INTRODUCTION
Extracellular matrix (ECM) is consisted of many components, such as collagen, glycoproteins, elastin, and proteoglycans that in addition to providing a scaffold for tissue, regulate many fundamental cellular processes such as proliferation, survival, migration and differentiation[1,2]. Many cell types require anchorage to ECM to proliferate[3]. If lacking attachment to ECM, they will undergo anoikis[2,4]. Integrins activate growth-promoting signaling pathways that are responsible for the anchorage, and two such pathways appear to be involved. One is that integrins facilitate growth factor-mediated activation of extracellular signal-regulated protein kinase (ERK); the other is that integrins activate the c-Jun NH2-terminal kinase (JNK)[2,5]. In addition, serine/threonine protein kinase, PKB, has emerged as a crucial regulator in the integrin pathway, which can be controlled through phosphatidylinositol-3’ kinase (PI3K)[6]. Integrins, often together with growth factor receptors, up-regulate cyclins D and E and down-regulate CKIs p21cip1, p27kip1, and p57kip2[2,7]. This action allows cells to pass through the G1/S transition and complete the cell cycle.
Many studies, however, have demonstrated that integrins give rise to growth inhibition rather than growth stimulation[8-13]. Integrin α5β1 has been often observed to be lost in cancerous areas other than in its normal counterpart tissues[14]. It is apparent from these studies that integrin signaling may play a major role in negative control of cell growth, which may be lost in some cancer cells, and the mechanisms of this effect are not completely known yet.
In this study we have further investigated the inhibition role of integrin α5β1 in human hepatocellular carcinoma cell line, SMMC-7721. We analyzed the effect of these cells with or without adhesion to ECM. These studies identified overexpression of β1 subunit or α5β1 inhibited cell cycle progression at S-phase, and this inhibition maybe resulted from the up-regulation of cdk2 inhibitors p21cip1 and p27kip1 and involved in the unoccupied β1 because of relative lack of its ligands.
MATERIALS AND METHODS
Materials and antibodies
Poly-HEME, wortmannin, FN and LN were all obtained from Sigma, and geneticin (G418) was purchased from Calbiochem (San Diego, CA). Monoclonal antibodies used were directly against cyclin D1 (Santa Cruz), cdk2 (Santa Cruz), integrin β1 (BD Transduction Laboratories, Lex ing to n, KY), phosphorylated FAK (anti-phosphotyrosine clone PT-66, Sigma) and β-actin (Santa Cruz). Goat anti-human integrin α5 polyclonal antibody was also purchased from Santa Cruz. Other antibodies used were those against FAK (Santa Cruz), p21cip1 (Santa Cruz), p27kip1 (Oncogene Research Products, Cambridge, MA), Ser473-phosphorylated form of PKB and PKB (Cell Signaling). Horseradish peroxidase conjugated anti-mouse, rabbit or goat IgG were purchased from Calbiochem (San Diego, CA).
Cell culture
Human hepatocellular carcinoma cell line SMMC-7721 was obtained from the Liver Cancer Institute, Zhongshan Hospital (Shanghai, China). SMMC-7721 cells were grown in RPMI 1640 medium (Gibco BRL) supplemented with 100 mL·L-1 calf bovine serum (CBS), 100 × 103 U·L-1 penicillin and 100 × 103 U·L-1 streptomycin sulfate. Integrin-overexpressing transfectant cell lines were maintained in the same medium as above plus 500 μg/mL geneticin (Gibco BRL).
Plasmid construction and stable transfections
pECE vector containing human full-length cDNA of integrin β1 was presented generously by Dr. Mara Brancaccio (Department of Genetics, University of Torino, Torino, Italy). Complementary DNA of β1 integrin cleaved from the pECE plasmid by EcoR I was subcloned into pcDNA3 vector to generate pcDNA3-β1. pcDNA3-α5 expression vector was presented kindly by Dr. Sue E. Craig (School of Biological Sciences, University of Manchester, Manchester, UK). Stable transfections were performed using LIPOFECTAMINETM 2000 (LF2000) reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. Briefly, logarithmically growing cells were transfected with 1 μg of plasmids and 2 μl of LF2000 reagent. At 48 h after transfection, the selective medium containing 1 mg/mL geneticin (G418) antibiotic (Calbiochem) was replaced, and then the cell clones were selected and identified.
Plating experiments
Mock and integrin transfected cells were seeded on the tissue culture plates coated with either FN (15 μg/mL) or LN (15 μg/mL), grown for 60 minutes, and followed by phase-contrast microscopy or protein extraction.
