Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1675
Revised: January 16, 2003
Accepted: February 17, 2003
Published online: August 15, 2003
AIM: To evaluate the possibility of using insulin as a carrier for carcinoma-targeted therapy mediated by receptor, and to investigate the expression of insulin receptor in human hepatocellular carcinoma and the receptor binding characteristics of insulin-IUdR (iododeoxyuridine).
METHODS: IUdR was covalently conjugated to insulin. Receptor binding assays of 125I-insulin to human hepatocellular carcinoma and its adjacent tissue were performed. Competitive displacements of 125I-insulin by insulin and insulin-IUdR to bind to insulin receptor were respectively carried out. Statistical comparisons between the means were made with paired t-test at a confidence level of 95%.
RESULTS: The data indicated that there were high- and low- affinity binding sites for 125I-insulin on both hepatocellular carcinoma and its adjacent tissue. Hepatocellular carcinoma had a significantly higher Bmax for high affinity binding site than its adjacent liver tissue (P < 0.05, t = 2.275). Insulin-IUdR competed as effectively as insulin with 125I-insulin for binding to insulin receptor. Values of IC501, C502, KI1 and KI2 for insulin-IUdR were 11.50 ± 2.83 nmol·L-1, 19.35 ± 5.11 nmol·L-1, 11.26 ± 2.65 nmol·L-1 and 19.30 ± 5.02 nmol·L-1 respectively, and for insulin were 5.01 ± 1.24 nmol·L-1,17.75 ± 4.86 nmol·L-1, 4.85 ± 1.12 nmol·L-1 and 17.69 ± 4.81 nmol·L-1, respectively. Values of IC501 and KI1 for insulin-IUdR were significantly higher than that for insulin (P < 0.01, t = 4.537 and 4.813).
CONCLUSION: It is possible to use insulin as a carrier for carcinoma-targeted therapy mediated by receptor.
- Citation: Ou XH, Kuang AR, Peng X, Zhong YG. Study on the possibility of insulin as a carrier of IUdR for hepatocellular carcinoma-targeted therapy. World J Gastroenterol 2003; 9(8): 1675-1678
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1675
Hepatocellular carcinoma is one of the most common cancers with highly uneven geographic distribution and its treatment by conventional methods is still difficult. In recent years receptor-based radiopharmaceuticals have been used for localization diagnosis and therapy of certain tumors[1-5]. Insulin may be a convenient carrier for anticancer drugs or radionuclides in targeted therapy of carcinoma, because several studies have shown there is an increased expression of insulin receptor on a variety of malignant tumor cells[6-11]. Moreover, insulin is internalized by cells possessing insulin receptor and reported in the cell nuclei subsequent to endocytosis[12-14].
S-iodo-z-deoxyuridine (IUdR), a thymidine analog, can be incorporated into the DNA of cells in the S phase. 125I-IUdR has been successfully applied for the treatment of bladder cancer and hepatic metastases[15-18]. Short-range Auger electrons emitted by 125I-IUdR can cause DNA double-strand broken and deliver a lethal radiation dose to the cell when it is incorporated into DNA. But when killing malignant cells, 125I-IUdR also brings damage to normal tissues, such as the bone marrow, the small intestine and the large intestine. To reduce the damage, we presented a strategy for IUdR targeting delivery to hepatocellular carcinoma which exploited the efficiency and specificity of internalization afforded by the process of receptor-mediated endocytosis. IUdR was primarily used in this investigation, because it had the identical chemical and biological properties with 125I-IUdR. The expression of insulin receptor in human hepatocellular carcinoma and the receptor binding characteristics of insulin-IUdR conjugate were investigated.
