Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1772
Revised: March 22, 2003
Accepted: April 1, 2003
Published online: August 15, 2003
AIM: To determine the changes of pS2 and ITF of TFF expression in gastric mucosa and the effect on ulcer healing of pS2, ITF to Water-immersion and restraint stress (WRS) in rats.
METHODS: Wistar rats were exposed to single or repeated WRS for 4 h every other day for up to 6 days.Gastric mucosal blood flow (GMBF) was measured by LDF-3 flowmeter and the extent of gastric mucosal lesions were evaluated grossly and histologically. Expression of pS2 and ITF mRNA was determined by RT-PCR. Immunohistochemistry was used to further detect the expression of pS2 and ITF.
RESULTS: WRS applied once produced numerous gastric mucosal erosions, but the number of these lesions gradually declined and GMBF restored at 2, 4, 8 h after stress. The area of gastric mucosal lesion was reduced by 64.9% and GMBF was increased by 89.8% at 8 h. The healing of stress-induced ulcerations was accompanied by increased expression of pS2 (0.51 ± 0.14 vs 0.77 ± 0.11, P < 0.01) and ITF (0.022 ± 0.001vs 0.177 ± 0.010, P < 0.01). The results were demonstrated further by immunohistochemistry of pS2 (0.95 ± 0.11 vs 1.41 ± 0.04, P < 0.01) and ITF (0.134 ± 0.001 vs 0.253 ± 0.01,P < 0.01). With repeated WRS, adaptation to this WRS developed, the area of gastric mucosal lesions was reduced by 22.0% after four consecutive WRS. This adaptation to WRS was accompanied by increased GMBF (being increased by 94.2%), active cell proliferation in the neck region of gastric glands, and increased expression of pS2 (0.37 ± 0.02 vs 0.77 ± 0.01, P < 0.01) and ITF (0.040 ± 0.001vs 0.372 ± 0.010, P < 0.01). The result was demonstrated further by immunohistochemistry of pS2 (0.55 ± 0.04 vs 2.46 ± 0.08, P < 0.01) and ITF (0.134 ± 0.001vs 0.354 ± 0.070, P < 0.01).
CONCLUSION: TFF may not only participate in the early phase of epithelial repair known as restitution(maked by increased cell migration),but also play an important role in the subsequent,protracted phase of glandular renewal(made by cell proliferation).
- Citation: Nie SN, Qian XM, Wu XH, Yang SY, Tang WJ, Xu BH, Huang F, Lin X, Sun DY, Sun HC, Li ZS. Role of TFF in healing of stress-induced gastric lesions. World J Gastroenterol 2003; 9(8): 1772-1776
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1772.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1772
Stress ulcer is a highly prevalent clinical complication. Fully understanding the mechanism of healing of stress-induced gastric lesions not only deepens our insights into stress ulcer, but also provides new ways for its prevention and treatment in clinical practice. The mechanism of the recovery of gastric mucosa after stress exposure has not been fully explained, the healing of stress ulcerations is a complex process which is affected by different factors. Current research has found that a variety of peptides are considered to play a crucial role in the control of mucosal integrity and repair. Among them, an important role was attributed to epidermal growth factor and transforming growth factor alpha[1-3].
Recently, a group of new peptides has been discovered, called TFF (trefoil factor family or trefoil peptides) because of their uniquely distinctive cysteine-rich “three-leaf” secondary structure, which probably protects these peptides from degradation by luminal acid and proteases within gastrointestinal tract. pS2 and intestinal trefoil factor (ITF) belong to the growing family of trefoil peptides[4,5].
The physiological role of TFF is poorly understood so far. The aim of the present study was to investigate the expression of pS2 and ITF in gastric mucosa of rats undergone WRS, and to probe the role of TFF in the early phase of epithelial repair of stress-induced gastric lesion.
