©The Author(s) 2025.
World J Hepatol. Nov 27, 2025; 17(11): 113756
Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.113756
Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.113756
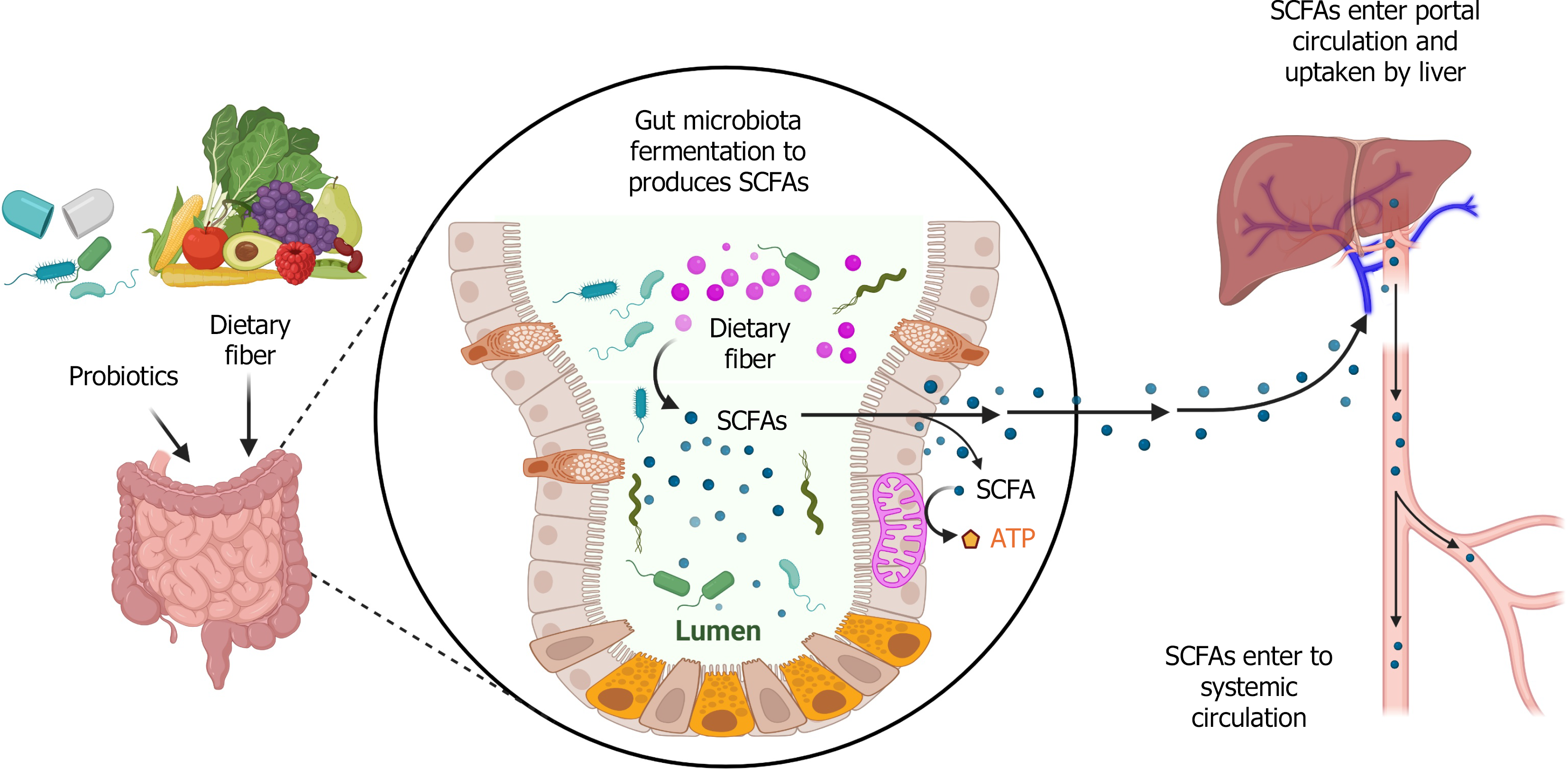
Figure 1 Short-chain fatty acids produced by gut microbiota and up taken by the liver.
