修回日期: 2025-04-21
接受日期: 2025-05-08
在线出版日期: 2025-05-28
代谢相关脂肪性肝病(metabolic dysfunction-associated fatty liver disease, MAFLD)是全球范围内日益流行的肝脏疾病, 其发病机制涉及肠道菌群失调、肠道屏障功能损伤以及肝脏炎症反应的异常激活. 肠道菌群作为人体最重要的微生物群落, 经过肠肝轴联通肝脏, 在MAFLD的发生发展过程中扮演着越来越重要的角色. 本文重点围绕肠道菌群如何通过破坏肠道黏膜屏障, 导致细菌及其代谢产物易位至肝脏, 进而激活肝脏免疫系统并引发炎症反应展开综述, 深入探讨肠道菌群在肠道黏膜屏障维护、破坏引发的炎症反应、免疫反应和脂质代谢、糖代谢、能量代谢等方面的作用, 以及其对肝脏免疫细胞功能和炎症通路的调节机制, 旨在为MAFLD的肠肝轴发病机制提供较多思路和证据.
核心提要: 肠道菌群参与肠道黏膜屏障甚或血管屏障的修复与损伤, 一旦某些因素, 如高脂饮食存在等使屏障易于破坏, 细菌及其代谢产物就可通过肠肝轴易位至肝脏, 激活肝脏免疫系统, 进而触发一系列炎症反应, 推动代谢相关脂肪性肝病发生进展. 明确肠道菌群对肠道黏膜甚或血管屏障的破坏作用及其对肝脏炎症、免疫反应和能量的调控作用, 有助于为代谢相关脂肪性肝病的肠肝轴发病机制提供较多思路和证据.
引文著录: 吴松婷, 鲁晓岚. 肠道菌群破坏肠道黏膜屏障调控代谢相关脂肪性肝病炎症、免疫与代谢紊乱的研究进展. 世界华人消化杂志 2025; 33(5): 337-343
Revised: April 21, 2025
Accepted: May 8, 2025
Published online: May 28, 2025
Metabolic dysfunction-associated fatty liver disease (MAFLD) is an increasingly prevalent liver disorder worldwide, with its pathogenesis involving gut microbiota dysbiosis, impaired intestinal barrier function, and abnormal activation of hepatic inflammatory responses. As the most significant microbial community in the human body, the gut microbiota interacts with the liver through the gut-liver axis and plays an increasingly significant role in the onset and progression of MAFLD. This review focuses on how the gut microbiota disrupts the intestinal mucosal barrier, leading to the translocation of bacteria and their metabolites to the liver, thereby activating the hepatic immune system and triggering inflammatory responses. Specifically, we delve into the role of the gut microbiota in maintaining intestinal mucosal integrity, the inflammatory and immune responses triggered by its disruption, and its influence on lipid, glucose, and energy metabolism. Additionally, we discuss the regulatory mechanisms by which the gut microbiota modulates hepatic immune cell function and inflammatory pathways. Through this comprehensive analysis, we aim to provide further insights and evidence regarding the gut-liver axis in the pathogenesis of MAFLD.
- Citation: Wu ST, Lu XL. Gut microbiota-mediated disruption of intestinal mucosal barrier: Implications in inflammation, immunity, and metabolism in metabolic dysfunction-associated fatty liver disease. Shijie Huaren Xiaohua Zazhi 2025; 33(5): 337-343
- URL: https://www.wjgnet.com/1009-3079/full/v33/i5/337.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v33.i5.337
代谢相关脂肪性肝病(metabolic dysfunction-associated fatty liver disease, MAFLD)曾被称为非酒精性脂肪性肝病, 此次更名更加突出了其与代谢紊乱的密切关联, 其疾病谱包括单纯性脂肪肝、脂肪性肝炎、肝纤维化、肝硬化甚至肝癌等一系列病理过程[1]. 随着全球肥胖、糖尿病等代谢性疾病的流行, MAFLD的发病率呈显著上升趋势, 已成为严重影响公众健康的全球性问题. 肠道菌群作为重要的环境因子, 从营养感知、能量摄入、糖脂代谢、炎症反应等方面干预宿主的功能状态, 越来越多的研究表明肠道菌群参与MAFLD的发病环节, 甚至是始动因素, 同时某些肠道菌群及代谢产物与MAFLD的疾病严重程度明显相关[2-4]. 肠道菌群的失衡(即生态失调)可能通过多种途径参与MAFLD的病理生理过程, 特别是在高脂饮食情况下, 某些细菌破坏肠道屏障的保护作用, 对机体和肝脏的代谢、免疫和炎症反应产生一系列重要的影响, 表现出肝脏脂质蓄积、氧化应激、线粒体功能障碍和细胞凋亡, 最终导致肝脏纤维化, 推动MAFLD的发生发展.
肠道屏障是维持肠道微环境稳态的关键防线, 主要由物理屏障、化学屏障、生物屏障和免疫屏障四大屏障组成, 这些屏障协同作用, 维持肠道炎症平衡和免疫稳态. 在MAFLD中, 肠道菌群的失衡可能导致肠道屏障功能受损, 使得肠道内的病原体和抗原物质进入血液循环, 激活炎症免疫系统, 诱导机体产生一系列后续反应, 引发病理结果.
