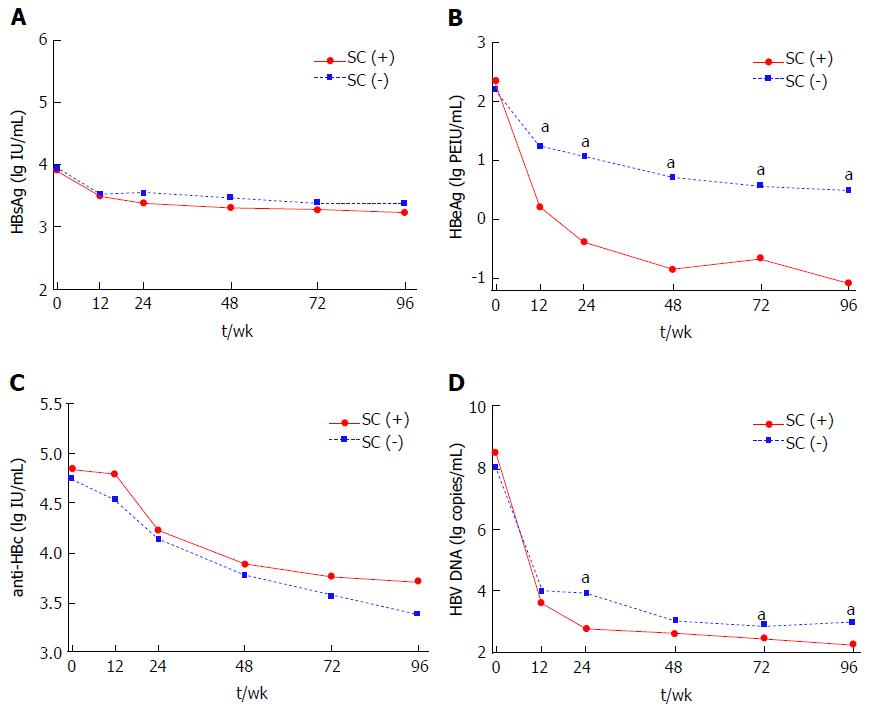
Figure 2 Dynamic changes of hepatitis B surface antigen (A), hepatitis B e antigen (B), hepatitis B core antibody (C) and hepatitis B virus DNA (D) levels from baseline to 96-wk in chronic hepatitis B patients received nucleos(t)ide analogues therapy stratified by hepatitis B e antigen seroconversion at 96-wk.
aP < 0.05; SC (+): HBeAg seroconversion; SC (-): Without HBeAg seroconversion; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; anti-HBc: hepatitis B core antibody; HBV: Hepatitis B virus; SC: HBeAg seroconversion.
- Citation: Gao YH, Meng QH, Zhang ZQ, Zhao P, Shang QH, Yuan Q, Li Y, Deng J, Li T, Liu XE, Zhuang H. On-treatment quantitative hepatitis B e antigen predicted response to nucleos(t)ide analogues in chronic hepatitis B. World J Hepatol 2016; 8(34): 1511-1520
- URL: https://www.wjgnet.com/1948-5182/full/v8/i34/1511.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i34.1511