Flow cytometry
Cells were starved by exposure to SFM for 48 h, the harvested cells were then grown in normal growth medium containing 10% CBS for 12-16 h, the time span covered the duration of normal S phase. At the end of incubation, the cells were digested with 2 mM EDTA in PBS and rinsed twice with ice-cold PBS solution, then fixed by adding them dropwise into 75% ice-cold ethanol while vortexing, followed by incubation on ice for 60 min. The fixed cells were washed with ice-cold PBS and incubated at 37 °C for 30 min in 0.5 mL PBS solution containing 20 μg/mL RNase A, 0.2% Triton X-100, 0.2 mM EDTA and 20 μg/mL of propidium iodide. DNA content was determined by FACS analysis (Becton Dickinson). The percentage of cells in G0/G1, S, and G2/M phases was determined using the Modfit program.
RNA isolation and RT-PCR
RT-PCR was performed to quantify the level of mRNA, which was isolated using Trizol system (Watson Biotechnologies, Shanghai, China) according to the manufacturer’s guidelines. Complementary DNA synthesis was performed essentially as described previously[15], except that 2 μg of total RNA was used for cDNA synthesis, and the primer used was oligo (dT)15. For amplification, 2-μl cDNA product was used in a final volume of 50 μl with 5 units of Taq polymerase (SABC, Luoyang, China). The primer pairs for p27kip1 and p21 cip1 were described previously[16,17]. Primers for β-actin[16] were used as the internal control. The expected product sizes were p27, 471 bp; p21, 159 bp and β-actin, 412 bp.
Cell lysis and immunoblotting
Cells cultured under the same conditions as cell cycle analysis were collected, and then washed twice with ice-cold PBS and lysed in 1×SDS lysis buffer (50 mM Tris (pH 6.8), 2% SDS, 10% glycerol, 100 μg/mL PMSF, 10 μg/mL leupeptin and 5 mM Na3VO4) for 10 min on ice. Cell lysates were boiled and clarified by centrifugation at 12000 g at 4 °C for 10 min. Protein concentration was determined with Hartree assay. Immunoblotting analyses using the enhanced chemiluminescence (ECL) detection system (Perfect, Shanghai, China) were carried out as described previously[18].
RESULTS
Overexpression of integrin α5β1 in SMMC-7721 cells
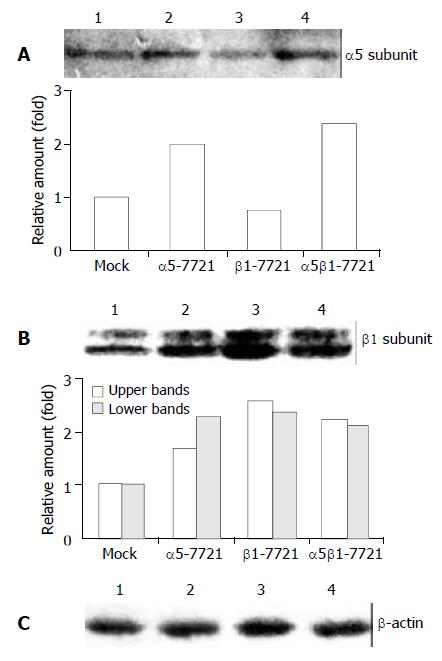
We transfected the full-length cDNA of genes ITGA5 (α5) or ITGB1 (β1) alone, or α5 and β1 together into a human hepatocellular carcinoma cell line, SMMC-7721, respectively. The pcDNA3 empty vector was the control plasmids, and cells transfected with pcDNA3 were regarded as the mocked cells. The overexpressed transfectant cell lines were mainly screened for increased expression of α5 or β1 in protein levels by Western analysis, and designated as α5-7721, β1-7721 and α5β1-7721, respectively. As shown in Figure 1A, integrin α5 expression was increased to 2-fold in α5-7721 or α5β1-7721 cells compared with the mocked cells. Meanwhile, β1-7721 and α5β1-7721 transfectants had more than 2.5-fold amount in integrin β1 protein level (Figure 1B). The β1 subunits appeared as two bands in Western blot because of variable post-translational modification (mainly N-glycosylation). The hypoglycosylated lower band (Figure 1B) was tentatively identified as biosynthetic precursor of β1 subunit. The band with lower migration rate (upper band in Figure 1B) of integrin β1 subunit was in hyperglycosylated forms, and mainly located in plasma membrane[19-21].
Figure 1 Integrin α5 and β1 protein levels in α5-, β1- and α5β1 transfected SMMC-7721 cells.