肠道黏膜屏障是肠道物理防御系统的关键组成部分, 其结构主要包括黏液层、肠道上皮细胞(intestinal epithelial cells, IECs)以及肠道血管屏障(gut vascular barrier, GVB). GVB作为阻止肠道菌群及其代谢产物进入血液循环的最后一道防线, 近年来逐渐成为研究热点, 寻找肠道菌群对GVB的破坏机制可能为MAFLD发病的机制研究提供新的参考. 研究证实GVB的破坏依赖于对WNT/β-catenin信号通路的干扰[5], 而肠道菌群对该信号通路具有重要的调控作用, 如小鼠伤寒沙门菌能依赖其毒力因子Spi2编码的Ⅲ型分泌系统, 抑制肠道内皮细胞中的β-catenin信号传导, 进而破坏GVB[6]; 艰难梭菌通过其外毒素介导的多种信号通路诱导血管内皮生长因子A产生, 增加GVB的通透性, 促进机体炎症的扩散[7]. 肠道黏膜屏障功能障碍尤其紧密连接蛋白(tight junction proteins, TJs)的破坏, 会导致肠道通透性增加, 例如大肠杆菌(Escherichia coli 25)可破坏肠道上皮细胞之间的TJs增加GVB的通透性[8], 这种病理状态称为"肠漏综合征", 肠道内的脂多糖(lipopolysaccharide, LPS)等内毒素会通过门静脉进入肝脏, 激活肝脏内的免疫细胞, 如枯否细胞(Kupffer cells, KCs), 引发免疫反应. 例如, Enterococcus gallinarum的定植可导致Hp/zonulin的诱导和TJs相关分子(如Cldn3和Ocln)的下调, 加剧屏障功能障碍, 细菌易位至肝脏上调肝脏自身抗原的表达[9]. 此外, Porphyromonas gingivalis干预已证实可以改变肠道菌群组成并抑制TJs的表达, 导致细菌及其代谢产物的全身易位[10].
此外, 肠道菌群还可以通过调控肠道内多种免疫细胞的分化和功能, 如T细胞、B细胞和树突状细胞(dendritic cells, DCs), 维持肠道免疫稳态. 在健康状态下, 肠道菌群通过促进免疫细胞的平衡分化, 帮助维持肠道黏膜免疫的非炎症性环境. Tang等[11]研究报道了肠道菌群尤其Enterobacter ludwigii产生的L-赖氨酸一方面激活DCs中的丝氨酸、甘氨酸、一碳代谢途径, 另一方面诱导DCs中的S-腺苷甲硫氨酸和脱氧沉默因子1样蛋白表达增加, 导致组蛋白H3赖氨酸79位点二甲基化增强, 进而影响Tgfb和Stat3基因启动子区域, 促进免疫耐受, 维持免疫稳态. 然而在某些病理环境下, 例如长期高脂饮食, 肠道菌群的失衡致肠道屏障功能受损, 使得肠道内的病原体和抗原物质进入血液循环, 激活免疫系统[12], 造成慢性炎症反应以及免疫耐受丧失. 而在MAFLD患者中, 肠道免疫细胞的功能异常可能导致免疫反应失调, 进一步加重肝脏损伤.
在健康状态下, 机体通过调节多种抗炎机制来维持炎症反应的平衡. 肠道菌群可以通过调节抗炎细胞因子如白细胞介素(Interleukin, IL)-10的分泌, 以及抗炎细胞如调节性T细胞(regulatory T cells, Tregs)的功能来发挥抗炎作用. 然而, 在MAFLD患者中, 肠道菌群的失调可能导致抗炎机制的失衡, 使得抗炎细胞因子的分泌减少, 抗炎细胞的功能受损. 例如, 一些研究发现, MAFLD患者肠道中产生IL-10的细菌数量减少, 导致IL-10的分泌不足, 无法有效抑制炎症反应[13,14]. 此外, Treg细胞的功能异常也可能导致免疫调节失衡, 促进炎症反应的持续发展. 一些研究表明炎性组织环境有利于肠道菌群的扰动, 表现为特定菌群的大量繁殖, 这些细菌往往具有更强的利用炎症肠道营养物质的能力, 例如柠檬酸杆菌和沙门氏菌最初利用毒力因子诱导肠道炎症, 在肠腔内获得生长优势[15,16].
