©The Author(s) 2026.
World J Hepatol. Jan 27, 2026; 18(1): 115048
Published online Jan 27, 2026. doi: 10.4254/wjh.v18.i1.115048
Published online Jan 27, 2026. doi: 10.4254/wjh.v18.i1.115048
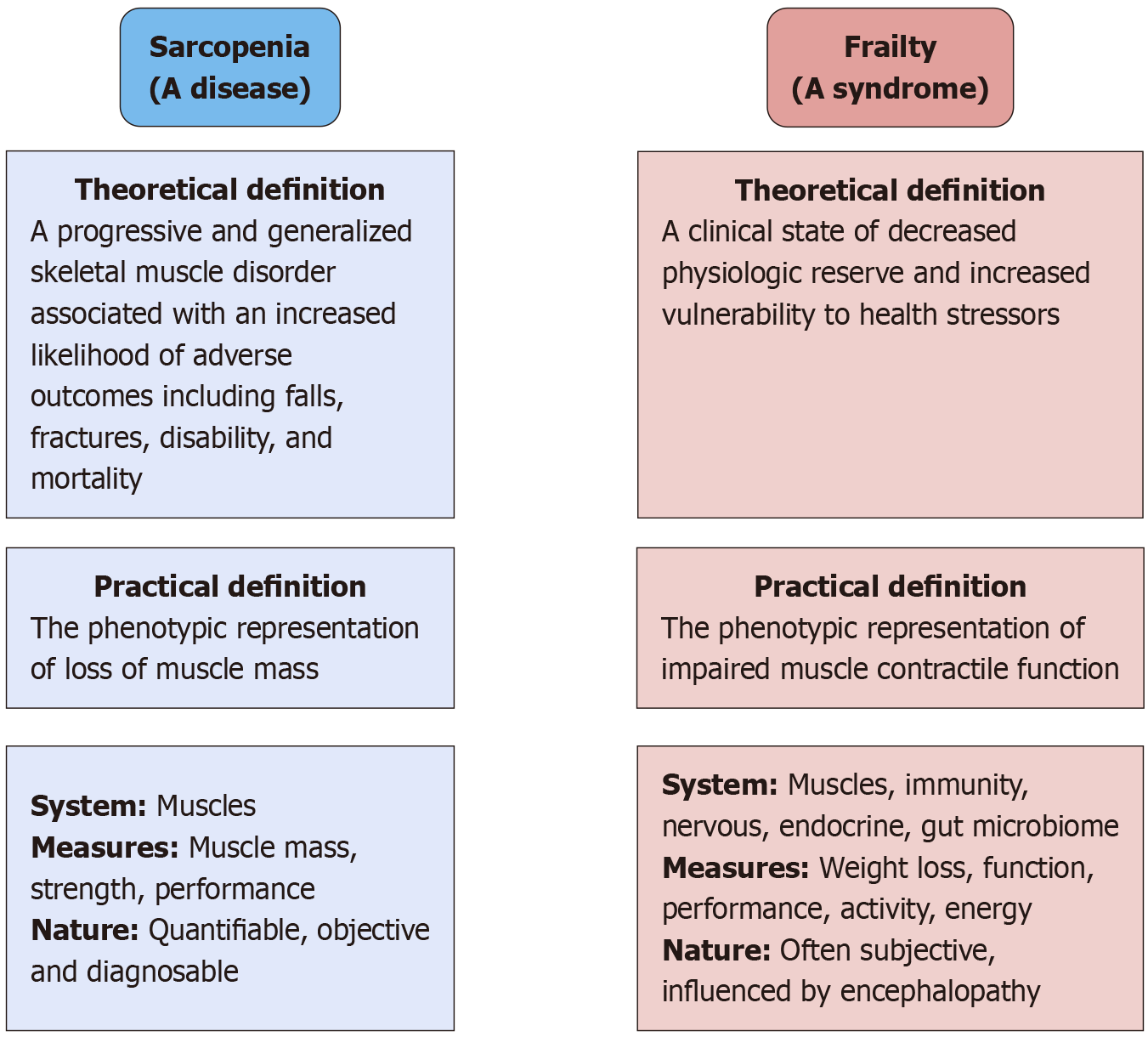
Figure 1 Conceptual distinctions between sarcopenia and frailty.
