Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.1900
Revised: March 27, 2003
Accepted: April 11, 2003
Published online: September 15, 2003
AIM: To investigate gene expression pattern of human γ-synuclein gene in human esophageal squamous cell carcinoma (ESCC) by using semi-quantitive reverse transcription polymerase chain reaction (RT-PCR), and to study the role of γ-synuclein in the development of human ESCC.
METHODS: Semi-quantitive RT-PCR of 27 pairs of specimens of human ESCC tissues and corresponding normal tissues was used to investigate the expression pattern of γ-synuclein in ESCC. 9706/γ-syn cells in which γ-synuclein was overexpressed were obtained through cloning γ-synuclein gene by PCR and transfecting it into ESCC 9706 cells, then selecting with G-418 for 14 days. The biological effects of γ-synuclein were measured and compared between 9706/γ-syn and 9706/vec cells by cell growth curve and soft agar assay.
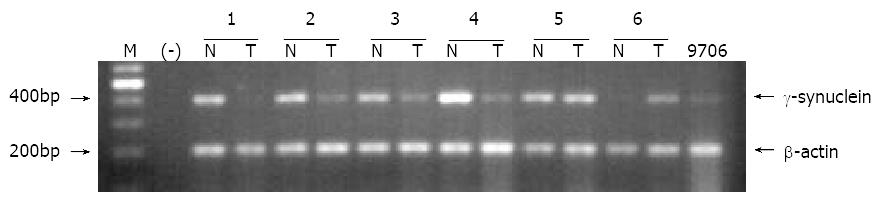
RESULTS: RT-PCR showed that γ-synuclein gene was expressed in all the 27 cases of normal epithelial tissues, while downregulation of γ-synuclein was observed in 16 out of the 27 cases (59.3%) of ESCC. There were also 6 cases of ESCC tissues with a high expression level of γ-synuclein mRNA. In functional analysis we found that over-expression of γ-synuclein in ESCC 9706 cells could inhibit the growth rate and transformation ability of ESCC 9706 cells.
CONCLUSION: The low expression level of γ-synuclein in human ESCC and the biological effects of γ-synuclein over-expression on ESCC 9706 cells suggest that γ-synuclein may play a role as a negative regulator in the development of human ESCC.
- Citation: Zhou CQ, Liu S, Xue LY, Wang YH, Zhu HX, Lu N, Xu NZ. Down-regulation of γ-synuclein in human esophageal squamous cell carcinoma. World J Gastroenterol 2003; 9(9): 1900-1903
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/1900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.1900
Synucleins are a family of small, highly conserved soluble proteins that are predominantly expressed in neural tissues and certain tumors[1,2]. There are at least three members of this family in vertebrates: α-, β-, γ-synuclein. All synucleins contain a highly conserved amino-terminal domain that includes several repeated domains displaying variation of a KTKEGV consensus sequence and a less conserved carboxy-terminal domain that includes a preponderance of acidic residues[1,2]. The α- and β-synuclein proteins are found primarily in brain tissues and in association with pathological lesions of neurodegenerative diseases[3,4]. While γ-synuclein, initially termed breast cancer-specific gene 1 (BCSG1)[5], is found in the peripheral nervous system and retina, and it may affect the integrity of the neurofilament network[4-6]. Furthermore, over-expression of γ-synuclein has been recently shown in several types of cancer, including breast and ovarian cancer, suggesting that it may be involved in a certain number of human cancers[5,7].
γ-synuclein gene was first isolated from a human breast tumor cDNA library, and therefore named breast cancer-specific gene 1 (BCSG1)[5], soon after it was determined to be a new member of the synuclein family[8]. The human γ-synuclein maps to chromosome region 10q23, and is composed of five exons and transcribed into an mRNA of about 1 kb, coding 127 amino acids[8,9]. It was reported that γ-synuclein was over-expressed in infiltrating breast ductal carcinoma and ovarian cancer[7]. Other studies have shown that over-expression of γ-synuclein in breast and ovarian cancer cells may enhance the motility and invasiveness in vitro and metastasis in vivo of breast cancer[10,11].