Dietary fiber or probiotics are fermented by gut microbiota to produce short-chain fatty acids (SCFAs). SCFAs mediate intestinal cell mitochondrial energy production. SCFAs are taken up by the liver through portal circulation and enter systemic circulation. SCFAs: Short-chain fatty acids; ATP: Adenosine triphosphate. This figure was created using BioRender.com (Supplementary material).
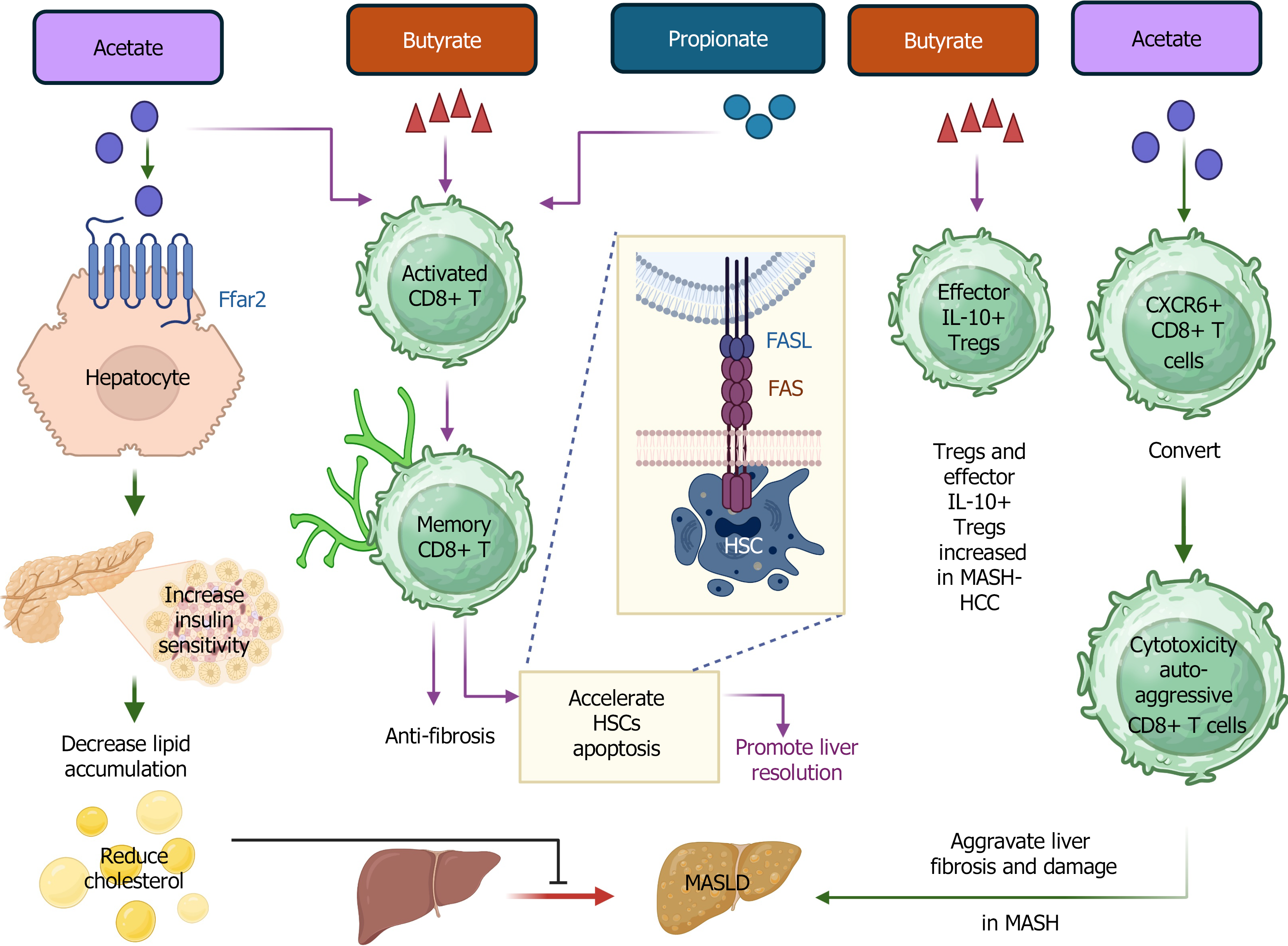
Figure 2 Graphic demonstration of the role of short-chain fatty acids in CD8+ T cells and Tregs during metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis.