(A) The expression level of α5 chain was increased in α5-7721 and α5β1-7721 cells. (B) Two forms of β1 integrin were due to different levels of β1-chain glycosylation. The hypoglycosylated lower band was tenta-tively identified as biosynthetic precursor of β1 subunit, the hyperglycosylated upper band was mature subunits, exposed in part on the cell surface (the 130-kDa product). Immunoblot assay showed that protein levels of the mature form were el-evated in the transfectants, especially in β1-7721 and α5β1-7721 cells. (C) The protein level of β-actin showed an equal loading amount in each well. Lane 1, mock cells; lane 2, α5-7721; lane 3, β1-7721; lane 4, α5β1-7721. Each point in the graphs was the mean value from three separate experiments.
Induction of S-phase delay by transfection of integrin β1 subunit
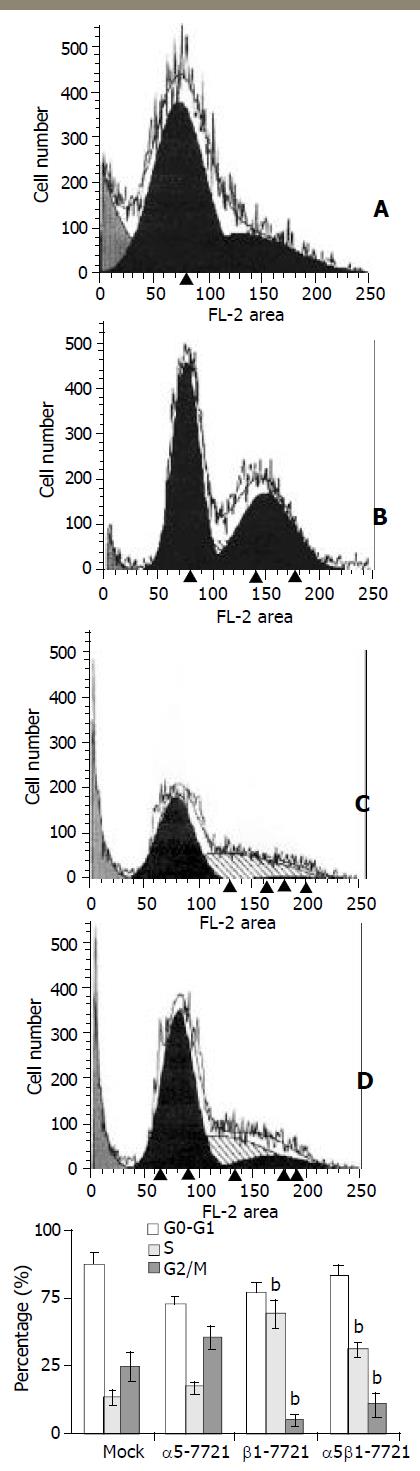
In the previous study, we showed that overexpression of integrin α5β1 or β1 subunit had negative effects on cell growth[11]. To elucidate the mechanisms of cell cycle perturbation induced by overexpressing integrins, flow cytometry analyses were applied. Cell cycle parameters were compared between transfected cells and mocked cells. Similar patterns of the cell cycle were found in α5-7721 and mocked cells (Figure 2). But in β1-7721 and α5β1-7721 transfectants, we observed a significant increase in fraction of cells in S phase of the cell cycle, as shown in Figure 2. This was accompanied by a decrease in proportion of cells in G2/M phases of the cell cycle. These changes were specific for the transfection events containing β1-plasmids, that is, at this point, the pattern of α5β1-7721 was similar to that of β1-7721 cells. These results were obtained from the cells synchronized partly in G0/G1 phase by exposure to serum-free medium. Therefore, these data showed that S-phase delay was probably due to enhanced production of exogenous β1 integrin.
Figure 2 S-phase delay was induced in β1- and α5β1-transfectant cells.
For cell cycle analysis, transfected and mocked cells were synchronized by exposure to SFM for 48 h, then grown in RPMI1640 medium containing 10% CBS and penicillin/streptomycin solution. Twelve or 16 h later, the cells were collected and analysed for flow cytometry as described under “Materials and Methods”. A, mocked cells; B, α5-7721 cells; C, β1-7721 cells; D, α5β1-7721 cells. Each bar in graph represented the mean ± SD obtained from three independent experiments. The S-phase delay was significantly different in β1-7721 and α5β1-7721 cells (n = 3, bP < 0.01 vs mocked cells).
