©2011 Baishideng Publishing Group Co.
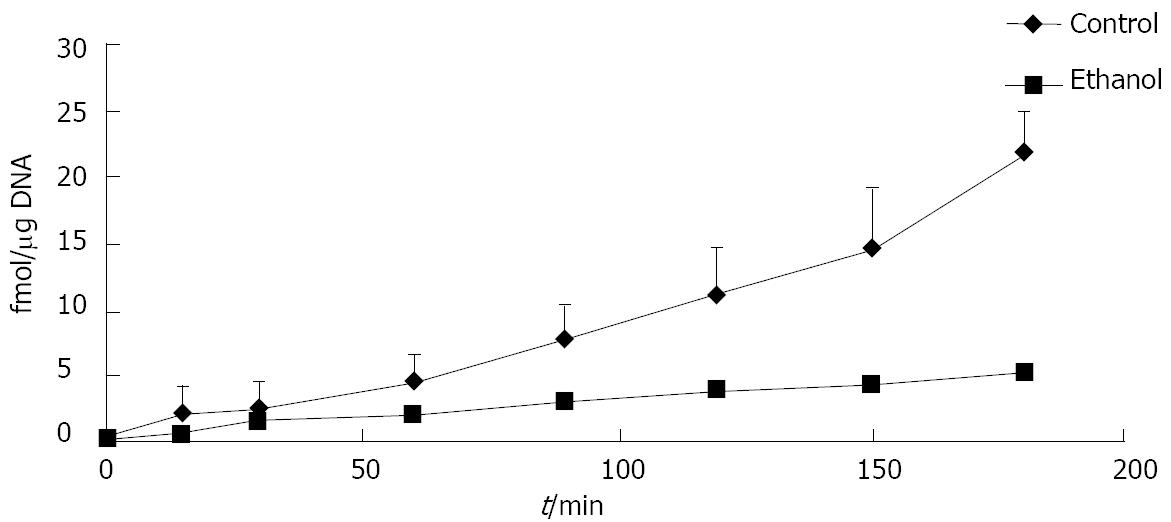
Figure 1 Ethanol administration delays degradation of iodinated cellular fibronectin in rats.
Isolated hepatocytes were obtained from rats fed a control or ethanol-containing liquid diet for 4-6 wk. The degradation of iodinated cellular fibronectin (125I-cFn) that has been internalized by the hepatocytes was monitored over a 3-h time period as described in Material and Methods. Data are presented as femtomoles bound per μg DNA and are means ± SEM (n = 3 experiments).
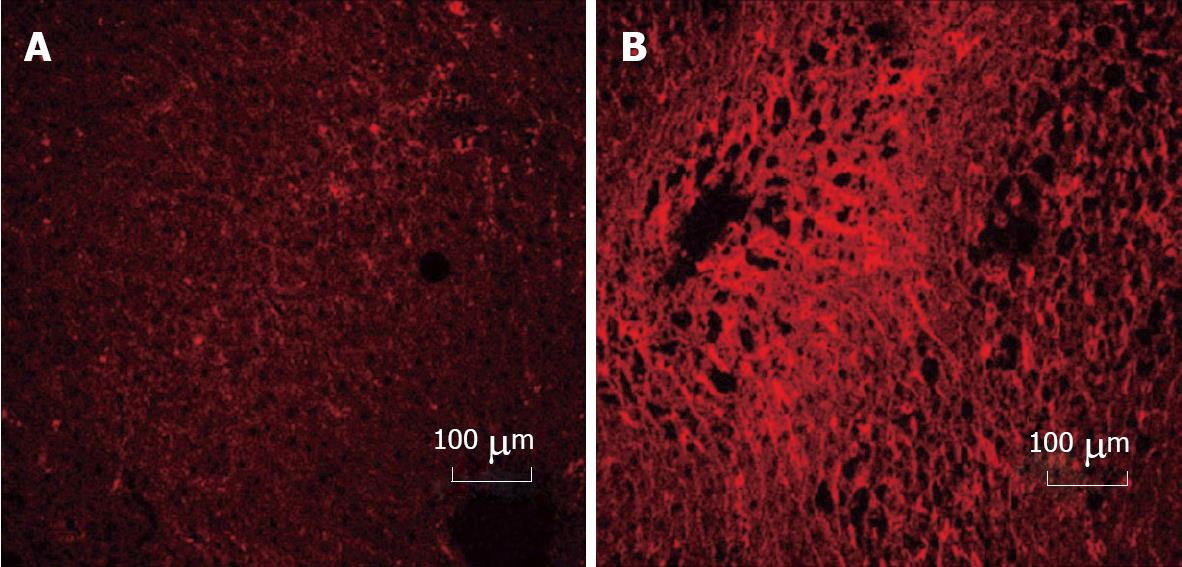
Figure 2 Immunohistochemical detection of cellular fibronectin in rat liver.