此外, 还有一类肠道菌群具有保护肠道黏膜屏障的作用, 如两歧双歧杆菌(Bifidobacterium bifidum; 以下简称B. bifidum), Xu等[17]的研究证实, 该菌不仅能逆转高脂饮食诱导的小鼠肥胖表型, 还可显著增强肠道黏膜屏障, 改善肝脏的组织学表现, 包括脂肪变性、气球样变性、炎症浸润及纤维化程度. B. bifidum参与色氨酸代谢的吲哚途径产生吲哚及其衍生物, 这些物质可以充当芳香烃受体(aryl hydrocarbon receptor, AHR)的配体[18,19]. AHR信号在维持肠道微环境稳态中起着关键作用, 它是肠道屏障免疫反应的核心调控因子, 研究证实AHR可直接作用于α-防御素1, 调节肠道菌群和免疫平衡, 从而缓解肠道炎症[20]. 此外, 研究还发现吲哚-3-乙酸等色胺酸代谢产物能够抑制巨噬细胞产生促炎介质, 包括肿瘤坏死因子-α(tumor necrosis factor, TNF-α)、IL-1β、单核细胞趋化蛋白等, 通过抗炎减轻MAFLD的发生发展[17,21]. 最新研究报道吲哚-3-丙酸通过与甲硫氨酸腺苷转移酶2A特异性结合, 促进S-腺苷甲硫氨酸合成, 进而促进USP16去泛素化酶的DNA甲基化, 导致Toll样受体(toll-like receptors, TLRs)4泛素化, 抑制NF-κB信号传导, 最终下调M1型巨噬细胞产生IL-1β[22]. 总之, 吲哚类物质通过激活AHR或调控甲基化相关通路, 协同抑制炎症介质或通路活化, 多层面发挥抗炎和调节免疫的作用, 促进MAFLD的缓解.
肠道菌群具有通过多种途径调节肝脏免疫细胞的功能. KCs是肝脏内主要的巨噬细胞, 在肝脏免疫监视和炎症反应中发挥着重要作用. 当肠道黏膜屏障及GVB受损时, 肠道内的细菌代谢产物如LPS等可以通过门静脉激活肝脏KCs, 使其分泌大量的促炎细胞因子(如TNF-α、IL-6等), 从而引发肝脏炎症和免疫反应. 肠道菌群失调还可通过AHR/TLR4/p-STAT3介导的线粒体氧化应激途径诱导肝脏M1型巨噬细胞极化, 进而触发一系列肝内免疫反应, 促进脂质在肝脏中不断沉积[23]. 此外, 肠道屏障功能的破坏会导致LPS突破肠黏膜屏障和黏膜下血管屏障, 进入血液循环, 随后通过门静脉到达肝脏, 激活KCs, 这一过程会上调肝脏内TLR4、MyD88和磷酸化NF-κB的表达, 触发过度炎症反应和免疫反应, 造成肝脏损伤[24,25], 而敲除TLR4可抑制TLR4/MyD88/NF-κB信号传导, 减少炎症和细胞凋亡, 进而减轻LPS诱导的肝损伤[26].
同时, 肠道菌群还可以调节肝内其他多种免疫细胞的功能, 包括自然杀伤(natural killer, NK)细胞、自然杀伤T(natural killer T cells, NKT)细胞、γδ T细胞、黏膜相关不变T(mucosa associated invariant T, MAIT)细胞等. NK细胞和NKT细胞不仅在抗病毒感染和抗肿瘤免疫中发挥着重要作用, 同时也参与了MAFLD的免疫病理过程, 肠道菌群的失衡可能导致NK细胞和NKT细胞功能异常, 影响肝脏的免疫防御和修复能力. 而MAIT细胞在不同肝脏疾病中扮演着不同角色, 通常被微生物核黄素衍生物激活, 也可通过细胞因子(如IL-12和IL-18)以MHC I类相关分子1非依赖方式激活, 在MAFLD中, MAIT细胞可通过诱导抗炎巨噬细胞极化起到疾病缓解作用[27], 而MAIT细胞缺陷Cd1d-/-/Mr1-/- MAFLD/MASH小鼠表现为更严重的肝脏脂质蓄积、脂肪变性和炎症[28]. 此外, 一项最新的研究表明洗涤粪菌移植亦可通过上调3型固有淋巴细胞(innate lymphoid cell type 3, ILC3s)上CXCR6的表达, 促进ILC3s通过CXCL16/CXCR6轴向肝脏归巢, 进而分泌IL-22减轻肝脏脂肪变性[29].
还有一些研究已经发现, 通过16SrRNA可以在肝脏组织中检测到肠道细菌的存在, 尤其在肝细胞癌中[30,31]. 这些研究提示, 肝脏内的炎症和系列免疫反应, 不仅仅是LPS的作用, 可能还有肠道细菌或其代谢产物的直接作用, 这方面的工作越来越引发大家的关注. 这些细菌产物是如何进入肝脏的, 除了肠黏膜屏障, GVB也是一个重要研究方面.
肠道菌群在调节宿主脂质代谢方面发挥重要作用. 胆汁酸和短链脂肪酸(short-chain fatty acids, SCFAs)是脂质消化和吸收的关键物质, 肠道菌群通过调控上述两种物质的代谢过程, 进而影响脂质的吸收和分解. 某些肠道菌群具备将初级胆汁酸代谢为次级胆汁酸的能力, 从而改变胆汁酸的组成和比例, 进一步影响脂质的代谢和转运[32]. 在MAFLD患者中, 肠道菌群的改变可能导致胆汁酸代谢异常, 使得胆汁酸的重吸收增加, 从而影响脂质的代谢和转运. 例如, 某些特定的肠道细菌(如Turicibacter属)可以通过调节胆汁酸代谢相关基因的表达, 促进胆汁酸的排泄, 减少脂质在肝脏的蓄积[33]. 在两项大规模的队列研究中发现人类肠道菌群的Ruminococcaceae UCG-002和UCG-003与心血管代谢疾病和血脂异常呈显著负相关, 而这种关联与某些胆汁酸(如异石胆酸、鼠胆酸和去甲胆酸)有关[34]. Xu等[35]使用血脂异常供体的粪菌移植建立人源化血脂异常小鼠模型, 发现Faecalibaculum和Ruminococcaceae UCG-010的丰度显著增加, 这一改变导致了血清胆酸、鹅脱氧胆酸和脱氧胆酸水平升高, 并通过肝脏法尼醇X受体(farnesoid X receptor, FXR)-小异二聚体伴侣抑制胆汁酸合成, 而在高脂饮食下Muribaculum的丰度下降, 肠道中猪脱氧胆酸减少, 并通过肠道FXR-成纤维细胞生长因子19促进胆汁酸合成, 增加脂质吸收.