p21cip1 and p27kip1 were up-regulated in β1 or α5β1 transfectant cell lines
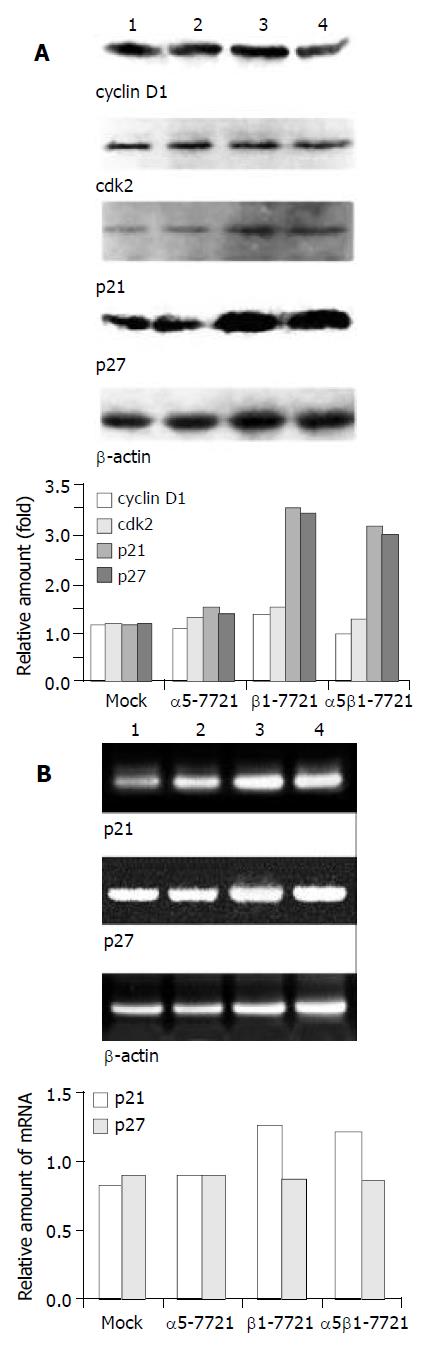
To clarify the mechanism by which S-phase delay was induced in β1-7721 and α5β1-7721 cells, we investigated whether cyclins and cdks were involved in this situation. Expression level of cyclin D1 was examined because this protein was always referred to as a sensor molecule to the extracellular cues[22]. It was shown that cyclin D1 expression was not changed, neither was the cdk2 protein (Figure 3A). As evidenced recently, enhanced p21cip1 and/or p27kip1 expression was considered to be associated with G1 cell cycle arrest[23,24], and under some circumstances, with S-phase delay in some cell types[25,26]. So, we further examined whether β1-chain overexpression could induce p21cip1 and p27kip1 in β1-7721 and α5β1-7721 cells. As shown in Figure 3A, a significant increase (2-fold amount) of p21cip1 protein levels was noted in β1-7721 and α5β1-7721 cells compared with the mocked cells or α5-7721 cells. Meanwhile, p27kip1 protein level was also increased to 2.5-fold. We also found that the mRNA level of p21cip1 increased, although that of p27 kip1 maintained the same as control (Figure 3B). Interestingly, we found that overexpression of β1 gene in SMMC-7721 cells induced S-phase delay in this study. The above findings showed that this S-phase delay might be attributed to the increased expression of p21cip1 and p27kip1.
Figure 3 Message RNA and/or protein levels of cell cycle regu-latory genes p21cip1 and p27 kip1 in β1-7721 and α5β1-7721 transfectants, but not that of cyclin D1 and cdk2.
(A) Immunoblot assay showed the protein level of cyclin D1 and cdk2 were not apparently affected, but p21cip1 and p27kip1 pro-tein levels were increased in β1-7721 and α5β1-7721 cells. The protein level of β-actin was detected to assess the loading amount in each well in SDS-PAGE gel. (B) Message RNA lev-els of p21cip1 and p27kip1 were assessed by RT-PCR, and nor-malized by that of β-actin. It was apparent that mRNA level of p21cip1 was increased in β1- and α5β1- transfected cells. However, the p27kip1 mRNA amount was the same as control. Each result represented three separate experiments. Lane 1, mocked cell; lane 2, α5-7721; lane 3, β1-7721; lane 4, α5β1-7721.
Phosphorylated form of PKB was inhibited in β1 and α5β1 transfectants and this might result in accumulation of p21cip1 and p27kip1
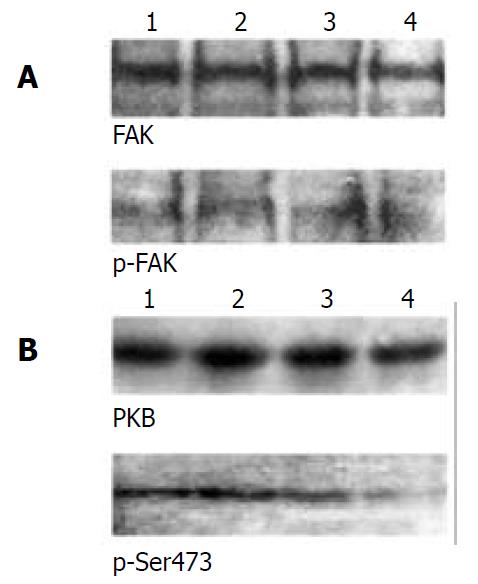
To determine how overexpressing α5β1 integrins transferred signals from membrane to cytosol or nucleus to modulate the expression of CKIs p21cip1 and p27kip1, we examined the two important signaling molecules mediated by integrins, FAK and PKB. The results showed that neither FAK nor its tyrosine phosphorylated form was affected (Figure 4A). However, levels of Ser473-phosphorylated form of PKB were decreased in β1-7721 and α5β1-7721 cells in comparison with the control cells, but total amount of PKB protein was not apparently affected (Figure 4B).