Short-chain fatty acids (SCFAs) bind with receptor free fatty acid receptor 2 on hepatocytes to enhance insulin sensitivity. SCFAs support memory CD8+ T cell survival and maintenance and accelerate the apoptosis of activated hepatic stellate cells. SCFAs increase the number of Tregs and IL-10+ effector Tregs in metabolic dysfunction-associated steatohepatitis (MASH)-hepatocellular carcinoma. SCFAs promote the conversion of CXCR6+ CD8 T cells to cytotoxic auto-aggressive CD8+ T cells in MASH. SCFA: Short-chain fatty acid; FFAR2: Free fatty acid receptor 2; HSC: Hepatic stellate cells; MASH: Metabolic dysfunction-associated steatohepatitis; MASLD: Metabolic dysfunction-associated steatotic liver disease; HCC: Hepatocellular carcinoma. This figure was created using BioRender.com (Supplementary material).
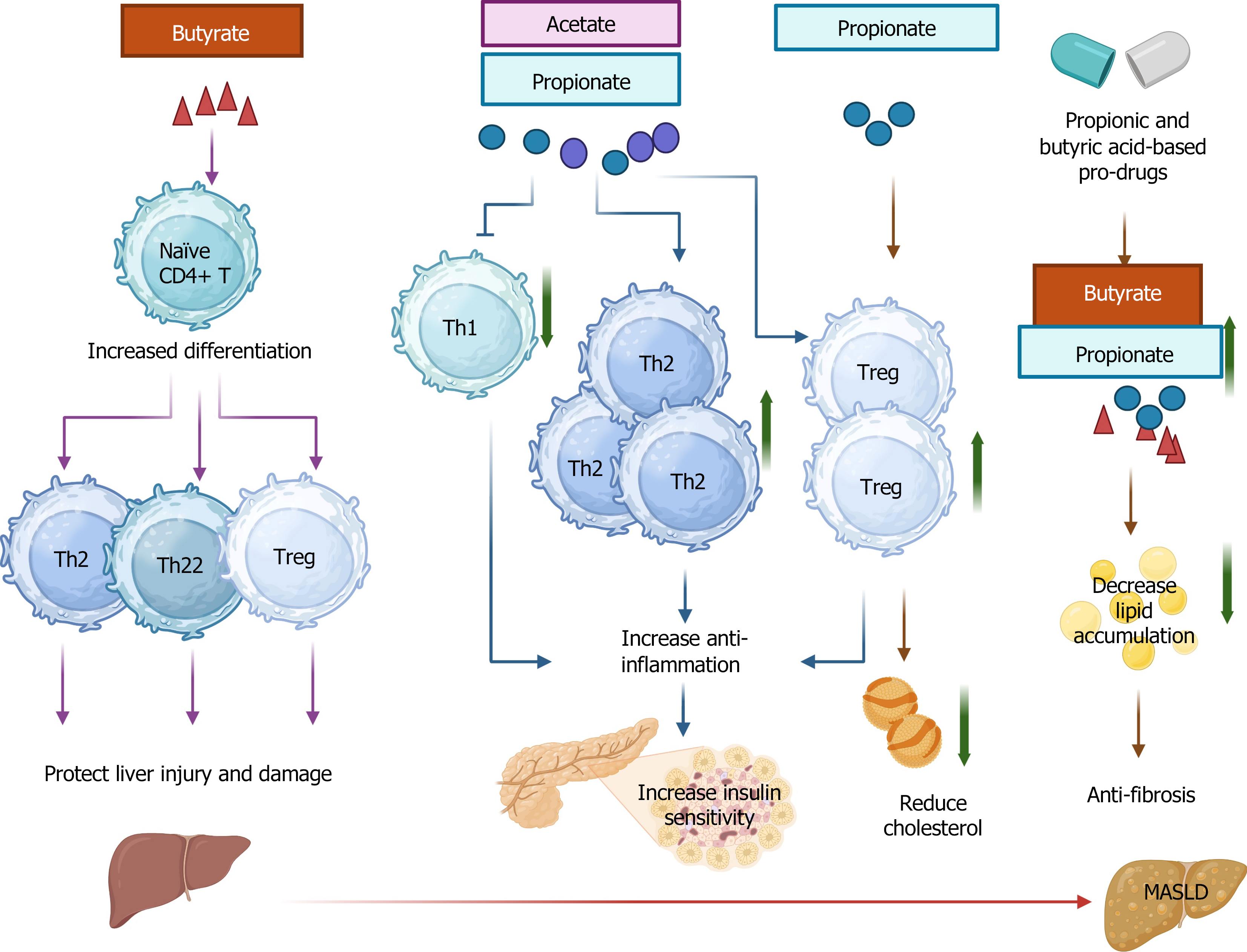
Figure 3 Graphic demonstration of the role of short-chain fatty acids in CD4 during metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis.