另一方面, 肠道菌群还可以通过调控SCFAs的生成来影响脂质代谢. SCFAs(如丁酸、乙酸和丙酸)是肠道菌群发酵膳食纤维的主要产物. 其中, 丁酸不仅是IECs主要的能量来源, 还能通过脂肪酸氧化为宿主提供能量. 此外, 丁酸能够刺激胰高血糖素样肽-1(glucagon-like peptide-1, GLP-1)和YY肽(PYY)的释放, 分别调节胰岛素生物合成和大脑饱腹感, 表现出控制血糖和抗肥胖的潜在效应. 与此一致的是, 动物实验表明, 使用丁酸前体药物-三丁酸甘油酯, 能够有效降低饮食诱导的肥胖、胰岛素抵抗和肝脏脂肪变性发生的风险[36]. 丙酸则通过抑制肝脏中脂肪酸的合成促进脂肪酸的氧化, 减少肝脏脂肪的堆积. 在MAFLD动物模型中, 补充含有产SCFAs细菌的益生菌可以改善脂质代谢紊乱, 减轻肝脏脂肪变性[37]. 此外, 肠道菌群还能通过代谢胆碱生成氧化三甲胺(trimethylamine N-oxide, TMAO), 在结肠中TMAO通过激活TLR4/MyD88/NF-κB通路、抑制Wnt/β-catenin通路, 破坏肠道屏障结构功能, 在肝脏中TMAO一方面诱导肝窦内皮细胞功能障碍、调节巨噬细胞极化, 另一方面通过KRT17促进体外脂肪肝细胞的脂质沉积和纤维化进程[25,38].
肠道菌群在糖代谢中也扮演着关键角色. 肠道菌群的失衡可能引发胰岛素抵抗(insulin resistance, IR)的发生和发展, 而IR也是MAFLD的重要病理特征之一. IR会使肝脏对胰岛素的敏感性降低, 进而增加肝脏葡萄糖的生成, 同时减少外周组织对葡萄糖的摄取和利用. 肠道菌群通过影响肠道屏障功能和内毒素血症来间接调控糖代谢. 当肠道屏障功能受损, 肠道内的内毒素如LPS进入血液循环, 激活免疫系统, 引发慢性炎症反应, 进而干扰胰岛素信号通路, 最终导致IR的形成. 研究表明, 支链氨基酸(branched-chain amino acids, BCAAs)浓度升高是IR和2型糖尿病风险增加的生物标志物[39], 某些特定细菌如Prevotella copri和Bacteroides vulgatus可升高血浆BCAAs水平, 诱导IR产生, 加剧葡萄糖不耐受[40]. 这些细菌通过改变宿主的氨基酸代谢, 影响胰岛素信号通路的正常功能, 从而推动糖代谢紊乱的进展. Liu等[41]最新的研究发现短期和长期高脂饮食均能显著提升白色脂肪组织mRNA的N6-甲基腺苷水平, 分别激活内源性逆转录病毒(endogenous retroviruses, ERVs)和长散在重复序列(long interspersed elements, LINEs), 并抑制组蛋白H3K9二甲基化, 通过肠道菌群介导的HGA-m6A-Ehmt2-ERV/LINE信号通路激活转座子, 最终引发葡萄糖不耐受和IR. 相反, 拟杆菌属、产芽孢梭菌产生的吲哚丙酸可改善胰岛素分泌及其敏感性, 降低2型糖尿病发生风险[42].
此外, 肠道菌群还可以通过调节肠道内分泌细胞分泌的激素来影响糖代谢. GLP-1是一种关键的肠促胰岛素激素, 它能够促进胰岛素的分泌, 抑制胰高血糖素的释放, 并延缓胃排空, 从而有效降低血糖水平. 某些肠道细菌能够刺激GLP-1的分泌, 进而改善宿主的糖代谢[43,44]. 最近的一项研究发现, 肠道游离脂肪酸受体4通过调控普通拟杆菌及其代谢物泛酸, 影响GLP-1-FGF21轴, 从而调节小鼠的糖偏好, 这一发现表明普通拟杆菌衍生的泛酸可能成为糖尿病治疗的新靶点[45].