Hepatocellular carcinoma and its adjacent liver tissue specimens were obtained from six patients at surgery whose diagnosis was confirmed by histopathology and immediately frozen at -70 °C for further use. Cell membrane fractions were prepared according to established techniques[19]. Tissues were cut into pieces, put into Tris-HCL buffer (pH 7.5) and homogenized. The cell membrane fractions purified by centrifugation in a discontinuity sucrose density gradient were stored at -70 °C. The protein concentration was determined according to Lowrry method[20].
Porcine insulin was radioiodinated with the Ch-T method and purified by polyacrylamide gel electrophoresis. The radiochemical purity of 125I-insulin was measured by TLC (thin layer chromatogray).
Insulin-IUdR was kindly synthesized by Department of Pharmaceutical, Sichuan University. Briefly, both IUdR (1.2 g) and succinic anhydride (1.5 g) were dissolved in 25 ml of pyridrine and the mixture was stirred at 70-80 °C for 24 hours. The solvents were evaporated under vacuum and the residue was crystallized from isopropanol.
Conjugation of IUdR-succinin to insulin was done as follows: IUdR-succinin (50 mg), EDC (100 mg) and HOBt (50 mg) were dissolved in 3 ml phosphate-buffered saline (pH = 8.9) and mixed with 1 ml phosphate-buffered saline (pH = 8.9) containing 25 mg insulin. The mixture was stirred at 4 °C for 48 hours. The precipitation was removed by filtration and pH of the solvent was adjusted to 5.5 with 0.5 mol·L-1 HCl. The reaction mixture was kept at 4 °C overnight. Thereafter, white solid precipitation was collected with filter and purified by polyacrylamine agarose gel electrophoresis. The solvents were evaporated under vacuum.
Isolated insulin-IUdR was analyzed by analytical HPLC (Beckman) with 4 × 200 mm ODS column. The mobile phase was 27% (v/v) acetonitrile and 73% 0.1 mol·L-1 NaH2PO4 (pH = 0.3) at a flow rate of 0.5 ml·min-1.
SDS-polyacrylamine gel electrophoresis (SDS-PAGE) was performed in 15% polyacrylamine gel to calculate the molecular weight of insulin-IUdR.
Cell membrane fractions (80 µg protein) of hepatocellular carcinoma or its adjacent liver tissue were incubated for 20 hours at 4 °C with binding buffer (NaCl 118 mmol·L-1 CaCl2 1.3 mmol·L-1 KCl 5 mmol·L-1 MgSO4 1.2 mmol·L-1 KH2PO4 1.2 mmol·L-1 pH 7.5) containing increasing concentration (5 × 103-5 × 105 cpm) of 125I-insulin. The free ligands were removed by centrifugation at 2000×g for 10 minutes after addition of 0.1 ml 0.3% bovine γ-globulin and 0.8 mL 15.8% PEG. Radioactivity of the membrane pellets was determined in a gamma counter for one minute as the total binding. The nonspecific binding was estimated by incubating membrane with 125I-insulin in the presence of 4.3 nmol unlabeled insulin. Specific binding was obtained by subtracting nonspecific binding from total binding.
Cell membrane fractions (80 µg protein) were incubated with 5 nmol 125I-insulin in the presence of increasing concentrations (10-12-10-7 mol/L) of the unlabeled insulin or insulin-IUdR. After incubated for 20 hours at 4 °C, the unbound ligands were removed as saturation binding assay. Radioactivity of the membrane pellets was determined in a gamma counter for one lmin as the total binding. The nonspecific binding was estimated by incubating membrane with 125I-insulin in the presence of 4.3 nmol unlabeled insulin.
Binding data were calculated on a computer with receptor binding analysis software. Values are presented as means ± SD. Statistical comparisons between the means were made with paired t-test at a confidence level of 95%.
The radiochemical purity of 125I-insulin was 98% and remained over 95% after 14 days stored at -20 °C.