Induction of gastric adaptation to WRS: Thirty male Wistar rats, weighing 210-250 g (purchased from Xipuer-Bikai Experimental Animal Co. LTD, Shanghai) which had been fasted for 24 h with free access to water, were used. The animals were deprived of water 1 h before the experiment and divided into normal control group (n = 6) and experimental control group (n = 24). After being fasted for 24 h, the rats of normal control group were lightly anesthetized with ether and tied up on the rat board, the abdomen was opened, the stomach was exposed and GMBF was measured in the oxyntic gland area, gastric mucosa was sampled. The rats of experimental control group were divided into four subgroups (6 in each group) and exposed to WRS[6] for 4 h. They were killed either immediately (0 time: namely 0 h) or after 2 h, 4 h, 8 h. GMBF was measured and gastric mucosa was sampled as described below.
The rats of experimental control group were divided into four subgroups (6 each group) and exposed to repeated WRS[7]: The rats of group I were lightly anesthetized with ether, tied up on the rat board and exposed to WRS for 4 h by placing in water at 20-23 °C to the rat’s xyphoid level at 10:00 AM on day 1. Then the rats were anesthetized with pentobarbital (30 mg·kg-1 i.p.), GMBF was measured and gastric mucosa was sampled. the rats of group II were treated similarly except that after WRS, they were removed from water and placed at room temprature, and refed with food and water until 10:00 AM the next day, and starved again for 24 h, WRS was repeated. the rats of group III and IV were exposed to the 3rd or 4th WRS as described above.
Measurement of GMBF: GMBF was measured by using laser Doppler flowmetry (LDF-3 flowmeter, Nankai University, Tianjin, China). In brief, the rats were anesthetized with pentobarbital (30 mg·kg-1 i.p.), the abdomen was opened, the stomach was exposed and transected, the gastric contents were gently evacuated to the exterior through the cut made in the forestomach.Then, an optical probe was placed gently 0.5 mm above perpendicular to the mucosal surface in the oxyntic gland area to monitor GMBF displayed in mV (value of Doppler signal voltage) on the digital panel of the flowmeter. When GMBF became stable, four points were selected for measurement (one point for 1 minute) and the average value was calculated and expressed as U/mV.
Appreciation of UI: Mucosal lesions were evaluated by the score systems reported by Nie S[7]. Briefly, after the measurement of GMBF, the stomach was dissected out and opened along the greater curvature. The stomach was then examined with a 10×magnifier for the presence of erosions and scored as follows: 1 point for small round hemorrhagic erosion, 2 points when the length of hemorrhagic erosion was less than 1 mm, 3 points when the length was 1-2 mm, 4 points when the length was 2-3 mm, 5 points when the length was longer than 4 mm. The score value multiplied 2 when the width of erosion was larger than 1 mm.
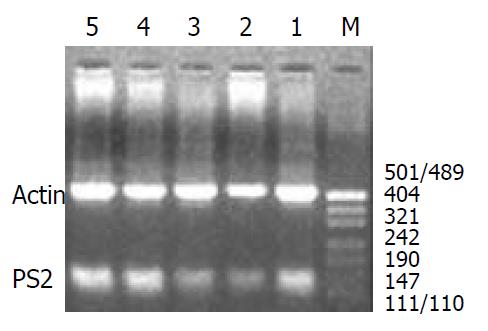
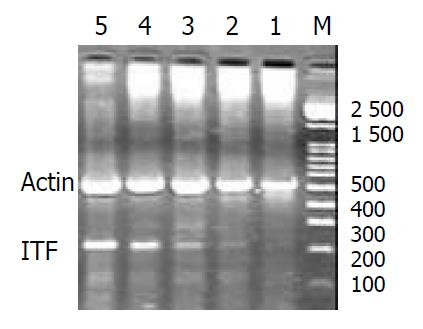
Reverse-transcriptase-polymerase chain reaction (RT-PCR) for detection of messenger RNA (mRNA) for pS2 and ITF: The stomachs were removed from rats with intact gastric mucosa and from those exposed to single or repeated stress. Mucosal specimens (about 100 mg) were scraped off using a slide glass and immediately snap frozen in liquid nitrogen and stored at -80 °C until analysis. Total RNA was isolated from mucosal samples using a guanidium isothiocyanate/phenol chloroform single step extraction kit from Stratagene (Gibco BRL,USA). Following precipitation, the RNA was resuspended in RNAse-free TE buffer and the concentration was estimated by absorbance at 260 nm wavelength. Furthermore, the quality of each RNA sample was determined by running the agarosefomaldehyde electrophoresis. RNA samples were stored at -80 °C until analysis.
