©The Author(s) 2015.
World J Hepatol. Oct 18, 2015; 7(23): 2449-2458
Published online Oct 18, 2015. doi: 10.4254/wjh.v7.i23.2449
Published online Oct 18, 2015. doi: 10.4254/wjh.v7.i23.2449
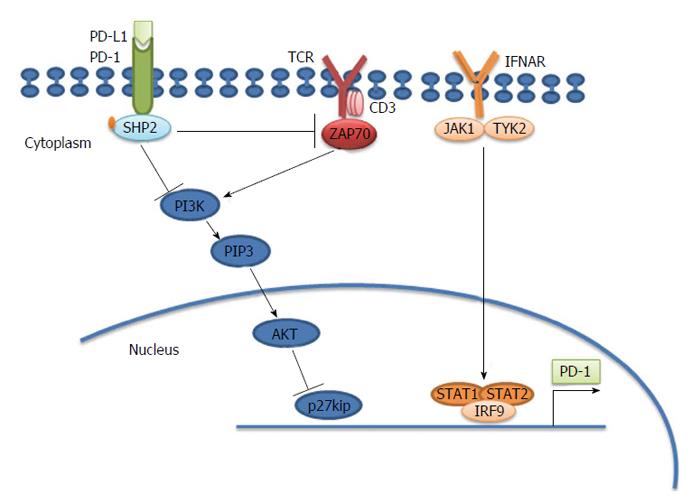
Figure 1 Programmed death-1 causes T cell exhaustion.
Programmed death-1 (PD-1) inhibits the T cell receptor (TCR) signaling pathway through src homology 2-containing protein-tyrosine phosphatase 2 (SHP2). PD-1 is located in the immune synapse at the T cell-antigen presenting cell (APC) interface. When its physiological ligand (PD-L1 or PD-L2) binds, PD-1 suppresses the activation and function of T cells through the recruitment of SHP-2, which dephosphorylates and inactivates ZAP70, a major integrator of TCR-mediated signaling. In chronically activated (“exhausted”) T cells, interferon (IFN)-α causes overexpression of PD-1 through the binding of the transcription factor IRF9 to the signal transducer and activator of transcription (STAT)1 and STAT2 promoters. PD-1 also results in accumulation of p27kip1, which is an inhibitor of cyclin dependent kinases to block cell cycle and proliferation[84]. ZAP70: Zeta-chain (TCR) associated protein kinase 70 kDa; IRF9: Interferon regulatory factor 9; JAK1: Janus kinase 1.
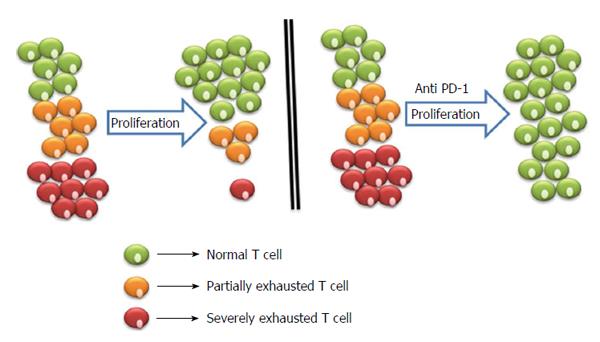
Figure 2 Proliferation of exhausted T cells and blockage strategies to reverse exhaustion.
Severely exhausted T cells (red cells) proliferate poorly in comparison to partially exhausted and normal T cells (yellow and green cells). Antibody blockade of the pathway with PD-1 and its ligand reverses exhaustion and restores the functional capacities of exhausted T cells. PD-1: Programmed death-1.
- Citation: Salem ML, El-Badawy A. Programmed death-1/programmed death-L1 signaling pathway and its blockade in hepatitis C virus immunotherapy. World J Hepatol 2015; 7(23): 2449-2458
- URL: https://www.wjgnet.com/1948-5182/full/v7/i23/2449.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i23.2449