Figure 4 Activation of PKB, but not FAK, was downregulated in β1- and α5β1- transfected cells.
(A) FAK activation was as-sessed by phosphotyrosine- specific antibody, followed by stripping and reprobing with anti-FAK antibody. (B) PKB and its Ser473-phosphorylated forms were determined by 10% SDS-PAGE with the equal loading amount. The loading amount control is shown in Figure 1C and Figure 3A. Lane 1, mock cells; lane 2, α5-7721; lane 3, β1-7721; lane 4, α5β1-7721. Results were representative of 4 repeated experiments.
In recent years, evidences have shown that activated PKB protein may phosphorylate p21cip1, and induce its degradation through the ubiquitin-26S proteasome pathway[6,27]. But evidences indicating p27kip1 phosphorylation by PKB are few. In this study, decrease of phosphorylated PKB (Figure 4B) and accumulation of p27 kip1 (Figure 3A) occurred concomitantly in β1-7721 or α5β1-7721 cells. To obtain support, we further performed an experiment to block the phosphorylated form of PKB. It is well known that Ser-473 phosphorylation of PKB, on behalf of its active forms, appears to be catalyzed by phosphoinositide-dependent kinase 1 (PDK1) and integrin-linked kinase (ILK)[28,29], and that PI3K is located upstream of both ILK and PKB[6]. So the PI3K inhibitor wortmannin was explored in this study. Following serum starvation, cells were cultured in the medium with 100 nM wortmannin for 24 h or 48 h. We investigated the level of phosphorylation of PKB and p27kip1 by immunostaining. The data showed that phosphorylation of PKB was blocked, and expression of p27kip1 was increased at the same time compared with control cells (Figure 5). Therefore, the decrease of phosphorylated form of PKB was at least in part, responsible for p27kip1 protein accumulation.
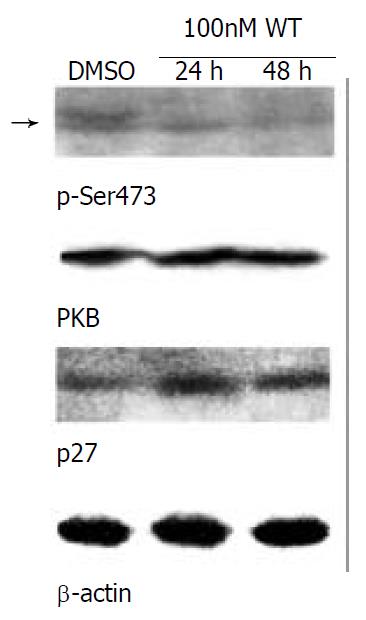
Figure 5 Ser473-phosphorylated form of PKB was decreased, but p27kip1 protein level was increased concomitantly in SMMC-7721 parental cells treated with the PI3K inhibitor wortmannin.
The parental SMMC-7721 cells were starved with serum-free medium for 48 h, then grown in normal medium/10% CBS containing DMSO (as control, 24 h) or 100 nM wortmannin for the indicated times. The amount of DMSO did not exceed 0.1%, which was determined not to damage the cells. Equal amount of wortmannin was added again after grown for 24 h. The level of Ser473-phosphorylated form of PKB (arrow) was declined, but p27kip1 protein level was elevated with the treatment of wortmannin in SMMC-7721 cells. Results were representative of at least 3 repeated experiments. Abbreviation: DMSO, dimethylsulfoxide; WT, wortmannin.