Short-chain fatty acids (SCFAs) promote naïve CD4+ T cell differentiation towards Th2, Th22, and Tregs to protect against liver injury and damage. SCFAs increase the ratio of Th17 to resting Treg, conferring an anti-fibrotic effect. SCFAs enhance anti-inflammatory effects through inhibition of Th1 and an increase of Th2 and Treg cells, improving insulin sensitivity. Increased levels of Treg can reduce cholesterol. Pro-drugs modulating SCFAs can decrease lipid accumulation and present an anti-fibrotic effect. MASLD: Metabolic dysfunction-associated steatotic liver disease. This figure was created using BioRender.com (Supplementary material).
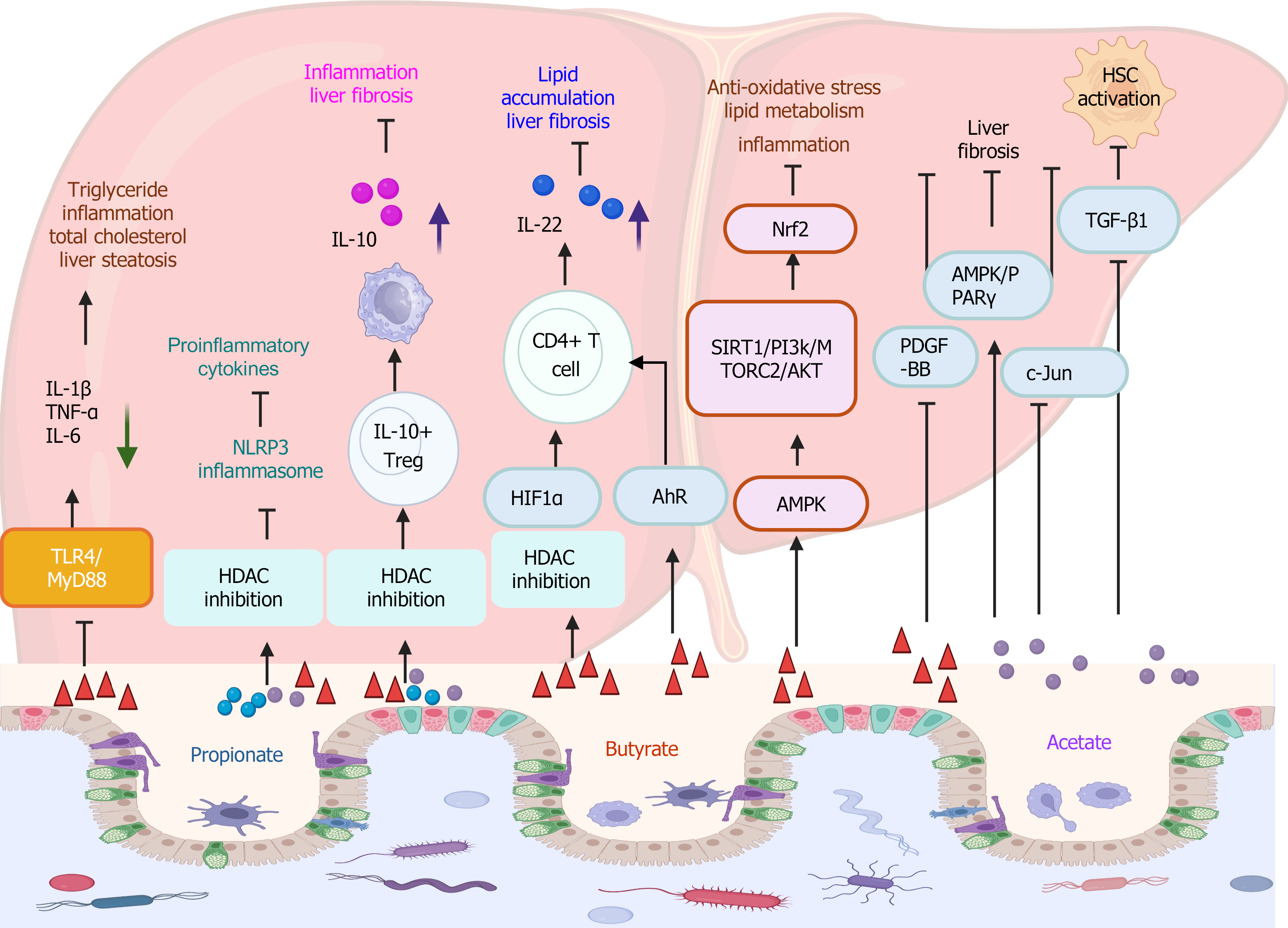
Figure 4 Short-chain fatty acid-associated signaling pathways in metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis.
Short-chain fatty acids (SCFAs) decrease the expression of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α by inhibiting the TLR4/MyD88 signaling pathway. Consequently, the triglyceride, total cholesterol, liver steatosis, and inflammation are decreased. SCFAs alleviate inflammation through inhibition of histone deacetylase and the NLRP3 signaling pathway. SCFAs promote IL10+ Treg to accelerate macrophage to induce elevated IL-10 production, conferring anti-inflammatory and anti-fibrosis function. SCFA promotes CD4+ T cells to produce IL-22 through HIFα and aryl hydrocarbon receptor signaling pathways. SCFA activates Nrf2 through the AMPK signaling pathway and its downstream signaling SIRT1/PI3k/MTORC2/AKT, presenting the anti-oxidative effect, and decreasing lipid metabolism and inflammation. SCFAs confer the anti-fibrotic effect through platelet-derived growth factor-BB inhibition, AMPK/PPARγ activation, and c-Jun inhibition signaling pathways. SCFA inhibits the activation of hepatic stellate cells via inhibiting the TGF-β1-mediated signaling pathway. TNF-α: Tumor necrosis factor-α; HDAC: Histone deacetylase; HSC: Hepatic stellate cells; IL: Interleukin. This figure was created using BioRender.com (Supplementary material).
- Citation: Zhang CY, Liu S, Sui YX, Yang M. Roles of short-chain fatty acids in metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis. World J Hepatol 2025; 17(11): 113756
- URL: https://www.wjgnet.com/1948-5182/full/v17/i11/113756.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i11.113756