Human esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors in China, and its etiology and pathogenesis remain to be determined[12]. However, it has been reported that many oncogenes and tumor suppressor genes are closely related to ESCC, such as c-myc, cyclin D1, p53[13-15]. In the present study, we first examined the expression pattern of γ-synuclein in 27 cases of ESCC. It was unexpected that γ-synuclein was down regulated in 16 out of 27 cases of ESCC compared to their corresponding normal tissues, rather than over-expressed in tumor tissues. In order to further explore the role of γ-synuclein in the development of ESCC, we cloned human γ-synuclein gene and transfected it into ESCC 9706 cell line. Our data demonstrated that the ectopic expression of γ-synuclein in ESCC cell line could inhibit cell growth in dish and colony formation in soft agar. In conclusion, unlike in breast and ovarian cancers, γ-synuclein might play a role as a tumor suppressor in the development of human ESCC.
Twenty-seven carcinomatous tissues and the corresponding normal tissues were obtained from surgically resected esophageal carcinoma in Cancer Hospital. Fresh samples were dissected manually to remove connective tissues and stored immediately at -80 °C until analysis. Carcinoma tissues were obtained from poorly, moderately, and well differentiated squamous epithelial cell carcinomas. The corresponding normal tissues were obtained from the distant edge of dissected esophagus. Each tissue sample contained over 80% of normal or tumor epithelial cells.
Total RNA was extracted from paired specimens of primary esophageal cancer and non-cancerous esophageal epithelial tissues with TRIZOL reagent (Invitrogen) according to the manufacturer’s protocol.
Five micrograms of total RNA extracted from paired esophageal carcinoma were used as a template respectively to synthesize cDNA in 25 μL reaction mixture with 2.5 mM oligo d(T)16 primers and M-MLV Reverse Transcriptase (Promega) at 37 °C for 1 h followed by 85 °C for 10 min. PCR was performed in 20 μL reaction mixture [1 × taq buffer (Dingguo Company), 100 ng template cDNA, 200 μM of each dNTPs, 0.5 μM of each primers and 2 U Taq] as follows: at 95 °C for 5 min followed by 20 cycles at 95 °C for 1 min, at 61 °C for 1 min, at 72 °C for 1 min, and the final step of extension was for 10 min at 72 °C. Sequences of the PCR primers for γ-synuclein are as follows: Upper primer: 5’CGGGATCCACCATGGATGTCTTCAAGAAG3’. Lower primer: 5’CCGCTCGAGCTAGTCTCCCCCACTCTG3’.
The reaction products were visualized by electrophoresis of 5 ul reaction mixture at 70 V for 40 min in 2% agarose gel containing 0.5 μg/mL ethidium bromide, and quantitated by densitometry using a dual-intensity transilluminator equipped with Gel-Pro Analyzer version 3.1. β-actin was used as internal control.
Human ESCC 9706 cell line (kindly provided by Dr. Mingrong Wang) was grown in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum (Gibico BRL)[16]. Transfection was performed in 80% confluent cells using LipofectAMINE (Invitrogen) according to the manufacturer’s protocol. Stable transfectants were obtained by G418 selection for about 14 days.
Total cell lysate was prepared in cell lysis buffer (1% NP-40, 137 mM NaCl, 20 mM Tris pH = 7.4, 1 mM DTT, 10% glycerin, 10 μg/mL Aprotinin, 2 mM sodium orthavanadate, 100 uM PMSF). After incubation for 30 min on ice, cells were scraped from the culture dish, and the mixture was centrifuged at 12000 g for 10 min at 4 °C. The protein concentration of the clarified lysate was determined using a BCA kit with BSA as a standard (Periece).