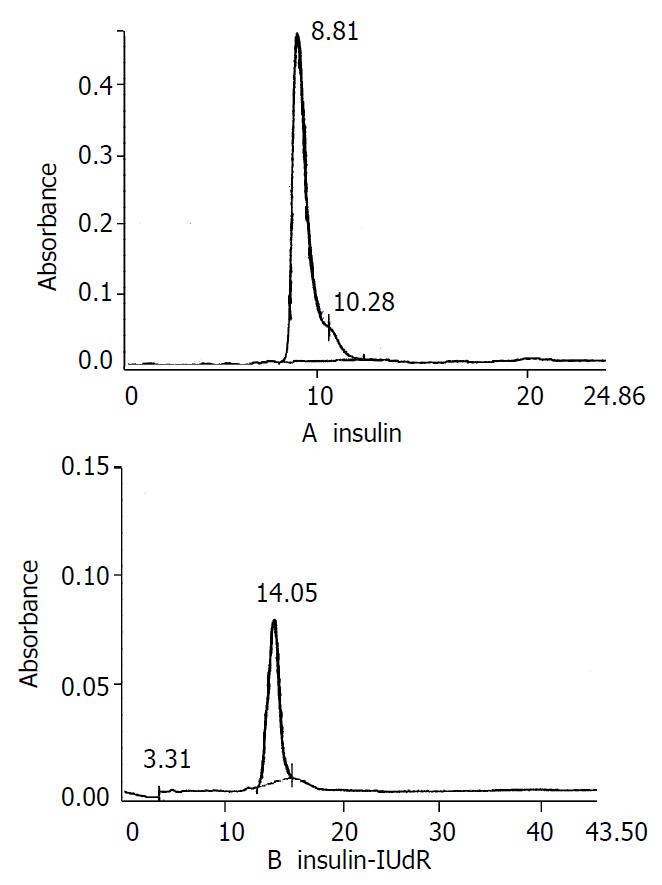
The recovery of insulin-IUdR was about 76.3%. HPLC showed that isolated insulin-IUdR (retention time 13.91 min) yield was about 98% (shown in Figure 1). The other 2% was the insulin (8.81 min) and IUdR (3.87 min).
The calculated molecular weight of insulin-IUdR was 7179Da according to its relative position to the molecular weight marker in the SDS-PAGE pattern.
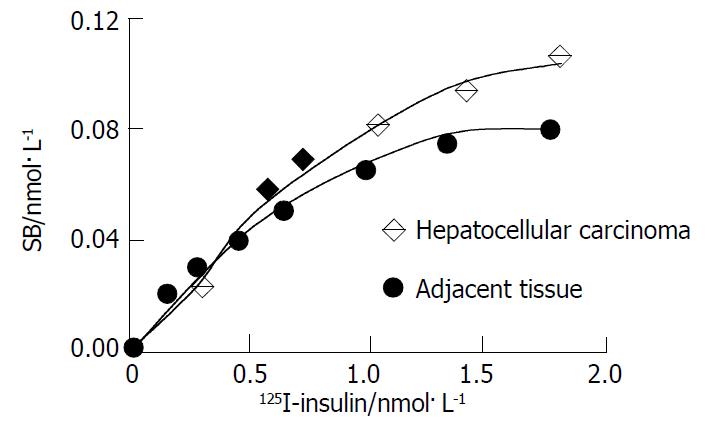
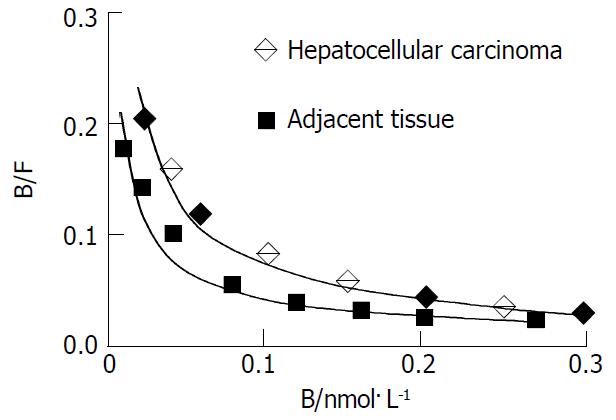
For cell membrane of hepatocellular carcinoma and its adjacent tissue, as concentration of the radioligand increased, binding amount went up and then plateaued rapidly (Figure 2). The Scatchard plot corresponding to the saturation binding curve had a slightly curvilinear shape (Figure 3). Curvilinear Scatchard plots were characteristic of insulin binding in many other systems and had been attributed to interaction of ligand with two or more classes of sites that exhibited different affinities. Using the RBA software, the Scatchard plot of hepatocellular carcinoma could be resolved in a component with Kd = 2.20 nmol·L-1 (Bmax = 0.36 nmol/L) and another component with Kd = 18.8 nmol·L-1 (Bmax = 1.58 nmol·L-1) by the computer. And that of adjacent tissue could be resolved in a component with Kd = 2.21 nmol·L-1 (Bmax = 0.33 nmol·L-1) and another component with Kd = 17.89 nmol·L-1 (Bmax = 1.09 nmol·L-1). Remarkably higher Bmax of high affinity insulin binding sites was identified in hepatocellular carcinoma (P < 0.05).
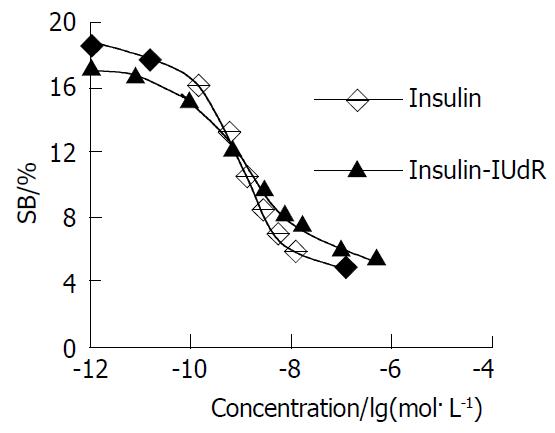