肠道菌群对宿主的能量代谢有着深远的影响. 它们能够通过发酵难以消化的碳水化合物, 生成额外的能量供宿主利用. 在MAFLD患者中, 肠道菌群的组成和功能改变可能导致能量代谢的失衡. 一些研究发现, MAFLD患者肠道中厚壁菌门与拟杆菌门的比例升高[46,47], 而厚壁菌门细菌具有更强的能量获取能力, 可能导致宿主能量摄入增加, 从而促进脂肪在肝脏的蓄积.
前述提到的B. bifidum亦可以调控宿主能量代谢. 功能宏基因组学分析表明, 该菌干预可显著富集线粒体电子传递链复合体I相关基因, 提示其通过增强有氧呼吸链功能提升能量代谢效率[48]. 在高脂饮食诱导的肥胖模型中, B. bifidum处理使小鼠腹股沟白色脂肪组织和性腺脂肪组织湿重显著降低, 表明其可能通过提高线粒体氧化磷酸化活性促使白色脂肪从能量储存模式向能量消耗模式转化[49].
肠道菌群还可以通过调节肠道内分泌细胞分泌的食欲调节激素(如胃饥饿素和瘦素)来影响宿主的能量摄入和消耗[50]. 胃饥饿素具有刺激食欲的作用, 而瘦素则能够抑制食欲, 帮助维持能量平衡. 然而, 肠道菌群的失衡可能扰乱这些激素的正常分泌和调节, 进而引发能量代谢紊乱.
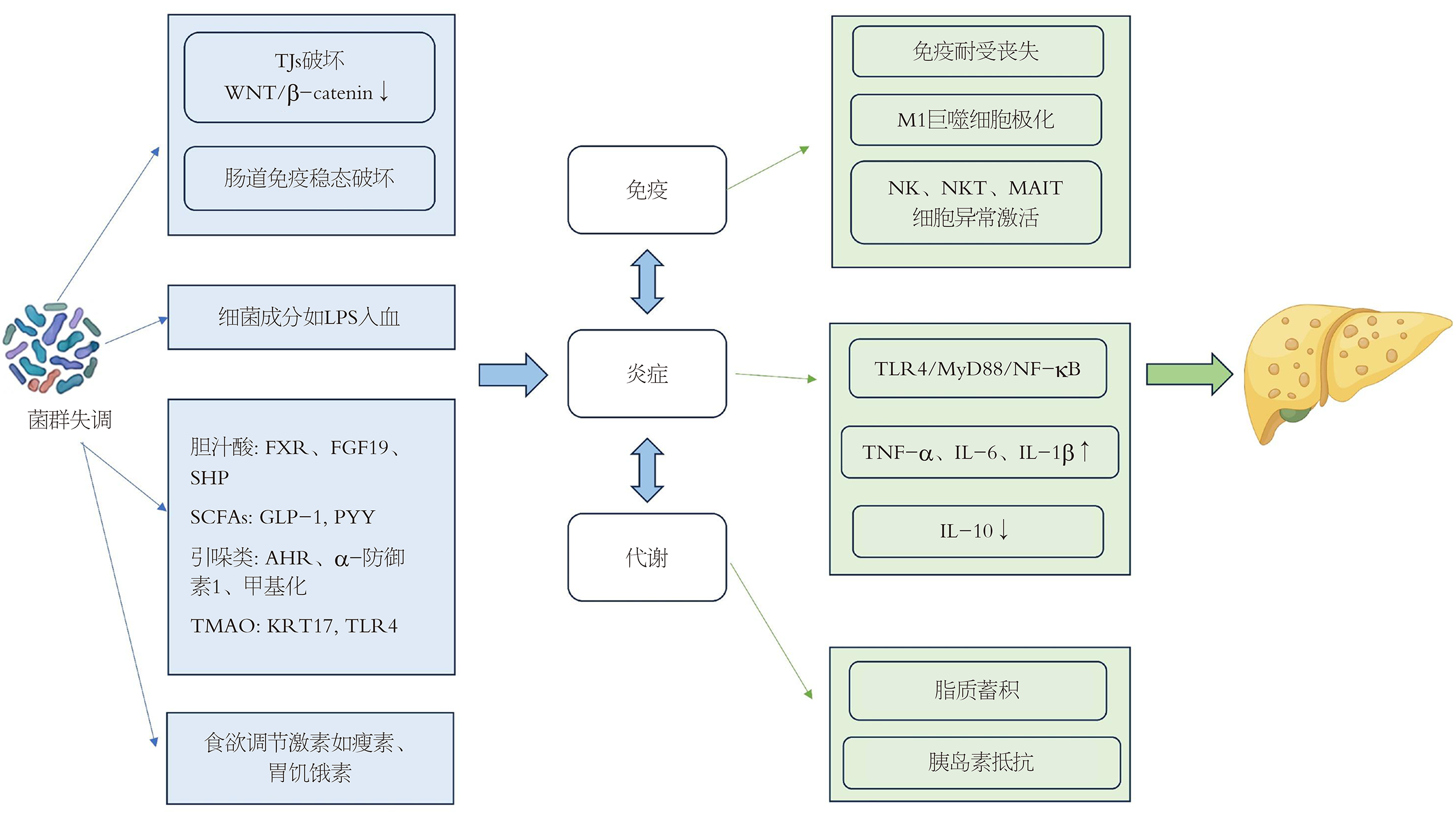
MAFLD的发病机制与肠道菌群失调密切相关, 如图1所示, 肠道菌群通过破坏肠道黏膜屏障甚或血管屏障, 导致细菌及其代谢产物易位至肝脏, 激活肝脏免疫系统引发炎症反应, 进而影响肝脏和机体脂质、糖和能量代谢过程. 所以, 肠肝轴在MAFLD发病中很可能起着启动和促进展的重要作用. 然而, 关于肠道菌群究竟是MAFLD的因还是果仍存在争议, 肝脏炎症及代谢异常亦可影响肠道微生态, 形成恶性循环. 因此, 肠肝轴更可能呈现双向反馈关系, 未来仍需更多的纵向研究明确其因果关系.