Inhibition of cell cycle was possibly due to the relative lack of ECM
Integrins, in general, promote the focal adhesion protein activities such as FAK and cell cycle progression[2]. So we ponder why overexpression of integrin β1 subunit can repress the level of phosphorylated PKB and the cell cycle. Here, we performed plating experiments to elucidate its mechanism. First, we observed the morphological change of cells that were plated on LN- or FN- coated dishes and grown for 60 minutes (Figure 6). It was found that β1-7721 and α5β1-7721 cells were prone to attachment and spreading, perhaps to the cell cycle progression. Next, we determined the changes of phosphorylated FAK and phosphorylation of PKB in cells attaching on LN and FN. As mentioned above (Figure 4A), total amount of FAK and its phosphorylated forms were not affected in cells cultured in the flasks without coating of LN or FN. But the level of tyrosine phosphorylated FAK was increased in cells, which were plated on LN- or FN- coated dishes and grown for the indicated times (Figure 7A, and data not shown for FN-coated dishes). Moreover, the level of Ser473 phosphorylated form of PKB was similar to that of phosphorylated FAK under the same condition (Figure 7B, and data not shown for FN-coated dishes). Finally, protein level of p27kip1 was declined in β1-7721 and α5β1-7721 cells (Figure 7C). These findings demonstrated that an important role of LN or FN in the molecular changes of β1-7721 or α5β1-7721 cells, especially, in PKB phosphorylation and p27kip1 protein levels. On the contrary, when attachment of the parental cells to ECM was blocked by plating them onto poly-HEME-coated petri dishes, the percentage of cells in S-phase was increased from 13.08% to 37.33% (Figure 8). That is, when the parental SMMC-7721 cells were prevented from interaction with ECM through the preparation of poly-HEME, the same effects of S-phase accumulation took place as that in β1-7721 or α5β1-7721 cells. These results suggested that S-phase delay induced by overexpressing β1 in SMMC-7721 cells might be the result of the relative lack of ECM.
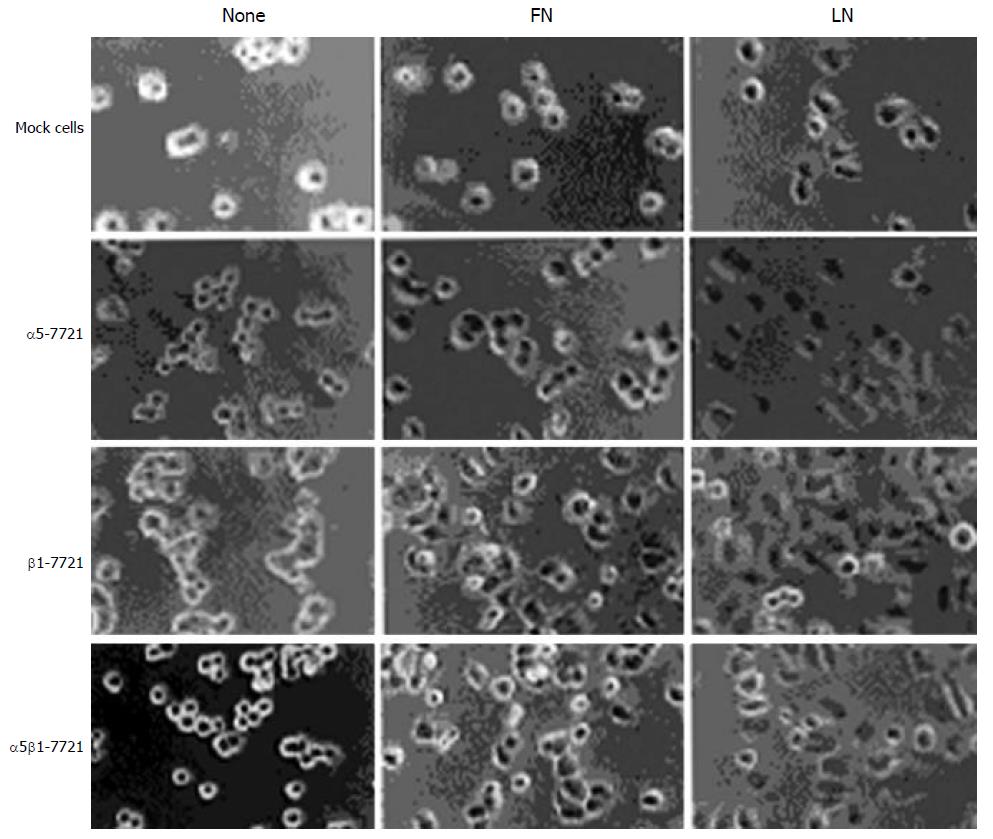
Figure 6 β1-7721 and α5β1-7721 cells subjected to spreading on fibronectin- or laminin- coated culture dishes.
The mocked and transfected cells were plated on FN- or LN- coated tissue culture plates in normal medium for 60 min, then cells on the plates were photographed by phase-contrast microscopy with a digital camera. Abbreviations: FN, fibronectin; LN, laminin.