Single-strand cDNA was generated from 5 µg of total cellular RNA using StrataScriptTM reverse transcriptase (Gibco BRL,USA ) and oligo (dT) primers (Gibco BRL,USA). Briefly, 5 µg of total RNA was used as the template to synthesize complementary DNA with 2.5 U of Maloney murine leukemia virus reverse transcriptase in 5 µl of buffer containing 10 mM Tris-HCl, pH 8.3; 50 mM KCl, 5 mM MgCl2; 1 mM of each deoxyribonucleoside triphosphate; 2.5 mM of oligo (dt) primers and 1.4 U µl-1 RNAse blocker. Reverse-transcription was performed at room temperature for 20 min, then at 37 °C for 15 min, at 90 °C for 5 min and at 5 °C for 10 min. The resulting complementary DNA was used as a template for subsequent polymerase chain reaction (PCR).
A 124-base pair (bp) fragment of pS2 was amplified from single-stranded DNA by polymerase chain reaction (PCR) using two oligonucleotide primers for pS2 sequence: Sense primer, 5’-CCATGGAGCACAAGGTGACCTG-3’ and antisense primer, 5’-GGGAAGCCACAATTTATTCT-3’. A 221-base pair (bp) fragment of ITF was amplified from single-stranded DNA by polymerase chain reaction (PCR) using two oligonucleotide primers to ITF sequence: Sense primer, 5’-ATGGAGACCAGAGCCTTCTGGAC-3’ and antisense primer, 5’-AGAGGTTTGAAGCACCAGGGC-3’. Concomitantly, amplification of the 521 bp fragment of rat β-actin was performed on the same RNA samples to assess RNA integrity, two oligonucleotide primers to β-actin sequence: Sense primer, 5’-TGGGACGATATGGAGAAGAT-3’ and antisense primer, 5’-ATTGCCGATAGTGATGACCT-3’. The nucleotide sequences of the primers for pS2 were based on the pulished cDNA sequences encoding pS2[8] and the nucleotide sequences of the primers for ITF were based on the published cDNA sequences encoding ITF [9]. The primers were synthesized by Bo-Ya Biotechnical Co. LTD, Shanghai, China.
Reaction mixture for PCR contained cDNA template (2 µl), 50 pmol of each primer, and 2.5 U of Termus aquaticus DNA (Promega) in 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, 0.5 mM dNTPs in a volume of 50 µl. RT blanks (no RNA included) were incubated in each analysis. The mixture was overlaid with 25 µl of mineral oil to prevent evaporation. Amplification was performed using a DNA thermal cycler for 35 cycles, each of which consisted of 2 min at 94 °C for denaturation, 45 s at 52 °C (pS2) and at 50 °C (ITF) for annealing, and 1 min at 72 °C for extension. The final cycle included extension for 5 min at 72 °C to ensure full extension of the product. The number of amplification cycles was previously determined to keep amplification in linear to avoid the “plateau effect” associated with increased numbers of PCR cycles. 8 µl of each PCR -product was electrophoresed on 1.6% agarose gel stained with ethidium bromide, and then visualized under UV light. Location of predicted PCR-product was confirmed by using DNA digest phix 174/Hae III as a stained size marker. The gel was then photographed under UV transillumination. In addition to size analysis by agarose gel electrophoresis,specificity of the primer pair for pS2 and ITF was assessed by sequencing PCR products. For quantification,we determined the intensity of polymerase chain reaction products on the negative film of gel photographs according to Konturek PC et al[10]. Expression of the products was quantified using video image analysis system (TanonGIS-1000, Tanon Technical Co, LTD, Shanghai, China). An index of messenger RNA expression was determined in each sample using the following equation according to Konturek PC et al[11].