Sarcopenia is recognized as a disease entity primarily affecting skeletal muscle with definitions anchored in quantifiable loss of muscle mass, strength, and performance. In contrast, frailty is defined as a broader clinical syndrome characterized by diminished physiologic reserve and vulnerability to external stressors. While sarcopenia can be diagnosed using objective measures, frailty encompasses multisystem dysfunction, including immune, endocrine, neurologic, and gut microbiome factors, and is often assessed through more subjective parameters.
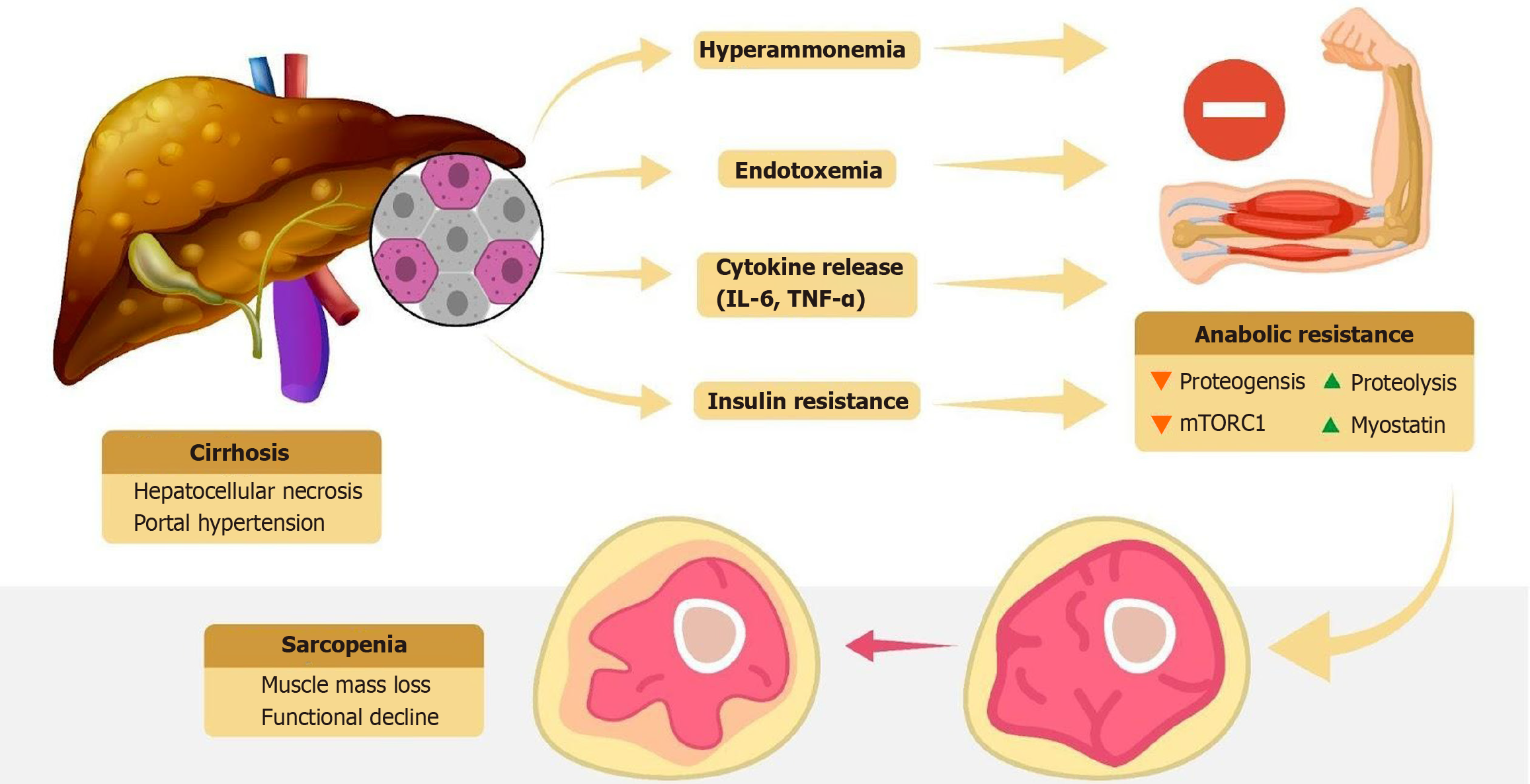
Figure 2 Pathophysiology of sarcopenia in cirrhosis.