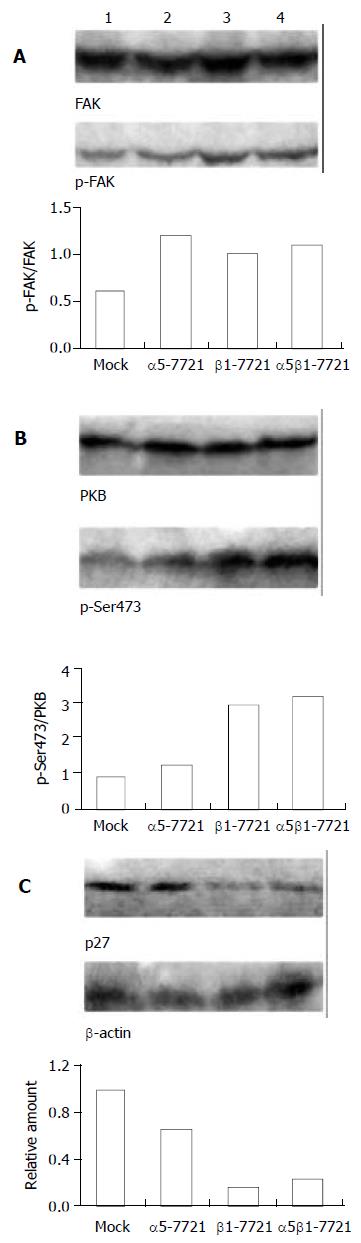
Figure 7 Effects of laminin on transfected cells.
Protein levels of phosphorylated FAK (A) and Ser473-phosphorylated form of PKB (B) were increased in the transfected cells, especially in β1-7721 and α5β1-7721 cells grown in the LN-coated culture dishes for 60 minutes. (C) Under the same condition, p27kip1 protein level was decreased in β1-7721 and α5β1-7721 cells. Lane 1, mock cells; lane 2, α5-7721; lane 3, β1-7721; lane 4, α5β1-7721. Each result represented at least 3 independent experiments.
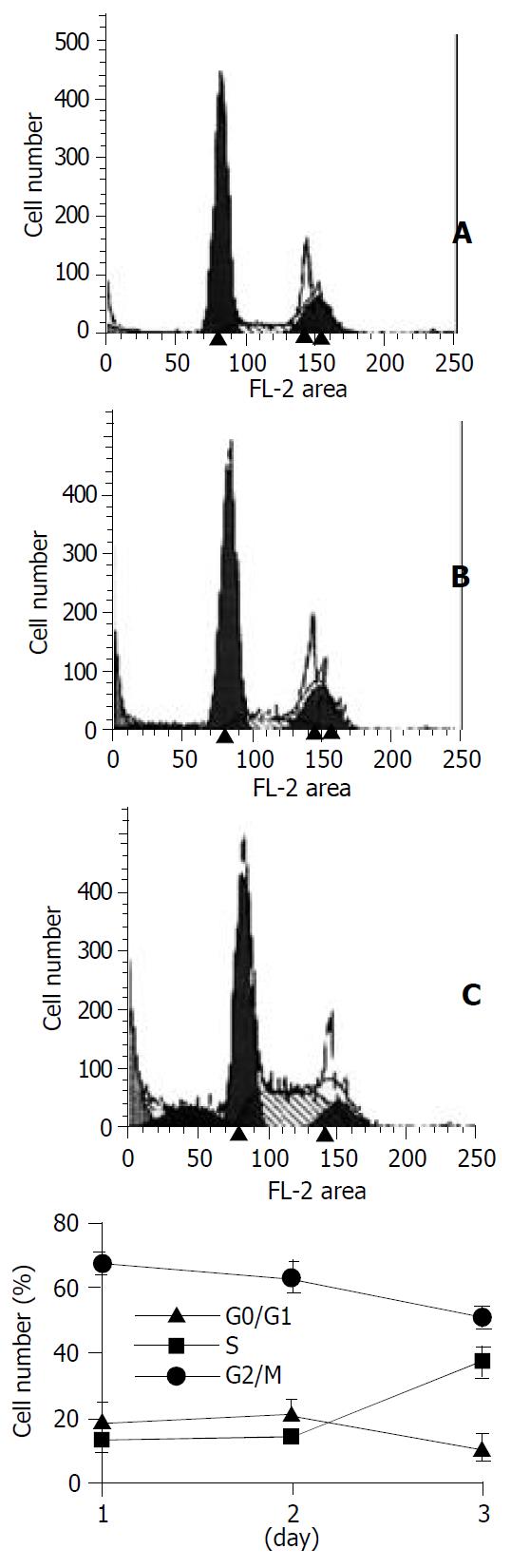
Figure 8 The percentage of cells in S phase was increased in SMMC-7721 cells plated on poly-HEME-coated petri dishes.