Immunocytochemistry: For histological assessment, the other half of the stomach was fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. For immunohistochemistry, serial sections obtained from these paraffin blocks were dewaxed and rehydrated. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 15 min. Sections were then incubated for 35 min with a specific monoclonal antibody against pS2 and ITF (Asgiraud, Italy), washed and incubated with biotinylated rabbit anti-mouse antibody. After 35 min incubation in avidin-biotin complex, the sections were incubated for 2 min in peroxidase substrate (diaminobenzidine, PBS, in addition to 0.3 percent of hydrogen peroxide) and counterstained with haematoxylin.
The intensity of pS2 and ITF staining (Mean score) for each cell was graded according to the criteria described by Nie et al[6]. as follows: 0 = no staining, I = weakly positive, II = moderately positive (cytoplasm positive but other cytoplasmic details also visible), or III = densely stained. The staining intensity was calculated in 300 consecutive cells in three regions of the gastric mucosa: Surface epithelium (top), neck region (neck) and basal portions of the gastric glands (base). The mean intensity per section and region was calculated. Negative control sections were processed immunohistochemically after replacing the primary antibody with an irrelevant monoclonal antibody or phosphate-buffered-saline (PBS).
Statistical analysis: Results were presented as means±SD. Statistical comparisons were made by Student’s t test.The linearly relevant analysis was applied to analyse the relationship between two variants, P values less than 0.05 were considered statistically significant.
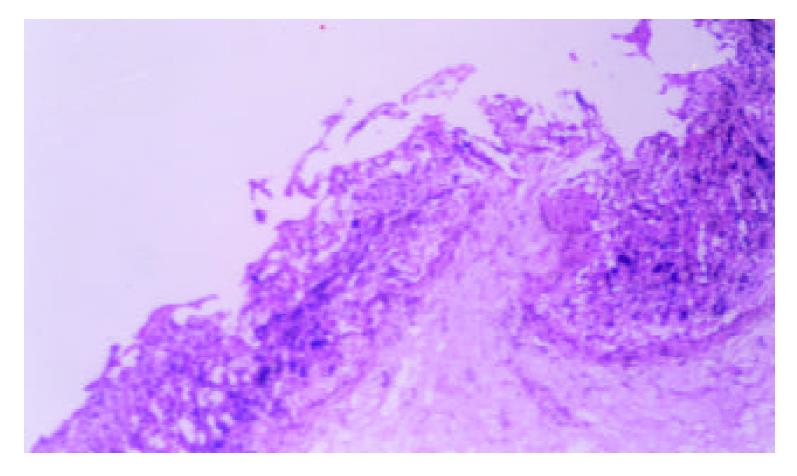
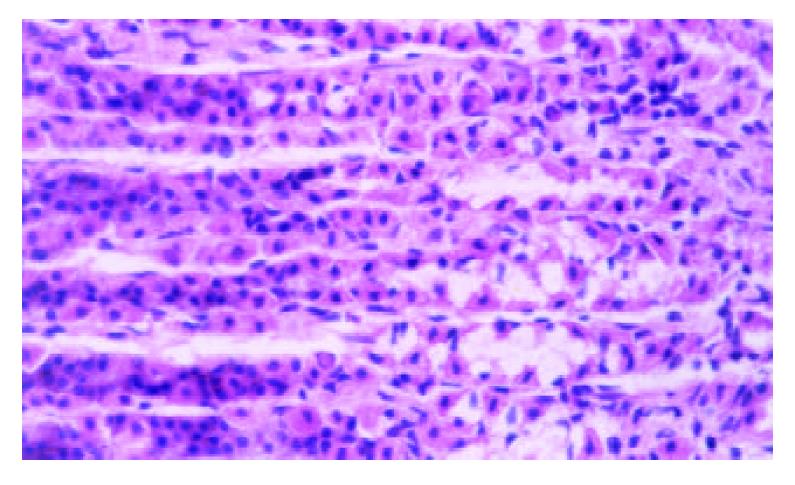
Gastric lesion induced by single or repeated WRS: WRS applied once produced numerous gastric mucosal erosions in oxyntic mucosa with the mean lesion number of 45.32 ± 1.41 per rat. No microscopic evidence of damage occurred in the forestomach. Microscopical examination of the mucosa after 4 h stress revealed widespread damage of the surface epithelium with many cells sloughed off into the gastric lumen and focal area of deep haemorrhagic necrosis (Figure 1). The number of stress lesions was gradually declined at 2, 4, 8 h after the end of stress. UI was reduced to about 20.8% of the initial number at 8 h after the end of stress (Table 1). With repeated WRS, adaptative cytoprotection against stress was developed, UI in II, III, IV groups markedly reduced as compared with group I (P < 0.01). UI after four consecutive WRS was 22% of UI after one WRS. Cell proliferation in the neck regions of gastric glands was activated (Figure 2, Table 2).