Equal amounts of total protein from cell lysate were resolved on 15% SDS-PAGE. For western blot analysis, proteins separated by SDS-PAGE were transferred to nitrocellulose (PROTRAN, Schleicher&Schuell). Firstly the filters were incubated in a blocking solution (5% fat-free dry milk, 2% BSA in TBS) for 1 h at room temperature. Then the filters were incubated with primary antibody, added with a blocking solution at 1:1000 dilution, for 1 h at room temperature. After washed three times with TTBS (0.5% Tween-20 in TBS), the filters were subsequently incubated for 40 min with HRP (horseradish peroxidase) conjugated secondary antibody (Zhongshan Company). The filters were washed as above and developed using a Luminol detection system (Santa Cruz). Before re-blotting, the filters were stripped by incubation for 30 min in stripping buffer (2% SDS, 62.5 mM Tris.Cl pH = 6.7, 100 mM 2-mercaptoethanol) at 50 °C.
Growth on plastic culture dish 2 × 104 cells from 9706/γ-syn and 9706/vec cell line were seeded in triplicate of 24-well plates and cultured in DMEM plus 1% FBS and 10% FBS. At each time point, cells were trypsinized to single cell suspension and counted on a Coulter counter set at > 10 μm in diameter.
Anchorage-independent growth in soft agar According to standard protocol, 200 ESCC 9706 cells were suspended in 1 mL of 0.3% agarose medium containing DMEM plus 10% FBS and layered onto a 2 mL bed of 0.6% agarose in a 35 mm dish with grids. Plates were incubated for about 14 days, and the colonies were stained with 0.2% p-iodonitroterazolium violet and photographed.
Expression pattern of γ-synuclein in human ESCC was examined using semi-quantitative RT-PCR approach. Figure 1 shows a 384 bp γ-synuclein fragment from ESCC tissues and relatively normal tissues, and a 200 bp β-actin fragment as an internal control by electrophoresis in 2% agarose gel. The γ-synuclein gene was expressed in all the 27 cases