Cellular fibronectin was detected by rhodamine conjugated antibody staining in liver sections from rats which had been fed control diet (A) or in ethanol-fed (B) animals for 12 wk. No staining was seen for isotype controls. Increased cellular fibronectin staining was observed (60% increase in intensity) in liver sections after ethanol administration. Values were determined using Carl Zeiss LSM 410 inverted confocal-laser scanning microscope.
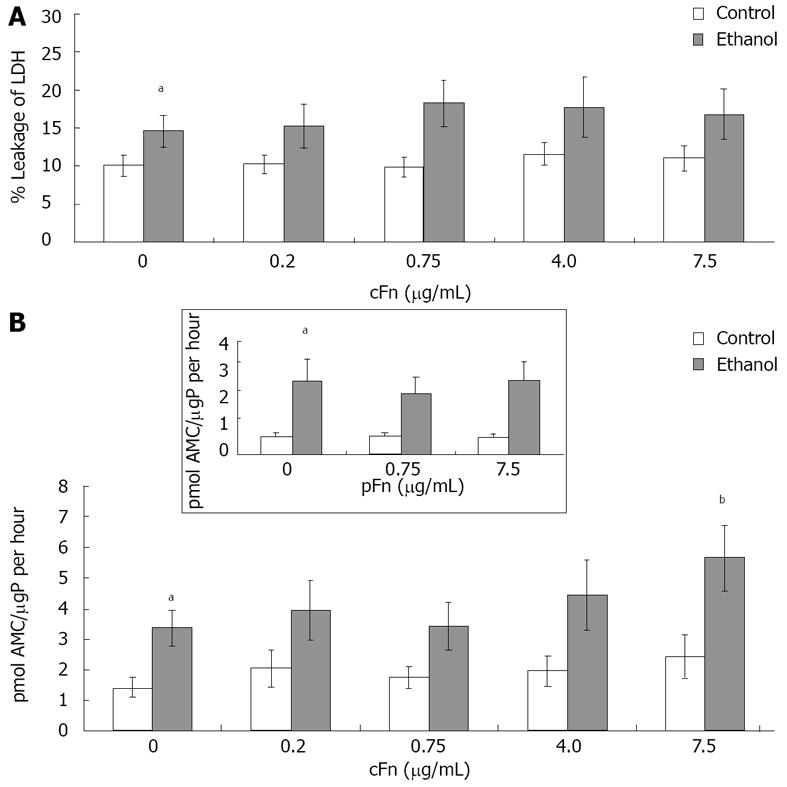
Figure 3 Effect of cellular fibronectin on hepatocyte viability.
A: Percentage leakage of the cytosolic lactate dehydrogenase (LDH) enzyme into the cell culture supernatant was used to test for severe irreversible cell damage (necrosis) as described in Material and Methods. Incidence of necrosis was consistently higher in cultured cells from ethanol-fed animals (n = 8 - 12); B: Caspase-3 activity in the cells was determined as described in Material and Methods. Basal level caspase-3 activity was significantly higher in hepatocytes from ethanol-fed animals when compared with controls. Enzyme activity is presented as picomoles of fluorogenic AMC product released upon cleavage of the caspase-3 substrate Ac-DEVD-AMC, per µg protein in the cell lysate in an hour. At the higher dose of exogenous cellular fibronectin (cFn) (7.5 μg/mL), caspase-3 activity was significantly induced in cells from the ethanol-fed animals. No changes were seen in the control cells (n = 5 - 14). Plasma fibronectin exhibited no effect on either cell type (inset). Data are represented as means ± SEM. Ethanol values significantly different from those of the control are expressed as aP < 0.05, and treatment significantly different from untreated cultures is expressed as bP < 0.05. μgP: μg protein.