| Group | GMBF (U/mV) | UI | Mean score (pS2) | pS2/β-actin | Mean score (ITF) | ITF/β-actin |
| Control experimental | 424.70 ± 7.72 | 0.00 | 1.65 ± 0.03 | 0.78 ± 0.11 | 0.003 ± 0.001 | 0.004 ± 0.0002 |
| 1 | 274.56 ± 13.0b | 45.32 ± 1.41 | 0.95 ± 0.11b | 0.51 ± 0.14b | 0.134 ± 0.001b | 0.022 ± 0.01b |
| 2 | 371.35 ± 15.27bd | 18.31 ± 1.47d | 1.63 ± 0.14d | 0.78 ± 0.13d | 0.259 ± 0.01bd | 0.287 ± 0.008bd |
| 3 | 417.451 ± 12.31d | 11.38 ± 1.31d | 1.53 ± 0.13bd | 0.71 ± 0.12d | 0.136 ± 0.04ad | 0.112 ± 0.009d |
| 4 | 401.32 ± 8.95d | 9.54 ± 1.27d | 1.41 ± 0.04bd | 0.77 ± 0.11ad | 0.235 ± 0.01bd | 0.177 ± 0.01ad |
| Group | GMBF(U/mV) | UI | Mean score (pS2) | pS2/β-actin | Mean score (ITF) | ITF/β-actin |
| Control experimental | 484.01 ± 10.97 | 0.00 | 2.01 ± 0.14 | 0.63 ± 0.01 | 0.0003 ± 0.001 | 0.004 ± 0.0004 |
| I | 321.87 ± 8.85b | 47.23 ± 1.20 | 0.55 ± 0.04b | 0.37 ± 0.02b | 0.134 ± 0.001b | 0.040 ± 0.001b |
| II | 418.35 ± 7.94bd | 30.54 ± 1.12d | 1.51 ± 0.03bd | 0.42 ± 0.01bd | 0.194 ± 0.05bd | 0.108 ± 0.009bd |
| III | 446.09 ± 10.98bd | 20.75 ± 1.54d | 2.55 ± 0.11bd | 0.72 ± 0.02bd | 0.281 ± 0.015bd | 0.265 ± 0.009bd |
| IV | 455.95 ± 11.81bd | 10.39 ± 1.18d | 2.46 ± 0.08bd | 0.77 ± 0.01bd | 0.354 ± 0.07bd | 0.372 ± 0.01bd |
Change of GMBF after single or repeated WRS: GMBF of normal rats was 424.70 ± 7.72U/mV. GMBF significantly decreased after single exposure to WRS and restored at 2, 4, 8 h after the end of stress. It increased up to 94.5% of normal value at 8 h after the end of stress (Table 1). GMBF of normal rats was 484.01 ± 10.97U/mV. GMBF significantly decreased after single exposure to WRS.With repetitive challenge with WRS, there was an adaptive increase of it in experimental group, and GMBF of groups II, III, IV markedly increased as compared with that of group I (P < 0.01). After the 4th time of WRS, the value of GMBF was almost restored to normal level (94.2% of normal value). There was a significantly negative relevance between GMBF and UI (r = -0.953, P < 0.01) (Table 2).