Competition displacement assays compared the ability of insulin-IUdR and unlabeled insulin to compete with 125I-insulin for binding to insulin receptor in hepatocellulor carcinoma (Figure 4). The conjugate competed as effectively as insulin for binding to the insulin receptor in a dose-dependent manner. IUdR had no effect on 125I-insulin binding (data shown in Table 1). Values of IC50 and KI for insulin-IUdR and insulin are shown in Table 2. Although there were increased IC501 and KI1, the data showed that most of the receptor-binding activity of insulin-IUdR remained.
| High affinity sites | Low affinity sites | |||
| Bmax (nmol·L-1) | Kd (nmol·L-1) | Bmax (nmol·L-1) | Kd (nmol·L-1) | |
| Hepatocellular carcinoma | 1.58 ± 0.16a | 2.20 ± 0.66 | 0.36 ± 0.11 | 18.80 ± 7.85 |
| Adjacent liver tissue | 1.09 ± 0.51 | 2.21 ± 0.72 | 0.33 ± 0.74 | 17.89 ± 7.87 |
In recent years, there have been several studies on target delivery of specific gene to living cells mediated by insulin receptor. For example, Ivanova et al[21] introduced a foreign DNA into early mouse and rabbit embryos by using insulin as a carrier, and Alexder carried out transfection of H-11 murine epithelial mammary cells as well as murine and sheep mammary glands using insulin-containing constructs that deliver DNA by receptor-mediated endocytosis[22]. Rosenkranz et al[23] synthesized a soluble construct consisting of a plasmid carrying the gene of SV40 large T-antigen and an insulin-poly-L-lysine conjugate which is able to selectively transfect PLC/ PRF/S human hepatoma cells possessing insulin receptors. Moreover, Zhang et al measured the levels of luciferase gene expression in human or rat glioma cells after targeting the PIL-encapsulated plasmid DNA via human insulin receptor, the human epidermal growth factor receptor, or rat transferrin receptor. The highest levels of gene expression were obtained after targeting the insulin receptor, and this may derive from the nuclear targeting properties of this receptor system[24].