The parental SMMC-7721 cells were plated on poly-HEME-coated petri dishes, and cultured in normal medium for 24 h (A), 48 h (B) or 72 h (C), respectively, then collected and analysed by flow cytometry. The cell cycle pattern (C) was simi-lar to that of β1-7721 or α5β1-7721 cells.
DISCUSSION
We examined the effects of overexpressed α5β1 or β1 on tumor cell proliferation, which provide the evidence that overexpression of α5β1 or β1 inhibits the proliferation of human hepatocellular carcinoma cell line SMMC-7721 and this inhibition may be related to the insufficient ligands for overexpressed β1 integrins. This finding also demonstrated that the inhibition of cell cycle was due to a specific growth arrest at S-phase that involved an increase in the protein level of p21cip1 and p27kip1.
This study showed that cyclin D1 expression was not affected by transfection events. So the early G1 phase progression may not be influenced under this condition. However, the protein levels of p21cip1 and p27kip1 were increased in β1 or α5β1 transfectant cells, which might be the major reason why S-phase delay occurred. It was previously reported that p21cip1 and p27 kip1 were assembly factors rather than inhibitors of cdk4/6 kinases[30], but were inhibitors of cdk2 kinase, which is the key kinase contributing to G1/S transition and S phase progression. One CKI protein, p21cip1, the first cyclin-dependent kinase inhibitor to be identified[31], has also a separate cdk2 binding site in its N-terminal region (amino acid 53-58) and optimal cyclin/cdk inhibition requires binding to this site as well as one of the cyclin binding domains. Furthermore, p21cip1 interacts with proteins such as PCNA, c-Myc and E2Fs that control DNA replication and other S phase events[32]. Meanwhile, evidences have shown that p27kip1 plays a key role in the regulation of the proliferation of tumor cells in response to signals from ECM[13]. Therefore, increased p21cip1 and p27kip1 proteins can induce S-phase delay in β1- and α5β1-transfected cells.
In many cell types, the intracellular concentration of p27kip1 is mainly controlled at the posttranscriptional level, and its degradation is initiated by phosphorylation with a target enzyme, cdk2[33] or other enzymes, such as PKB [6], and completed by the ubiquitin-proteasome pathway. p21cip1 protein can also be phosphorylated by PKB[6]. In this study, we found that PKB level of phosphorylated form was decreased in β1-or α5β1- transfected cells, accompanied by the increase of p21cip1 and p27kip1 to a certain extent. These findings indicate that the decrease of active form of PKB may interpret the accumulation of p21cip1 and p27kip1bona fide, which in turn, interferes with the cell cycle at S phase.
This study suggested that overexpression of β1 or α5β1 integrin in SMMC-7721 cells could induce S-phase delay. The mechanism underlying this phenomenon may be due to two kinds of possibilities. One is “integrin-mediated death” (IMD) described by Stupack and his colleagues[34,35], that is, an unligated integrin promotes apoptosis of cells. It is well known that α5β1 integrin, in general, acts as the effector protein of cell proliferation, such as endothelial cells in the vascular system[36]. In this study, however, we showed that overexpression of β1 or α5β1 induced S-phase delay. When cells were plated on FN/LN-coated culture dishes, they were subjected to attachment and spreading compared with the mocked and α5-7721 cells. Moreover, for the parental cells, they underwent S-phase delay and apoptosis when they were deprived of attachment by plating them on poly-HEME-coated petri dishes. Therefore, we postulated that the relative lack of ECM might be involved in S-phase delay triggered by overexpression of β1 or α5β1 integrin gene in SMMC-7721 cells. Another possibility is the trans-dominant integrin inhibition, which is defined as the occupancy of one integrin by its ligand, can inhibit the functions of other integrins[37-40]. For example, integrin α5β1 is essential for angiogenesis[36], but αvβ3 may suppress the functions of integrin α5β1, if β3 integrins prevail in the endothelial cells[41]. It was reported that the basal integrin repertoire in hepatocellular carcinoma cells was characterized by the expression of several potential laminin receptors of the integrin family, such as α1β1, α2β1 and α6β1[42-47]. So the overexpression of β1 may preferentially dimerize with α1, α2 and α6, the subunits of the receptors of laminin or collagen (they were lost in this in vitro model). Therefore, if these α subunits are occupied, the functions of integrin α5β1 may be suppressed, including its capacity to block cell cycle arrest.
ACKNOWLEDGEMENTS
We are grateful to Dr. Mara Brancaccio from the University of Torino (Torino, Italy), and Dr. Sue E. Craig from the University of Manchester (Manchester, UK) for their gifts of the plasmids. We also acknowledge the Chinese Medicine Board (CMB) in New York, U.S.A. for its kind support to our research.