Expressions of pS2 and ITF mRNA and immunohistochemical staining for expression of pS2 and ITF proteins during recovery from stress damage: The expressions of pS2 and ITF could be detected in normal gastric mucosa. They were expressed mainly in the regenerative zone of cytoplasm and weaker expressions were found at the basal portions of the gastric glands. The expressions of pS2 and ITF in single WRS significantly decreased and was absent in the necrotic region, whereas repeated WRS significantly increased expression of pS2 and ITF. In addition to the regenerative zone,other areas including the lumen of gastric glands also expressed pS2 and ITF.
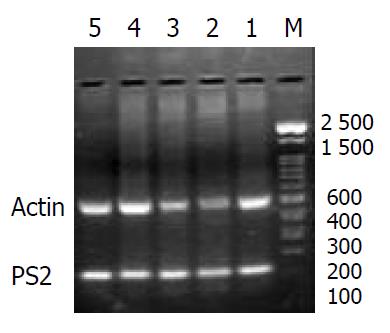
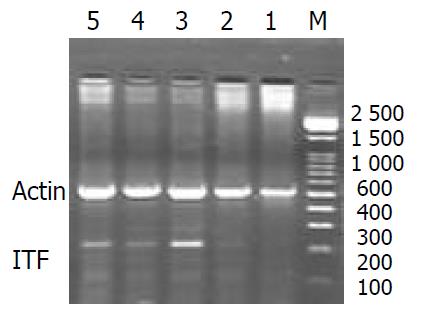
The expressions of pS2 and ITF mRNA were increased during the healing after single WRS (pS2: 0.51 ± 0.14 vs 0.77 ± 0.11, P < 0.01; ITF: 0.022 ± 0.001 vs 0.177 ± 0.010, P < 0.01) (Figure 3, Figure 4). The same results were observed by immunohistochemistry (pS2: 0.95 ± 0.11 vs 1.41 ± 0.04, P < 0.01; ITF: 0.134 ± 0.001vs 0.253 ± 0.01, P < 0.01). With repeated WRS, adaptative cytoprotection against stress was developed.The expression of pS2 and ITF mRNA was increased by using RT-PCR (pS2: 0.37 ± 0.02 vs 0.77 ± 0.01, P < 0.01; ITF: 0.040 ± 0.001vs0.372 ± 0.010, P < 0.01) (Figure 5, Figure 6) an d immunohistochemistry (pS2: 0.55 ± 0.04 vs 2.46 ± 0.08, P < 0.01; ITF: 0.134 ± 0.001vs 0.354 ± 0.070, P < 0.01). There was a significantly negative relevance between expressions of pS2 or ITF and UI (r = -0.921, P < 0.01; r = -0.965, P < 0.01), and positive relevance was found between expressions of pS2 or ITF and GMBF (r = 0.826, P < 0.05; r = 0.854, P < 0.05) (Table 2).
The cytoprotective functions in protecting gastrointestinal tract against ongoing damage may be accomplished in several ways, and evidences for participation in both the early phase of epithelial repair known as restitution (marked by increased cell migration but no proliferation), and in the subsequent, protracted phase of glandular renewal (marked by proliferation, differentiation and migration) have been published[12-14].
This study assessed for the first time immunohistochemical and RT-PCR analyses of pS2, ITF expression in rat gastric mucosa after exposure to water immersion and restrained stress. Our observation showed that expression of pS2 and ITF in gastric mucosa was enhanced shortly after the stress, leading us to hypothesize that this process might be mediated by pS2 and ITF.