In the present study, we presented a procedure for tumor treatment which combined the targeted transportation function afforded by insulin receptor mediating endocytosis with radiotoxicity of 125I-IUdR to cells at the S phase. IUdR is usually administrated by local infusion or intratumor injection. Systemic therapy is not indicated because of potential severity of undesirable side effects. Insulin used as a carrier might offer an advantage in this aspect. Firstly, most of 125I-IUdR is directly delivered into the liver by insulin. Thereby, other actively dividing normal tissue can efficiently avoid being damaged. Secondly, because of overexpression of insulin receptor in tumor cells, there will be very little conjugate entering normal liver cells. At last, Auger electron’s range is about 10 nm, it is hardly harmful to cells until it is incorporated in the DNA of cells. Since IUdR can only be incorporated in the DNA of cells at the S phase[25], non-dividing tissues possessing insulin receptor will not be injured.
We found that hepatocellular carcinoma bound more 125I-insulin than its adjacent liver tissue. Amir kurtaran has successfully applied 123I-insulin for in vivo scintigraphy in the diagnosis of hepatocellular carcinoma[26]. Kuang Anren reported that 131I-insulin was localized within H22 hepatoma in mice, and cleared rapidly from the rest of the body[27]. Schars reported that insulin receptor might play a role in the regulation of hepatocellular carcinoma[28]. Our finding was consistent with theirs. The results demonstrated that escalated binding site for 125I-insulin was expressed in hepatocellular carcinoma. The Bmax values indicated that the increased binding of 125I-insulin in hepatocellular carcinoma was due to an apparent increase in high affinity insulin receptor rather than an increase in low affinity insulin receptor. Our studies also showed that high affinity insulin receptor, constituting 87.72% of insulin receptor on cell surface of hepatocellular carcinoma, predominated over low affinity insulin receptor. These observations indicated that more IUdR covalently linked to insulin could be transferred to the carcinoma through mediation of the overexpressed high affinity insulin receptor.
It has also been verified in in vitro studies that insulin-IUdR is capable of binding specifically to insulin receptor in human hepatocellular carcinoma with high affinity. Chakrabarti et al conjugated 125I-IUdR with T101 antibody via polylysine, but the conjugate remained only 68% immunoactivity[29]. The conjugate used was prepared by covalently linking IUdR to insulin via succine. This complex has an advantage over the conjugate linked via poly-L-lysine[30] or albumin [31] or other large molecules. Both succine and IUdR are small molecules. The small size of the complex may result in less interference in insulin binding to its receptor or other cellular proteins and may permit this complex to be processed intracellularly in a manner more identical to that of unlabelled insulin.
Why insulin-IUdR has an increased IC50 and KI for high affinity insulin receptor but not for low affinity receptor is unclear. It is possible that the functional sites of structural microheterogeneity in the two receptors have different sensitivity to the structural change of ligand. Competition binding assays showed that insulin-IUdR still maintained the greater part of affinity for insulin receptor. Therefore, it is not necessary to administer insulin-IUdR in a micromolar range, which may result in glucopenia.
SDS-polyacrylamine agarose gel electrophoresis showed the molecular weight of insulin-IUdR was 7179 Da, which was equal to the total weight of one molecule of insulin (Mr = 5800 Da) and three molecules of IUdR (Mr = 454 Da). It might be concluded that the ratio of insulin molarity to the IUdR is 1:3. On the basis of this molar ratio, it is calculated that for administration 100-300 MBq 125I-IUdR, about 10-9 mol insulin was required. Administration of such a low dose of insulin in therapy would not result in glucopenia.