尽管肠道菌群在MAFLD发生发展中起着关键作用, 但开发基于肠道菌群的精准治疗仍存在不少的挑战, 一方面由于肠道菌群与宿主之间的相互作用涉及免疫、代谢等多个层次, 如何精准解析这些复杂的相互作用仍是一个难题; 另一方面, 由于个体差异性使得标准化的治疗方案很难统一, 肠道菌群这一庞大群体非常复杂, 人类对它的了解还很不够, 干预的有效性及安全性仍需要大量基础研究和临床试验验证. 未来应结合多组学技术全面揭示肠道菌群与MAFLD之间的复杂关系, 揭秘肠道菌群与人体代谢的关联, 探讨菌群入肝的路径. 同时, 积极开发基于肠道菌群的精准干预策略, 探索其在MAFLD早期诊断和预防中的个体化应用, 将为MAFLD的防治提供新的思路和方案.
学科分类: 胃肠病学和肝病学
手稿来源地: 上海市
同行评议报告学术质量分类
A级 (优秀): A
B级 (非常好): B, B
C级 (良好): C
D级 (一般): 0
E级 (差): 0
科学编辑: 刘继红 制作编辑:郑晓梅
| 2. | Chen J, Vitetta L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int J Mol Sci. 2020;21. [PubMed] [DOI] |
| 3. | Wang L, Cao ZM, Zhang LL, Li JM, Lv WL. The Role of Gut Microbiota in Some Liver Diseases: From an Immunological Perspective. Front Immunol. 2022;13:923599. [PubMed] [DOI] |
| 4. | Schwenger KJP, Copeland JK, Ghorbani Y, Chen L, Comelli EM, Guttman DS, Fischer SE, Jackson TD, Okrainec A, Allard JP. Characterization of liver, adipose, and fecal microbiome in obese patients with MASLD: links with disease severity and metabolic dysfunction parameters. Microbiome. 2025;13:9. [PubMed] [DOI] |
| 5. | Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216-1228. [PubMed] [DOI] |
| 6. | Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830-834. [PubMed] [DOI] |
| 7. | Huang J, Kelly CP, Bakirtzi K, Villafuerte Gálvez JA, Lyras D, Mileto SJ, Larcombe S, Xu H, Yang X, Shields KS, Zhu W, Zhang Y, Goldsmith JD, Patel IJ, Hansen J, Huang M, Yla-Herttuala S, Moss AC, Paredes-Sabja D, Pothoulakis C, Shah YM, Wang J, Chen X. Clostridium difficile toxins induce VEGF-A and vascular permeability to promote disease pathogenesis. Nat Microbiol. 2019;4:269-279. [PubMed] [DOI] |
| 8. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [PubMed] [DOI] |
| 9. | Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta SS, Knight JR, Jain D, Goodman AL, Kriegel MA. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156-1161. [PubMed] [DOI] |
| 10. | Kinashi Y, Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front Immunol. 2021;12:673708. [PubMed] [DOI] |
| 11. | Tang Q, Fan G, Peng X, Sun X, Kong X, Zhang L, Zhang C, Liu Y, Yang J, Yu K, Miao C, Yao Z, Li L, Zhang ZS, Wang Q. Gut bacterial L-lysine alters metabolism and histone methylation to drive dendritic cell tolerance. Cell Rep. 2025;44:115125. [PubMed] [DOI] |
| 12. | Nian F, Wu L, Xia Q, Tian P, Ding C, Lu X. Akkermansia muciniphila and Bifidobacterium bifidum Prevent NAFLD by Regulating FXR Expression and Gut Microbiota. J Clin Transl Hepatol. 2023;11:763-776. [PubMed] [DOI] |
| 13. | Si W, Zhao X, Li R, Li Y, Ma C, Zhao X, Bugno J, Qin Y, Zhang J, Liu H, Wang L. Lactobacillus rhamnosus GG induces STING-dependent IL-10 in intestinal monocytes and alleviates inflammatory colitis in mice. J Clin Invest. 2025;135. [PubMed] [DOI] |
| 14. | Shen Z, Luo W, Tan B, Nie K, Deng M, Wu S, Xiao M, Wu X, Meng X, Tong T, Zhang C, Ma K, Liao Y, Xu J, Wang X. Roseburia intestinalis stimulates TLR5-dependent intestinal immunity against Crohn's disease. EBioMedicine. 2022;85:104285. [PubMed] [DOI] |
| 15. | Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907-915. [PubMed] [DOI] |
| 16. | Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, Núñez G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336:1325-1329. [PubMed] [DOI] |
| 17. | Xu J, Xia Q, Wu T, Shao Y, Wang Y, Jin N, Tian P, Wu L, Lu X. Prophylactic treatment with Bacteroides uniformis and Bifidobacterium bifidum counteracts hepatic NK cell immune tolerance in nonalcoholic steatohepatitis induced by high fat diet. Gut Microbes. 2024;16:2302065. [PubMed] [DOI] |
| 18. | Min BH, Devi S, Kwon GH, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, Yoon SJ, Park HJ, Eom JA, Jeong MK, Hyun JY, Stalin N, Park TS, Choi J, Lee DY, Han SH, Kim DJ, Suk KT. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024;16:2307568. [PubMed] [DOI] |