of normal esophageal epithelial tissues, while down-regulation of γ-synuclein gene was observed in 16 out of the 27 cases (59.3%) of ESCC. There were also 6 cases of ESCC tissues with high expression level of γ-synuclein mRNA (Table 1). These results indicated that γ-synuclein might play a negative role in carcinogenesis of human ESCC.
| γ-synuclein RT-PCR | Cases (percentage) |
| Down-regulated | 16 (59.3%) |
| Up-regulated | 6 (22.2%) |
| No change | 5 (18.5%) |
| Total | 27 (100%) |
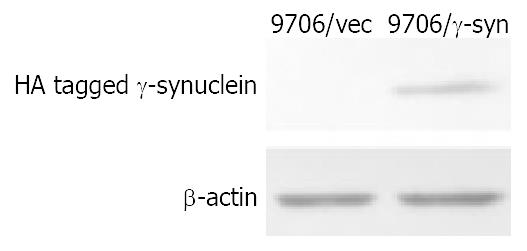
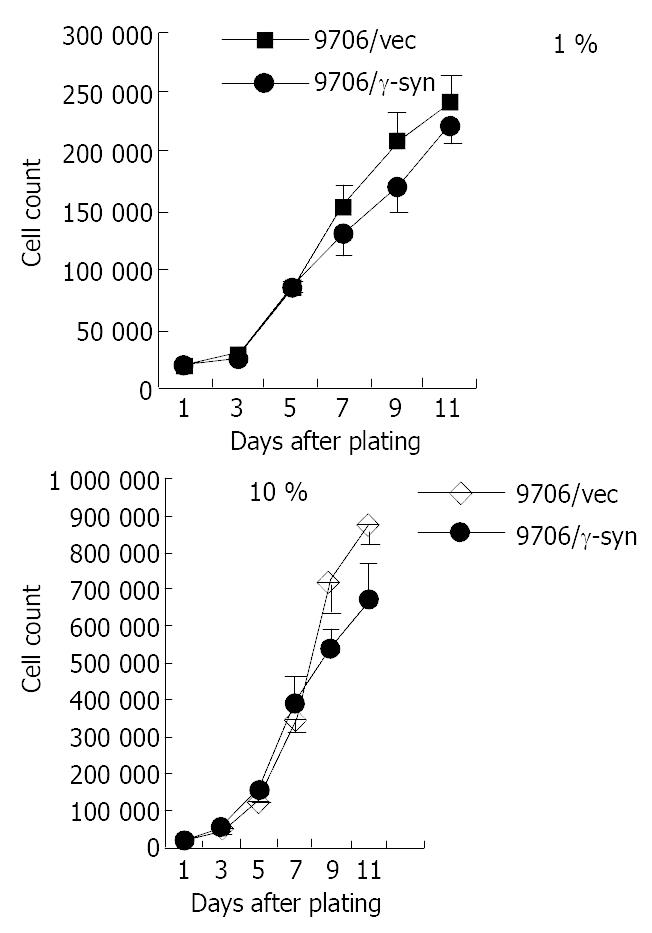
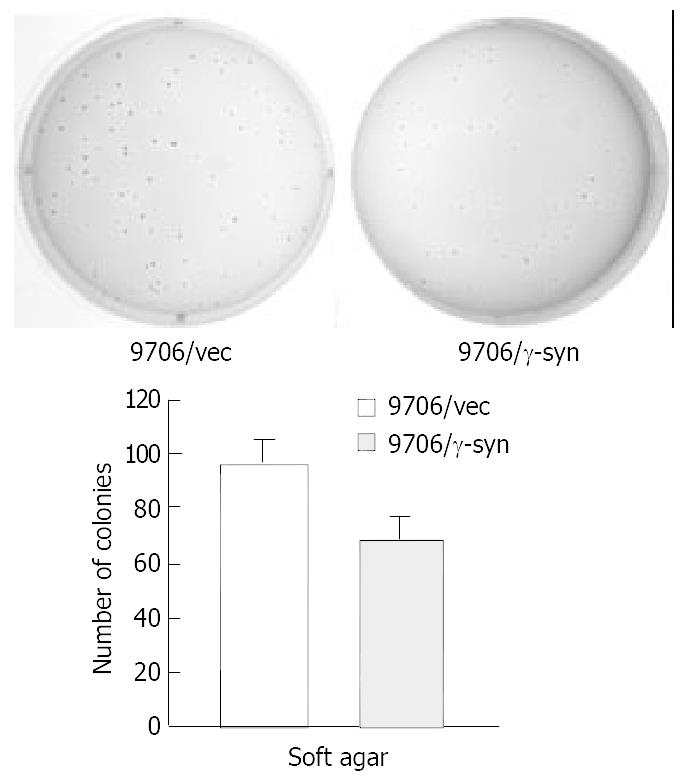
RT-PCR results led us to investigate the role of γ-synuclein in carcinogenesis of human ESCC. Firstly we amplified the γ-synuclein cDNA fragment from fetal brain cDNA and cloned it into the pcDNA3.0 vector to generate HA-tagged γ-synuclein expression plasmids. Then we transfected γ-synuclein expression plasmid and pcDNA3.0 vector into ESCC 9706 cells respectively. After 14 days selection with G418 (300 μg/mL), we obtained the stable transfectants named 9706/γ-syn and 9706/vec cells. The expression of transfected γ-synuclein was verified by Western blot analysis (Figure 2). To determine whether γ-synuclein over-expression affected the growth of ESCC 9706 cells, cells from 9706/γ-syn and 9706/vec were seeded in triplicate of 24-well plate in DMEM plus 1% FBS and 10% FBS. According to the growth curve we observed that 9706/γ-syn cells grew more slowly than control cells, whether in low or high serum medium in dishes (Figure 3). Furthermore, we confirmed the negative effect of γ-synuclein on ESCC 9706 cells by soft agar assay in which 9706/γ-syn cells displayed much smaller and fewer colonies than 9706/vec cells (Figure 4), indicating that γ-synuclein had the ability to inhibit the transformation activity of ESCC 9706 cells, and might act as a negative regulator in the development of human ESCC.