In summary, hepatocellular carcinoma overexpressing insulin receptor and insulin-IUdR conjugate can specifically bind to insulin receptor. This new conjugate holds promise for therapy of hepatocellular carcinoma, but further investigation into transportation of IUdR and its interactions with tumor cells during subsequent intracellular processing would be a desirable prerequisite.
| 1. | Menda Y, Kahn D. Somatostatin receptor imaging of non-small cell lung cancer with 99mTc depreotide. Semin Nucl Med. 2002;32:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Virgolini I, Traub T, Novotny C, Leimer M, Füger B, Li SR, Patri P, Pangerl T, Angelberger P, Raderer M. New trends in peptide receptor radioligands. Q J Nucl Med. 2001;45:153-159. [PubMed] |
| 3. | Rao PS, Thakur ML, Pallela V, Patti R, Reddy K, Li H, Sharma S, Pham HL, Diggles L, Minami C. 99mTc labeled VIP analog: evaluation for imaging colorectal cancer. Nucl Med Biol. 2001;28:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Thakur ML, Marcus CS, Saeed S, Pallela V, Minami C, Diggles L, Le Pham H, Ahdoot R, Kalinowski EA. 99mTc-labeled vasoactive intestinal peptide analog for rapid localization of tumors in humans. J Nucl Med. 2000;41:107-110. [PubMed] |
| 5. | Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7:971-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 173] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA. Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology. 2002;143:3259-3267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Vella V, Sciacca L, Pandini G, Mineo R, Squatrito S, Vigneri R, Belfiore A. The IGF system in thyroid cancer: new concepts. Mol Pathol. 2001;54:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Maune S, Görögh T. [Detection of overexpression of insulin receptor gene in laryngeal carcinoma cells by using differential display method]. Laryngorhinootologie. 2000;79:438-441. [PubMed] |
| 9. | Spector SA, Olson ET, Gumbs AA, Friess H, Büchler MW, Seymour NE. Human insulin receptor and insulin signaling proteins in hepatic disease. J Surg Res. 1999;83:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Frittitta L, Vigneri R, Stampfer MR, Goldfine ID. Insulin receptor overexpression in 184B5 human mammary epithelial cells induces a ligand-dependent transformed phenotype. J Cell Biochem. 1995;57:666-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Freund GG, Kulas DT, Way BA, Mooney RA. Functional insulin and insulin-like growth factor-1 receptors are preferentially expressed in multiple myeloma cell lines as compared to B-lymphoblastoid cell lines. Cancer Res. 1994;54:3179-3185. [PubMed] |
| 12. | Shah N, Zhang S, Harada S, Smith RM, Jarett L. Electron microscopic visualization of insulin translocation into the cytoplasm and nuclei of intact H35 hepatoma cells using covalently linked Nanogold-insulin. Endocrinology. 1995;136:2825-2835. [PubMed] |
| 13. | Harada S, Smith RM, Smith JA, Jarett L. Inhibition of insulin-degrading enzyme increases translocation of insulin to the nucleus in H35 rat hepatoma cells: evidence of a cytosolic pathway. Endocrinology. 1993;132:2293-2298. [PubMed] |
| 14. | Harada S, Loten EG, Smith RM, Jarett L. Nonreceptor mediated nuclear accumulation of insulin in H35 rat hepatoma cells. J Cell Physiol. 1992;153:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Chiou RK, Dalrymple GV, Baranowska-Kortylewicz J, Holdeman KP, Schneiderman MH, Harrison KA, Taylor RJ. Tumor localization and systemic absorption of intravesical instillation of radio-iodinated iododeoxyuridine in patients with bladder cancer. J Urol. 1999;162:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Morgan RJ, Newman EM, Doroshow JH, McGonigle K, Margolin K, Raschko J, Chow W, Somlo G, Leong L, Tetef M. Phase I trial of intraperitoneal iododeoxyuridine with and without intravenous high-dose folinic acid in the treatment of advanced malignancies primarily confined to the peritoneal cavity: flow cytometric and pharmacokinetic analysis. Cancer Res. 1998;58:2793-2800. [PubMed] |