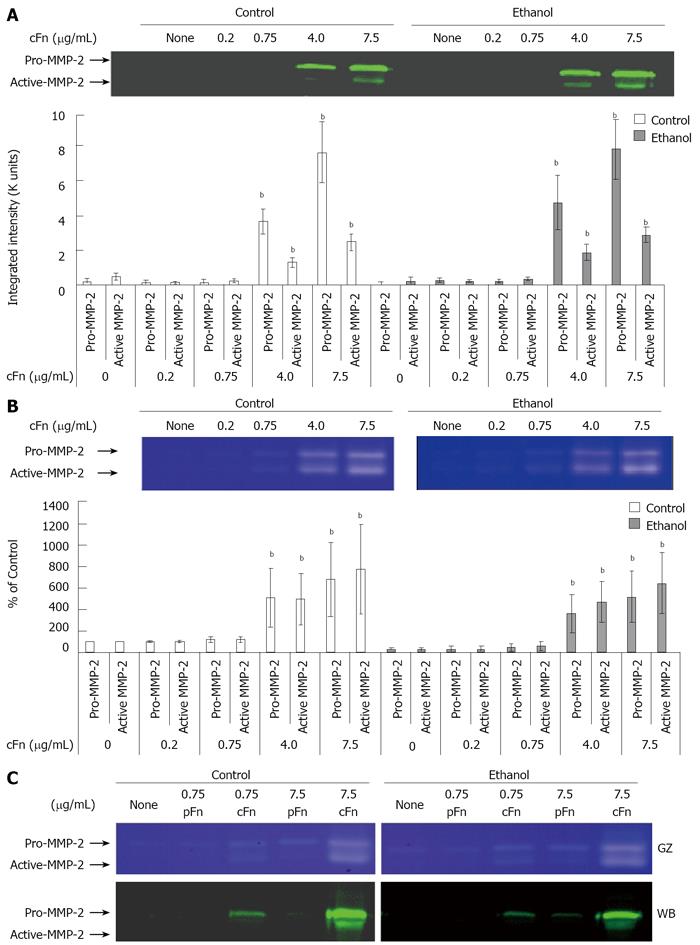
Figure 4 Effect of cellular fibronectin on the secretion of pro- and active-matrix metalloproteinase-2 by cultured hepatocytes isolated from control and ethanol-fed rats.
Protein and activity levels of pro- and active-matrix metalloproteinase-2 (MMP-2) in the cell culture media of hepatocytes after 20 h of treatment with cellular fibronectin (cFn) were determined as described in Material and Methods. In the presence of higher concentrations of exogenous cFn (4 μg/mL and 7.5 μg/mL), cells from both control and ethanol-fed animals released elevated levels of pro- and active-MMP-2 (A) with corresponding higher MMP-2 activity (B). Plasma fibronectin treatment produced no effect on either cell type (C). GZ: gel zymography; WB: western blot. Data are represented as means ± SEM. Ethanol values significantly different from those of the control are expressed as aP < 0.05, and treatment significantly different from untreated cultures is expressed as bP < 0.05. (n = 3 - 11 experiments).
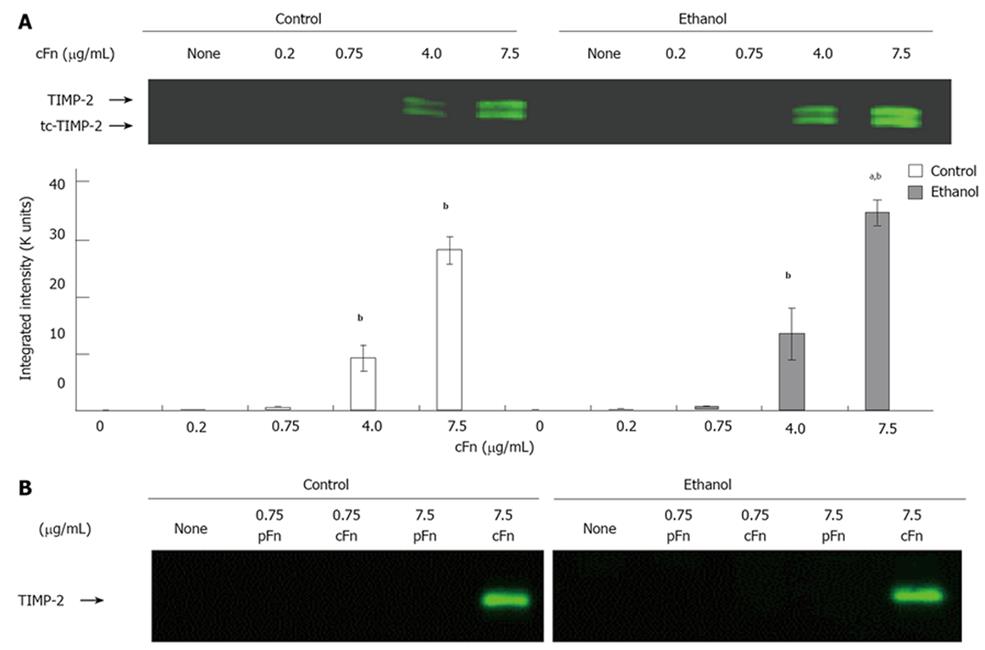
Figure 5 Effect of cellular fibronectin on the secretion of tissue inhibitor of metalloproteinase-2 by cultured hepatocytes isolated from control and ethanol-fed rats.