Pathophysiological mechanisms linking cirrhosis to sarcopenia, including hyperammonemia, endotoxemia, cytokine release, and insulin resistance, all of which contribute to anabolic resistance, muscle mass loss, and functional decline. IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α.
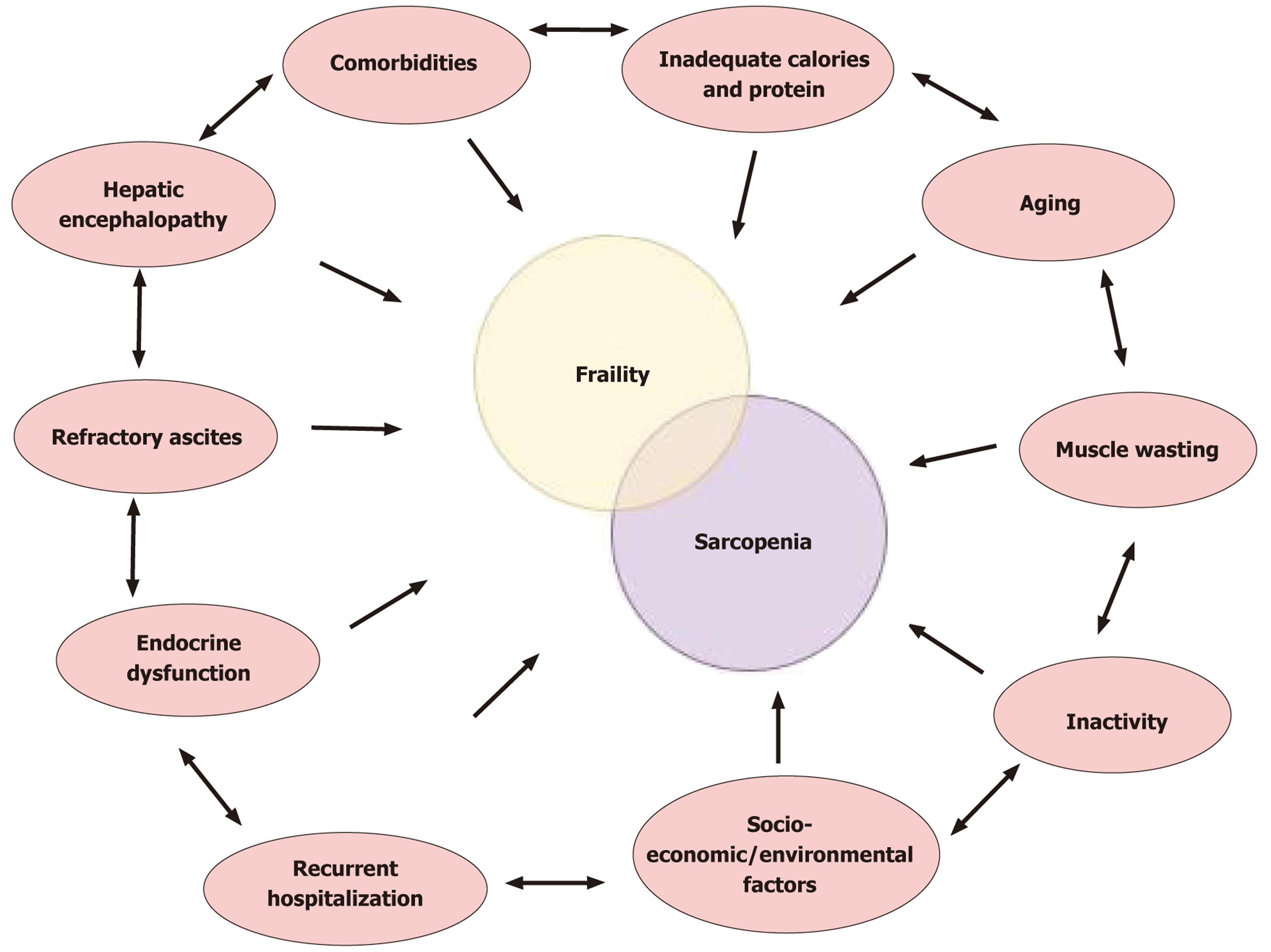
Figure 3 Conceptual interplay between frailty, sarcopenia, and their shared determinants.