The importance of trefoil peptides in the process of response to the damage action of strong irritants has not yet been evaluated.The members of the trefoil peptide family, including pS2 and ITF, share a common structural feature, which is a motif of six cysteine residues termed a trefoil or a P domain. There are increasing evidences that pS2 and ITF are important in maintaining the integrity of gastric mucosa and involved in the repair of ulcerated areas in gastrointestinal tract[15-19]. This is supported by an observation that increased expressions of pS2 and ITF were found in the ulcer-associated cell lineage (UACL), which is a glandular structure that develops in the area of gastrointestinal tract adjacent to ulcerated mucosa[20]. This is supported by the findings obtained from in vitro study which showed that pS2 and ITF exhibited a mitogenic effect on different cell lines[21,22]. Moreover, exogenously recombinant TFF has been shown to significantly attenuate the extent of acute mucosal injury induced by a variety of ulcerogens such as 96% ethanol, indomethacin or stress[23], indicating that this peptide does exhibit gastroprotective activity.
The present study showed that, WRS applied once produced numerous gastric mucosal erosions. UI gradually declined at 2, 4, 8 h after the end of stress, the expression of pS2 and ITF was increased during the healing of stress-induced ulceration, there was not a correlation between the expression of pS2 or ITF and UI.
The facts that pS2 or ITF is over-expressed in gastric mucosa immediately after stress injury and that this peptide stimulates cell migration, strongly suggest that it might mediate the early phase healing of acute gastric lesion called restitution[24-28].
It has also been proposed that trefoil peptide family contribute to gastric mucosal defence and repair by affecting cell proliferation[29,30].
In the present study, we found this adaptation was accompanied by an increased mucosal cell proliferation and enhanced expressions of mRNA for pS2 and ITF, suggesting the involvement of pS2 and ITF in the adaptation process.
The major finding of this report was the demonstration for the first time that gastric adaptation to WRS involved overexpressions of mRNA for pS2 and ITF and an increased rate of cell proliferation in gastric mucosa, and enhanced cell proliferation was preceded by overexpressions of pS2 and ITF mRNA, although the expressions of mRNA for pS2 and ITF decreased in initial phase after exposure of gastric mucosa to WRS, suggesting that this trefoil peptide contributes to cell proliferation.
The present study also showed that with repeated WRS, adaptative cytoprotection against stress was developed, the mucosal lesions reduced markedly after 2nd, 3rd and 4th WRS. The expression of pS2 and ITF was increased. There was a significantly negative relevance between expressions of pS2 or ITF and UI.
After the 4th WRS, GMBF was almost restored to normal level. Therefore, during the process of tolerant cytoprotection, GMBF, UI and expression of pS2 and ITF showed regular changes and there was a good relationship beween them.
Indeed, we have confirmed that WRS-adapted mucosa exhibits an augment GMBF, but it is not clear whether this factor could directly or indirectly account for the mucosal adaptation, or what could be the mechanism of this mucosal hyperemia in the stomach. TGFα has been shown to increase GMBF[2,31], while TFF can promote synthesis of TGFα. So hyperemia observed during the development of adaptation can be mediated, at least in part by the release of this peptide.
| 1. | Brzozowski T, Konturek SJ, Majka J, Dembinski A, Drozdowicz D. Epidermal growth factor, polyamines, and prostaglandins in healing of stress-induced gastric lesions in rats. Dig Dis Sci. 1993;38:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Konturek SJ, Brzozowski T, Majka J, Dembinski A, Slomiany A, Slomiany BL. Transforming growth factor alpha and epidermal growth factor in protection and healing of gastric mucosal injury. Scand J Gastroenterol. 1992;27:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Konturek PC, Ernst H, Brzozowski T, Ihlm A, Hahn EG, Konturek SJ. Expression of epidermal growth factor and transforming growth factor-alpha after exposure of rat gastric mucosa to stress. Scand J Gastroenterol. 1996;31:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Podolsky DK. Mechanisms of regulatory peptide action in the gastrointestinal tract: trefoil peptides. J Gastroenterol. 2000;35 Suppl 12:69-74. [PubMed] |