The level of tissue inhibitor of metalloproteinase (TIMP)-2 released into the cell culture media of hepatocytes after 20 h of treatment with cellular fibronectin (cFn) was determined as described in the Material and Methods. At the higher concentrations of exogenous cFn (4 μg/mL and 7.5 μg/mL), hepatocytes from both control and ethanol-fed animals secreted significantly higher levels of TIMP-2 forms than corresponding untreated cells, and those incubated in the presence of low concentrations of cFn. The cells from ethanol-fed animals cultured in the presence of 7.5 μg/mL cFn were significantly more responsive than those of the controls (A). Plasma fibronectin treatment produced no effect on either cell type (B). Data are represented as means ± SEM. Ethanol values significantly different from those of the control are expressed as aP < 0.05, and treatment significantly different from untreated cultures is expressed as bP < 0.05. (n = 11 experiments).
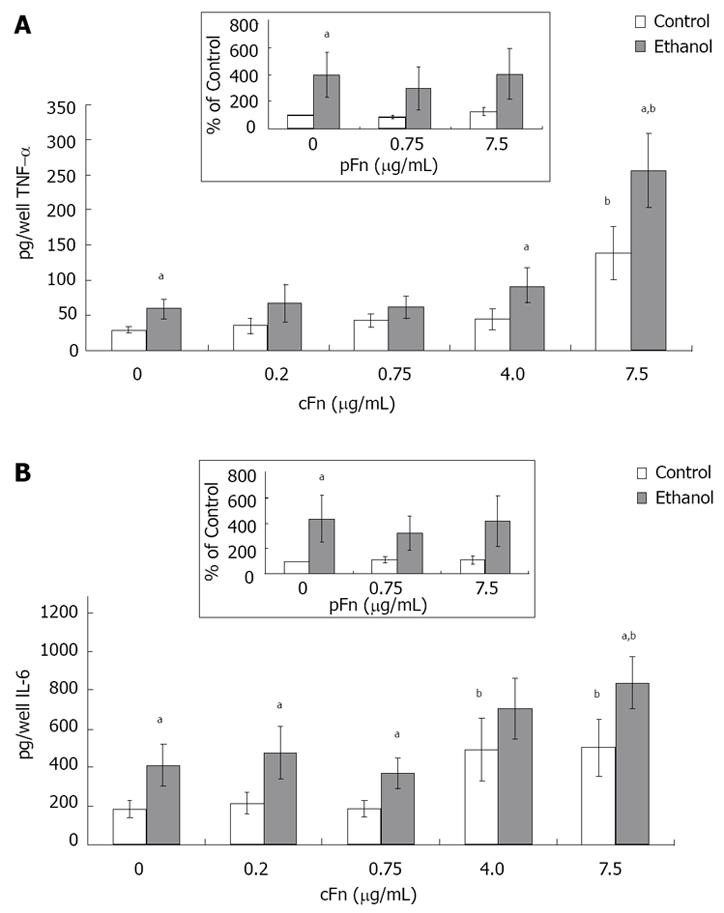
Figure 6 Secretion of cytokines tumor necrosis factor-alpha and interleukin-6 by cultured hepatocytes isolated from the livers of rats that were pair-fed control and ethanol diets and stimulated with cellular fibronectin.