Human esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors in China[12]. Although the etiological agents have yet to be identified, studies have shown a series of genetic events involving the development of this cancer[13-15,17]. Identification of the genetic changes in ESCC as well as in other cancers will provide diagnostic and prognostic markers and molecular targets for treatment, intervention and prevention of human cancers.
It was reported that γ-synuclein was over-expressed in breast and ovarian cancer[5,7]. In the current study, we intended at first to detect the expression of γ-synuclein in esophageal squamous cell carcinoma (ESCC). However, γ-synuclein was unexpectedly down regulated in some 60% tumor samples (16 out of 27 cases), compared to the corresponding normal tissues, rather than over-expressed in tumor tissues such as in breast and ovarian cancer. On the other hand, γ-synuclein was detected in all the 27 normal esophageal tissues. Furthermore, consistent with our observation that the expression of γ-synuclein was lower in tumor tissue than in surrounding non-tumor tissue in ESCC, after transfection of human ESCC cells into ESCC 9706 cells and selection with G-418, the transfectants expressing γ-synuclein (9706/γ-syn) grew remarkably slower than 9706/vec cells in low or high serum medium in dishes (Figure 3). It also displayed much smaller and fewer colonies than 9706/vec cells in soft agar (Figure 4), indicating that γ-synuclein has the ability to inhibit the transformation activity of ESCC 9706 cells. Therefore our present study suggests that γ-synuclein may be a negative regulator in tumorigenesis of human ESCC, unlike that in breast and ovarian cancer as an oncogene.
It is not unexpected to find out that the biological and biochemical functions of the same gene are different in different cells or tissues from different tumors, since the development of tumors is a complex multigene-related phenomenon and the result of a multistep process involving a series of genetic events. As shown in our study, the manner and effect of γ-synuclein in human ESCC were different from those in breast and ovarian cancer. Moreover it was previously shown by others that in A2780 and OVCAR5 cells (ovarian cancer cell lines) with over-expressed γ-synuclein, the activated ERK1/2 was increased 2- to 3-fold. In contrast, in HEK293 cells (human embryonic kidney cells), γ-synuclein over-expression did not increase ERK activation level[18]. Likewise, the same status was also observed in many other studies, among which GKLF/KLF4 (gut-enriched Krüppel-like factor/Krüppel-like factor 4) gene was a good example. GKLF/KLF4 encodes a zinc finger transcription factor and belongs to a big family[19]. It is the same as γ-synuclein as it is over-expressed in breast cancer[20], while down regulated in ESCC[21]. In addition, Annexin I (lipocortin I) gene is another very similar case. Annexin I is a phospholipid binding protein which is implicated in various cell activities, including proliferation, differentiation and apoptosis[22]. Studies showed that annexin I protein was actually over-expressed in human tumors including breast cancer, hepatocellular carcinoma[23,24]. However, it was also found that annexin I protein expression was decreased in human esophageal and prostate cancers[25,26]. p21 (Waf1/Cip1), one of the best broad-specificity inhibitors of CDK, acts as a suppressor in most tumors. But in some tumors, including ESCC, p21 (Waf1/Cip1) is over-expressed, acting as an oncogene to promote carcinogenesis and tumor progression[27].
Despite the fact that the mechanism by which γ-synuclein protein is involved in carcinogenesis of human tumors remains unclear, the data presented here have shown that γ-synuclein is down-regulated in ESCC, and is over-expressed in breast and ovarian cancer[5,7]. To shed new light on the role of γ-synuclein in oncogenesis, more studies are certainly needed to determine how γ-synuclein is down-regulated or up-regulated in tumors and what the physiological functions of γ-synuclein are during the development of human tumors.