| 19. | Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43:1522-1535. [PubMed] [DOI] |
| 20. | Palrasu M, Kakar K, Marudamuthu A, Hamida H, Thada S, Zhong Y, Staley S, Busbee PB, Li J, Garcia-Buitrago M, Nagarkatti M, Nagarkatti P. AhR Activation Transcriptionally Induces Anti-Microbial Peptide Alpha-Defensin 1 Leading to Reversal of Gut Microbiota Dysbiosis and Colitis. Gut Microbes. 2025;17:2460538. [PubMed] [DOI] |
| 21. | Taverniti V, Cesari V, Gargari G, Rossi U, Biddau C, Lecchi C, Fiore W, Arioli S, Toschi I, Guglielmetti S. Probiotics Modulate Mouse Gut Microbiota and Influence Intestinal Immune and Serotonergic Gene Expression in a Site-Specific Fashion. Front Microbiol. 2021;12:706135. [PubMed] [DOI] |
| 22. | Han Z, Fu J, Gong A, Ren W. Bacterial indole-3-propionic acid inhibits macrophage IL-1β production through targeting methionine metabolism. Sci China Life Sci. 2025;68:1118-1131. [PubMed] [DOI] |
| 23. | Zhang J, Liu H, Shen Y, Cheng D, Tang H, Zhang Q, Li C, Liu M, Yao W, Ran R, Hou Q, Zhao X, Wang JS, Sun X, Zhang T, Zhou J. Macrophage AHR-TLR4 cross-talk drives p-STAT3 (Ser727)-mediated mitochondrial oxidative stress and upregulates IDO/ICAM-1 in the steatohepatitis induced by aflatoxin B(1). Sci Total Environ. 2024;923:171377. [PubMed] [DOI] |
| 24. | Coste SC, Orășan OH, Cozma A, Negrean V, Sitar-Tăut AV, Filip GA, Hangan AC, Lucaciu RL, Iancu M, Procopciuc LM. Metabolic Dysfunction-Associated Steatotic Liver Disease: The Associations between Inflammatory Markers, TLR4, and Cytokines IL-17A/F, and Their Connections to the Degree of Steatosis and the Risk of Fibrosis. Biomedicines. 2024;12. [PubMed] [DOI] |
| 25. | Nian F, Chen Y, Xia Q, Zhu C, Wu L, Lu X. Gut microbiota metabolite trimethylamine N-oxide promoted NAFLD progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int Immunopharmacol. 2024;142:113173. [PubMed] [DOI] |
| 26. | Chen SN, Tan Y, Xiao XC, Li Q, Wu Q, Peng YY, Ren J, Dong ML. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol Sin. 2021;42:1610-1619. [PubMed] [DOI] |
| 27. | Li Y, Huang B, Jiang X, Chen W, Zhang J, Wei Y, Chen Y, Lian M, Bian Z, Miao Q, Peng Y, Fang J, Wang Q, Tang R, Gershwin ME, Ma X. Mucosal-Associated Invariant T Cells Improve Nonalcoholic Fatty Liver Disease Through Regulating Macrophage Polarization. Front Immunol. 2018;9:1994. [PubMed] [DOI] |
| 28. | Zheng Q, Xue C, Gu X, Shan D, Chu Q, Wang J, Zhu H, Chen Z. Multi-Omics Characterizes the Effects and Mechanisms of CD1d in Nonalcoholic Fatty Liver Disease Development. Front Cell Dev Biol. 2022;10:830702. [PubMed] [DOI] |
| 29. | Zhong HJ, Zhuang YP, Xie X, Song JY, Wang SQ, Wu L, Zhan YQ, Wu Q, He XX. Washed microbiota transplantation promotes homing of group 3 innate lymphoid cells to the liver via the CXCL16/CXCR6 axis: a potential treatment for metabolic-associated fatty liver disease. Gut Microbes. 2024;16:2372881. [PubMed] [DOI] |
| 30. | Xue C, Jia J, Gu X, Zhou L, Lu J, Zheng Q, Su Y, Zheng S, Li L. Intratumoral bacteria interact with metabolites and genetic alterations in hepatocellular carcinoma. Signal Transduct Target Ther. 2022;7:335. [PubMed] [DOI] |
| 31. | Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, Rozeman EA, Blank CU, Ronai A, Shaoul R, Amit A, Dorfman T, Kremer R, Cohen ZR, Harnof S, Siegal T, Yehuda-Shnaidman E, Gal-Yam EN, Shapira H, Baldini N, Langille MGI, Ben-Nun A, Kaufman B, Nissan A, Golan T, Dadiani M, Levanon K, Bar J, Yust-Katz S, Barshack I, Peeper DS, Raz DJ, Segal E, Wargo JA, Sandbank J, Shental N, Straussman R. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973-980. [PubMed] [DOI] |