Frailty and sarcopenia in cirrhosis converge around impaired muscle health, leading to overlap in their underlying drivers. External contributors, including nutritional deficiency, aging, inactivity, comorbid illnesses, endocrine disruption, hepatic encephalopathy, recurrent hospitalization, and socioeconomic or environmental factors, can individually predispose patients to frailty, sarcopenia, or both (arrows pointing inward). Moreover, these determinants may reinforce each other through bidirectional interactions (arrows within the outer ring), thereby amplifying their impact on muscle decline and clinical outcomes.
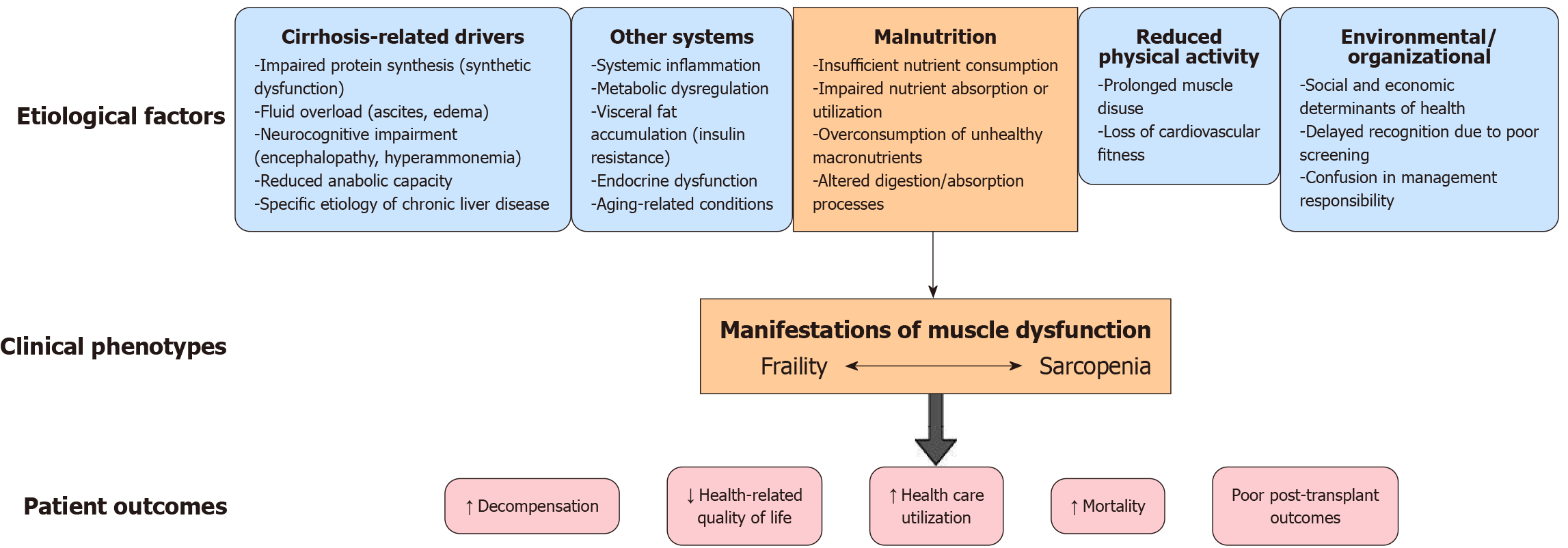
Figure 4 Pathophysiological framework linking cirrhosis to frailty and sarcopenia.