| 17. | Sondak VK, Robertson JM, Sussman JJ, Saran PA, Chang AE, Lawrence TS. Preoperative idoxuridine and radiation for large soft tissue sarcomas: clinical results with five-year follow-up. Ann Surg Oncol. 1998;5:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Daghighian F, Humm JL, Macapinlac HA, Zhang J, Izzo J, Finn R, Kemeny N, Larson SM. Pharmacokinetics and dosimetry of iodine-125-IUdR in the treatment of colorectal cancer metastatic to liver. J Nucl Med. 1996;37:29S-32S. [PubMed] |
| 19. | Yan XQ, Cheng ML, Liu BW, Lan TH. Study on binding assay of insulin receptor in liver cell membrane. Zhonghua Heyixue Zazhi. 1986;6:91-93. |
| 20. | Cheng YQ. Shengwu huaxue shiyan fangfa he jishu.1. Beijing: Science Press. 2002;164-166. |
| 21. | Ivanova MM, Rosenkranz AA, Smirnova OA, Nikitin VA, Sobolev AS, Landa V, Naroditsky BS, Ernst LK. Receptor-mediated transport of foreign DNA into preimplantation mammalian embryos. Mol Reprod Dev. 1999;54:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Sobolev AS, Rosenkranz AA, Smirnova OA, Nikitin VA, Neugodova GL, Naroditsky BS, Shilov IN, Shatski IN, Ernst LK. Receptor-mediated transfection of murine and ovine mammary glands in vivo. J Biol Chem. 1998;273:7928-7933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Rosenkranz AA, Yachmenev SV, Jans DA, Serebryakova NV, Murav'ev VI, Peters R, Sobolev AS. Receptor-mediated endocytosis and nuclear transport of a transfecting DNA construct. Exp Cell Res. 1992;199:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Boado RJ, Pardridge WM. Marked enhancement in gene expression by targeting the human insulin receptor. J Gene Med. 2003;5:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Toyohara J, Hayashi A, Sato M, Tanaka H, Haraguchi K, Yoshimura Y, Yonekura Y, Fujibayashi Y. Rationale of 5-(125)I-iodo-4'-thio-2'-deoxyuridine as a potential iodinated proliferation marker. J Nucl Med. 2002;43:1218-1226. [PubMed] |
| 26. | Kurtaran A, Li SR, Raderer M, Leimer M, Müller C, Pidlich J, Neuhold N, Hübsch P, Angelberger P, Scheithauer W. Technetium-99m-galactosyl-neoglycoalbumin combined with iodine-123-Tyr-(A14)-insulin visualizes human hepatocellular carcinomas. J Nucl Med. 1995;36:1875-1881. [PubMed] |
| 27. | Kuang AR, Zhou LY, Liang ZL, Tan TZ, Wang CJ. Evaluation of hepatoma targeting ability with radioiodiated insulin in H22 bearing mice. Zhonghua Heyixue Zazhi. 1999;19:179-180. |
| 28. | Scharf JG, Dombrowski F, Ramadori G. The IGF axis and hepatocarcinogenesis. Mol Pathol. 2001;54:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Chakrabarti MC, Paik CH, Carrasquillo JA. Preparation and in vitro studies of [125I]IUDR-T101 antibody conjugate. Cancer Biother Radiopharm. 1999;14:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Wagner E, Ogris M, Zauner W. Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv Drug Deliv Rev. 1998;30:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 378] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Huckett B, Ariatti M, Hawtrey AO. Evidence for targeted gene transfer by receptor-mediated endocytosis. Stable expression following insulin-directed entry of NEO into HepG2 cells. Biochem Pharmacol. 1990;40:253-263. [PubMed] |
Edited by Xu XQ and Zhu LH