| 32. | Ridlon JM, Gaskins HR. Another renaissance for bile acid gastrointestinal microbiology. Nat Rev Gastroenterol Hepatol. 2024;21:348-364. [PubMed] [DOI] |
| 33. | Lynch JB, Gonzalez EL, Choy K, Faull KF, Jewell T, Arellano A, Liang J, Yu KB, Paramo J, Hsiao EY. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat Commun. 2023;14:3669. [PubMed] [DOI] |
| 34. | Jiang Z, Zhuo LB, He Y, Fu Y, Shen L, Xu F, Gou W, Miao Z, Shuai M, Liang Y, Xiao C, Liang X, Tian Y, Wang J, Tang J, Deng K, Zhou H, Chen YM, Zheng JS. The gut microbiota-bile acid axis links the positive association between chronic insomnia and cardiometabolic diseases. Nat Commun. 2022;13:3002. [PubMed] [DOI] |
| 35. | Xu H, Fang F, Wu K, Song J, Li Y, Lu X, Liu J, Zhou L, Yu W, Yu F, Gao J. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 2023;11:262. [PubMed] [DOI] |
| 36. | Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570-575. [PubMed] [DOI] |
| 37. | van der Hee B, Wells JM. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021;29:700-712. [PubMed] [DOI] |
| 38. | Nian F, Zhu C, Jin N, Xia Q, Wu L, Lu X. Gut microbiota metabolite TMAO promoted lipid deposition and fibrosis process via KRT17 in fatty liver cells in vitro. Biochem Biophys Res Commun. 2023;669:134-142. [PubMed] [DOI] |
| 39. | Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311-326. [PubMed] [DOI] |
| 40. | Cunningham AL, Stephens JW, Harris DA. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021;13:50. [PubMed] [DOI] |
| 41. | Liu Y, Liu J, Ren R, Xin Z, Luo Y, Chen Y, Huang C, Liu Y, Yang T, Wang X. Short-term and long-term high-fat diet promote metabolic disorder through reprogramming mRNA m(6)A in white adipose tissue by gut microbiota. Microbiome. 2025;13:75. [PubMed] [DOI] |
| 42. | Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem. 2018;124:306-312. [PubMed] [DOI] |
| 43. | Zeng Y, Wu Y, Zhang Q, Xiao X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio. 2024;15:e0203223. [PubMed] [DOI] |
| 44. | Jiang Y, Yang J, Xia L, Wei T, Cui X, Wang D, Jin Z, Lin X, Li F, Yang K, Lang S, Liu Y, Hang J, Zhang Z, Hong T, Wei R. Gut Microbiota-Tryptophan Metabolism-GLP-1 Axis Participates in β-Cell Regeneration Induced by Dapagliflozin. Diabetes. 2024;73:926-940. [PubMed] [DOI] |
| 45. | Zhang T, Wang W, Li J, Ye X, Wang Z, Cui S, Shen S, Liang X, Chen YQ, Zhu S. Free fatty acid receptor 4 modulates dietary sugar preference via the gut microbiota. Nat Microbiol. 2025;10:348-361. [PubMed] [DOI] |
| 46. | Ha S, Wong VW, Zhang X, Yu J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2024;74:141-152. [PubMed] [DOI] |
| 47. | Zhang L, Liu ZX, Liu YH, Chen Y, Chen J, Lu CH. Auricularia auriculaPolysaccharides Exert Anti-inflammatory Effects in Hepatic Fibrosis by the Gut-Liver Axis and Enhancing SCFA Metabolism. J Agric Food Chem. 2025;73:4617-4629. [PubMed] [DOI] |
| 48. | Feng C, Zhang W, Zhang T, He Q, Kwok LY, Tan Y, Zhang H. Heat-Killed Bifidobacterium bifidum B1628 May Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, and the Anti-Inflammatory Effect Is Associated with Gut Microbiota Modulation. Nutrients. 2022;14. [PubMed] [DOI] |
| 49. | Kim G, Yoon Y, Park JH, Park JW, Noh MG, Kim H, Park C, Kwon H, Park JH, Kim Y, Sohn J, Park S, Kim H, Im SK, Kim Y, Chung HY, Nam MH, Kwon JY, Kim IY, Kim YJ, Baek JH, Kim HS, Weinstock GM, Cho B, Lee C, Fang S, Park H, Seong JK. Bifidobacterial carbohydrate/nucleoside metabolism enhances oxidative phosphorylation in white adipose tissue to protect against diet-induced obesity. Microbiome. 2022;10:188. [PubMed] [DOI] |
| 50. | Han H, Yi B, Zhong R, Wang M, Zhang S, Ma J, Yin Y, Yin J, Chen L, Zhang H. From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators. Microbiome. 2021;9:162. [PubMed] [DOI] |