Multiple pathways, including cirrhosis-specific mechanisms (synthetic dysfunction, ascites, encephalopathy), systemic drivers (inflammation, metabolic dysregulation, endocrine abnormalities), malnutrition, reduced physical activity, and social or organizational barriers, converge to produce muscle dysfunction. This dysfunction manifests along a continuum of frailty and sarcopenia, ulti
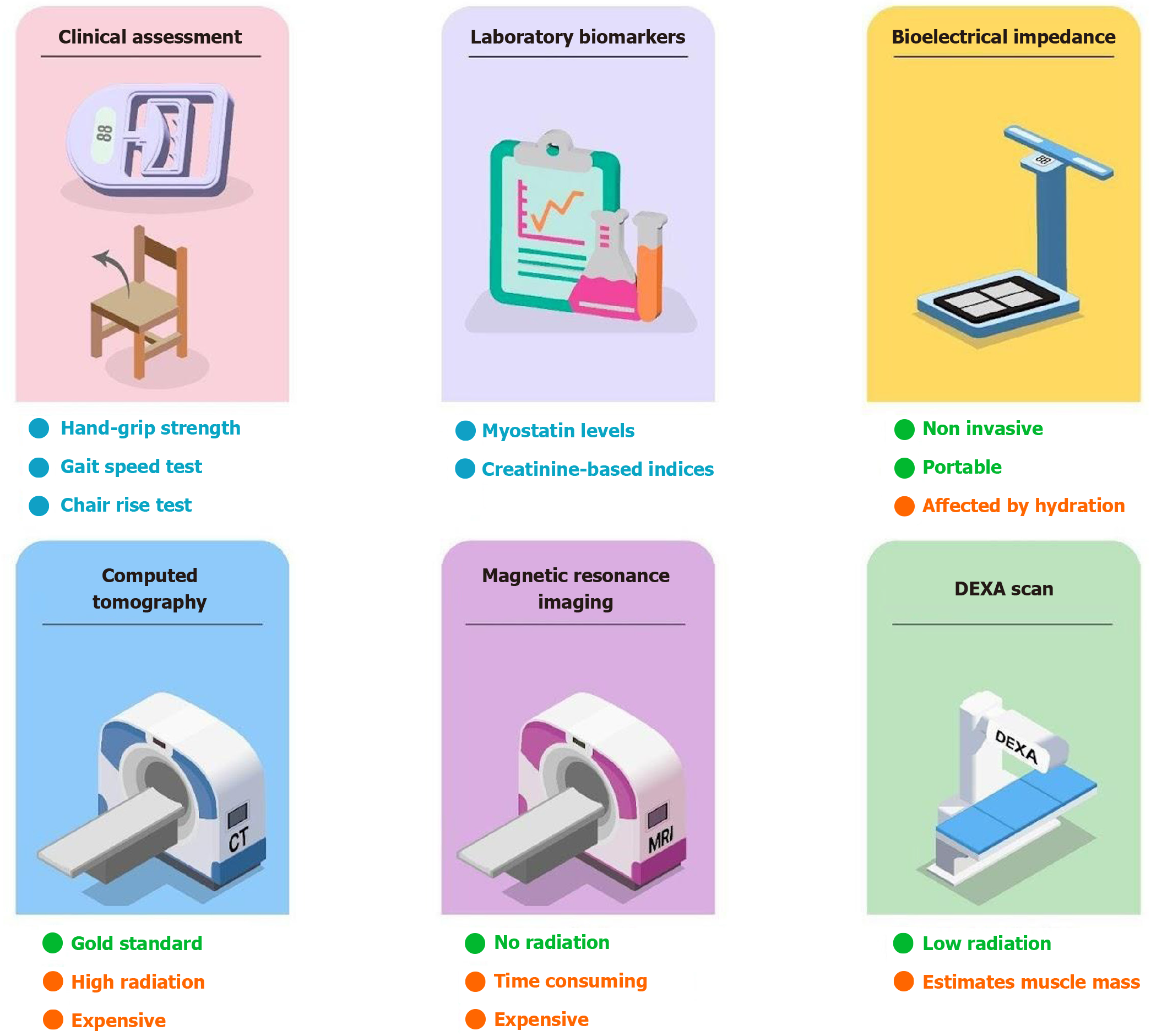
Figure 5 Diagnostic methods for sarcopenia.
Diagnostic modalities for sarcopenia in cirrhosis range from bedside clinical assessments and laboratory biomarkers to advanced imaging techniques, each with distinct advantages and limitations.