Hepatocytes were cultured in the presence of different concentrations of exogenous cultured hepatocytes (cFn) (0 μg/mL, 0.2 μg/mL, 0.75 μg/mL, 4.0 μg/mL and 7.5 μg/mL) for 20 h. After this time, the level of each cytokine (pg/well) released by the cells into the culture supernatant was determined by ELISA, as described in Material and Methods. In the presence of 7.5 μg/mL cFn, cells from both control and ethanol-fed animals released elevated levels of tumor necrosis factor-alpha (TNF-α) (A) and Interleukin-(IL)-6 (B) compared with corresponding untreated cells, with hepatocytes from ethanol-fed rats secreting significantly higher levels of both cytokines compared with controls. Plasma fibronectin treatment produced no effect on either cell type (inserts, A and B). Data are represented as means ± SEM. Ethanol values significantly different from those of the control are expressed as aP < 0.05, and treatment significantly different from untreated cultures is expressed as bP < 0.05. (n = 8 - 15 experiments).
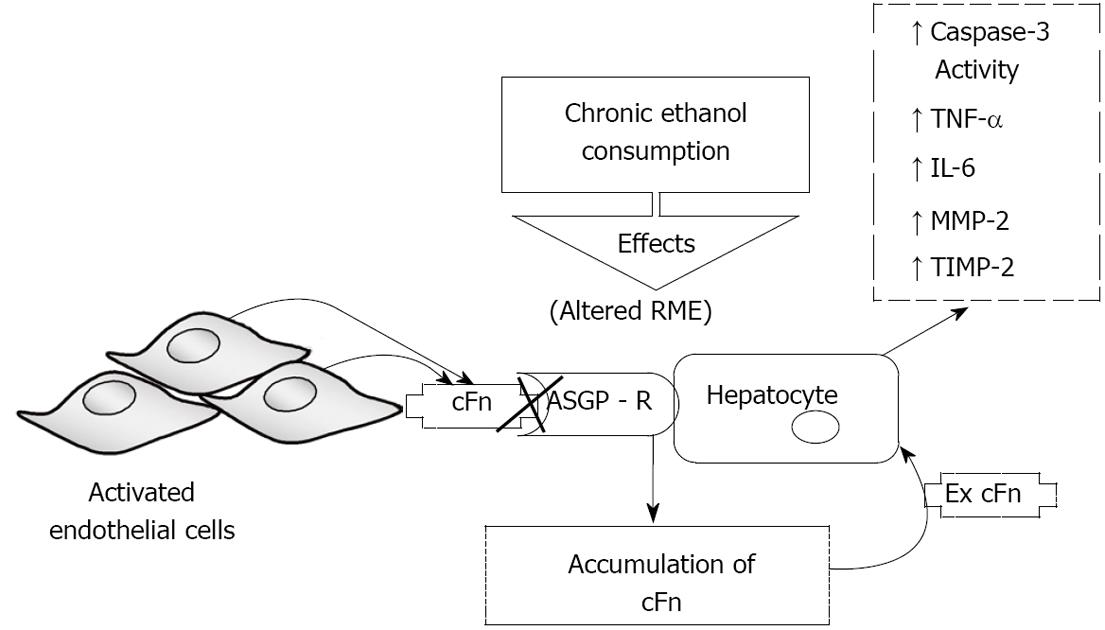
Figure 7 Schematic representation of the proposed model of ethanol-induced liver injury linking altered asialoglycoprotein receptor clearance of cellular fibronectin with hepatocyte activation by the accumulating protein.
Subsequently, there is an increase in caspase-3 activity and an elevated secretion of pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), as well as secretion of matrix metalloproteinase-2 (MMP-2) and its corresponding inhibitor tissue inhibitors of metalloproteinase-2 (TIMP-2). RME: receptor-mediated endocytosis; ASGP-R: asialoglycoprotein receptor.
- Citation: Aziz-Seible RS, McVicker BL, Kharbanda KK, Casey CA. Cellular fibronectin stimulates hepatocytes to produce factors that promote alcohol-induced liver injury. World J Hepatol 2011; 3(2): 45-55
- URL: https://www.wjgnet.com/1948-5182/full/v3/i2/45.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i2.45