| 5. | Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Nie SN, Li ZS, Zhan XB, Gong YF, Tu ZX, Gong YF. Role of the pS2 in healing of stress-induced gastric lesions. Weichangbingxue. 2002;7:20-23. |
| 7. | Nie S, Li Z, Zhan X, Tu Z, Xu G, Gong Y, Man X. [Role of the pS(2) in gastric mucosa adaptative cytoprotection from stress]. Zhonghua Yixue Zazhi. 2002;82:172-175. [PubMed] |
| 8. | Itoh H, Tomita M, Uchino H, Kobayashi T, Kataoka H, Sekiya R, Nawa Y. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996;318:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Nie SN, Li ZS, Zhan XB, Xu GM, Tu ZX, Gong YF. Role of the pS2,ITF in the early phase of epithelial repaire of stress-induced gastric lesion. Jiefangjun Yixue Zazhi. 2002;27:182-185. |
| 10. | Brzozowski T, Konturek PC, Konturek SJ, Stachura J. Gastric adaptation to aspirin and stress enhances gastric mucosal resistance against the damage by strong irritants. Scand J Gastroenterol. 1996;31:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Konturek PC, Brzozowski T, Pierzchalski P, Kwiecien S, Pajdo R, Hahn EG, Konturek SJ. Activation of genes for spasmolytic peptide, transforming growth factor alpha and for cyclooxygenase (COX)-1 and COX-2 during gastric adaptation to aspirin damage in rats. Aliment Pharmacol Ther. 1998;12:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495-G499. [PubMed] |
| 13. | Wright NA. Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Philos Trans R Soc Lond B Biol Sci. 1998;353:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Podolsky DK. Healing the epithelium: solving the problem from two sides. J Gastroenterol. 1997;32:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Ulaganathan M, Familari M, Yeomans ND, Giraud AS, Cook GA. Spatio-temporal expression of trefoil peptide following severe gastric ulceration in the rat implicates it in late-stage repair processes. J Gastroenterol Hepatol. 2001;16:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 17. | Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, Wright NA. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | McKenzie C, Thim L, Parsons ME. Topical and intravenous administration of trefoil factors protect the gastric mucosa from ethanol-induced injury in the rat. Aliment Pharmacol Ther. 2000;14:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Cook GA, Thim L, Yeomans ND, Giraud AS. Oral human spasmolytic polypeptide protects against aspirin-induced gastric injury in rats. J Gastroenterol Hepatol. 1998;13:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Alison MR, Chinery R, Poulsom R, Ashwood P, Longcroft JM, Wright NA. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995;175:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Chinery R, Coffey RJ. Trefoil peptides: less clandestine in the intestine. Science. 1996;274:204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 525] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 188] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Saitoh T, Mochizuki T, Suda T, Aoyagi Y, Tsukada Y, Narisawa R, Asakura H. Elevation of TFF1 gene expression during healing of gastric ulcer at non-ulcerated sites in the stomach: semiquantification using the single tube method of polymerase chain reaction. J Gastroenterol Hepatol. 2000;15:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Poulsen SS, Thulesen J, Christensen L, Nexo E, Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999;45:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Dossinger V, Kayademir T, Blin N, Gött P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell Physiol Biochem. 2002;12:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Ito S, Lacy ER, Rutten MJ, Critchlow J, Silen W. Rapid repair of injured gastric mucosa. Scand J Gastroenterol Suppl. 1984;101:87-95. [PubMed] |
| 28. | Konturek PC, Brzozowski T, Konturek SJ, Elia G, Wright N, Sliwowski Z, Thim L, Hahn EG. Role of spasmolytic polypeptide in healing of stress-induced gastric lesions in rats. Regul Pept. 1997;68:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Konturek PC. Physiological, immunohistochemical and molecular aspects of gastric adaptation to stress, aspirin and to H. pylori-derived gastrotoxins. J Physiol Pharmacol. 1997;48:3-42. [PubMed] |
| 30. | Modlin IM, Poulsom R. Trefoil peptides: mitogens, motogens, or mirages. J Clin Gastroenterol. 1997;25 Suppl 1:S94-100. [PubMed] |
| 31. | Tepperman BL, Soper BD. Effect of epidermal growth factor, transforming growth factor alpha and nerve growth factor on gastric mucosal integrity and microcirculation in the rat. Regul Pept. 1994;50:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Edited by Zhao P and Wang XL