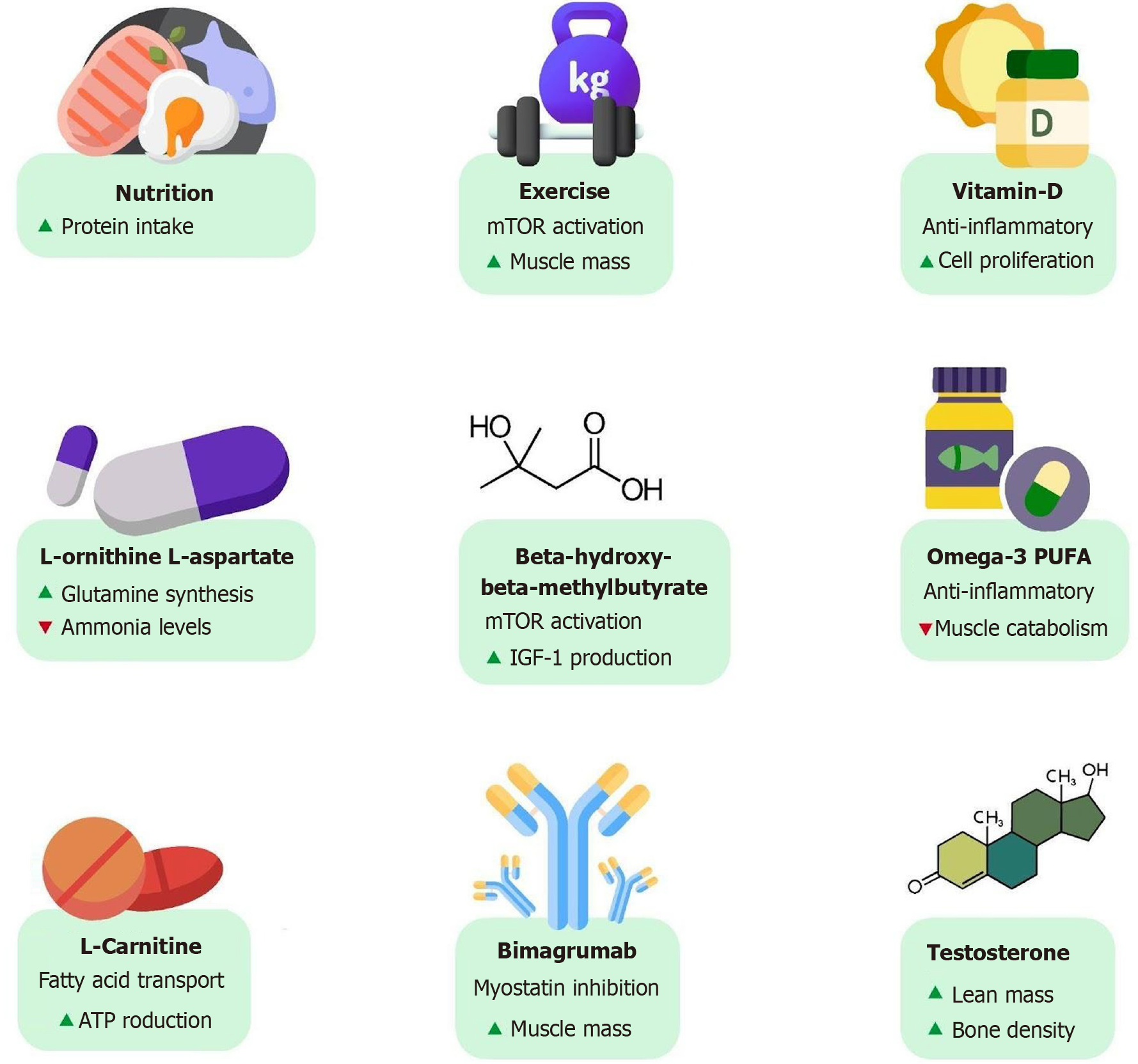
Figure 6 Existing and emerging therapies.
Emerging therapeutic strategies for sarcopenia in cirrhosis include nutritional support, exercise, vitamin and amino acid supplementation, pharmacological agents, and anabolic therapies that target muscle mass, strength, and function.
- Citation: Goyal MK, Chowdhary R, Vohra C, Patel M, Kalra S, Mehta M, McNulty R, Goyal K, Vuthaluru AR, Goyal O. Current management strategies for sarcopenia and frailty in cirrhosis: Missing link in transplant candidacy. World J Hepatol 2026; 18(1): 115048
- URL: https://www.wjgnet.com/1948-5182/full/v18/i1/115048.htm
- DOI: https://dx.doi.org/10.4254/wjh.v18.i1.115048