We are grateful to Dr. Mingrong Wang for generously providing the human ESCC 9706 cell line.
| 1. | Lavedan C. The synuclein family. Genome Res. 1998;8:871-880. [PubMed] |
| 2. | George JM. The synucleins. Genome Biol. 2002;3:REVIEWS3002. [PubMed] |
| 3. | Mukaetova-Ladinska EB, Hurt J, Jakes R, Xuereb J, Honer WG, Wischik CM. Alpha-synuclein inclusions in Alzheimer and Lewy body diseases. J Neuropathol Exp Neurol. 2000;59:408-417. [PubMed] |
| 4. | Li J-, Henning Jensen P, Dahlström A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113:463-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, Joseph BK, Rosen C, Shi YE. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997;57:759-764. [PubMed] |
| 6. | Buchman VL, Hunter HJ, Pinõn LG, Thompson J, Privalova EM, Ninkina NN, Davies AM. Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system. J Neurosci. 1998;18:9335-9341. [PubMed] |
| 7. | Bruening W, Giasson BI, Klein-Szanto AJ, Lee VM, Trojanowski JQ, Godwin AK. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000;88:2154-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Lavedan C, Leroy E, Dehejia A, Buchholtz S, Dutra A, Nussbaum RL, Polymeropoulos MH. Identification, localization and characterization of the human gamma-synuclein gene. Hum Genet. 1998;103:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Ninkina NN, Alimova-Kost MV, Paterson JW, Delaney L, Cohen BB, Imreh S, Gnuchev NV, Davies AM, Buchman VL. Organization, expression and polymorphism of the human persyn gene. Hum Mol Genet. 1998;7:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Jia T, Liu YE, Liu J, Shi YE. Stimulation of breast cancer invasion and metastasis by synuclein gamma. Cancer Res. 1999;59:742-747. [PubMed] |
| 11. | Liu J, Spence MJ, Zhang YL, Jiang Y, Liu YE, Shi YE. Transcriptional suppression of synuclein gamma (SNCG) expression in human breast cancer cells by the growth inhibitory cytokine oncostatin M. Breast Cancer Res Treat. 2000;62:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wang DX, Li W. Advances in esophageal neoplasms etiology. Shijie Huaren Xiaohua Zazhi. 2000;8:1029-1030. |
| 13. | Mandard AM, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res. 2000;462:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Raja S, Godfrey TE, Luketich JD. The role of tumor suppressor genes in esophageal cancer. Minerva Chir. 2002;57:767-780. [PubMed] |
| 15. | Zou JX, Wang LD, Shi ST, Yang GY, Xue ZH, Gao SS, Li YX, Yang CS. p53 gene mutations in multifocal esophageal precan-cerous and cancerous lesions in patients with esophageal cancer in high-risk northern China. Shijie Huaren Xiaohua Zazhi. 1999;7:280-284. |
| 16. | Han Y, Wei F, Xu X, Cai Y, Chen B, Wang J, Xia S, Hu H, Huang X, Han Y. [Establishment and comparative genomic hybridization analysis of human esophageal carcinomas cell line EC9706]. Zhonghua Yixue Yichuanxue Zazhi. 2002;19:455-457. [PubMed] |
| 17. | Zhou J, Zhao LQ, Xiong MM, Wang XQ, Yang GR, Qiu ZL, Wu M, Liu ZH. Gene expression profiles at different stages of human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:9-15. [PubMed] |
| 18. | Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J Biol Chem. 2002;277:35050-35060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009-20017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 542] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, Ruppert JM. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488-6495. [PubMed] |
| 21. | Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN, Wu M. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966-970. [PubMed] |
| 22. | Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1567] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 23. | Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997;15:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M. Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology. 1996;24:72-81. [PubMed] |
| 25. | Xia SH, Hu LP, Hu H, Ying WT, Xu X, Cai Y, Han YL, Chen BS, Wei F, Qian XH. Three isoforms of annexin I are preferentially expressed in normal esophageal epithelia but down-regulated in esophageal squamous cell carcinomas. Oncogene. 2002;21:6641-6648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J. Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000;60:6293-6297. [PubMed] |
| 27. | Roninson IB. Oncogenic functions of tumour suppressor p21(Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 325] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
Edited by Zhu LH