Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1278
Revised: January 4, 2003
Accepted: January 8, 2003
Published online: June 15, 2003
AIM: To investigate the role of nuclear factor-κB (NF-κB) inhibitor caffeic acid phenethy1 ester (CAPE) in the proliferation, collagen synthesis and apoptosis of hepatic stellate cells (HSCs) of rats.
METHODS: The HSCs from rats were isolated and cultured in Dulbecco's Modified Eagle's Medium (DMEM) and treated with CAPE. The proliferation and collagen synthesis of HSCs were determined by 3H-TdR and 3H-proline incorporation respectively, and the expression of type I, III procollagen genes was further explored by in situ hybridization. Apoptosis cell indices (AIs) were examined using terminal deoxynucleotidyl transferase- mediated DIG-dUTP nick end labeling (TUNEL).
RESULTS: In activated HSC in culture, CAPE significantly inhibited 3H-TdR and 3H-proline incorporation by HSCs at concentrations of 5 μmol/L and 10 μmol/L respectively. CAPE also reduced the type I procollagen gene expression (P < 0.05) at higher concentration. Apoptosis of HSC was induced by CAPE and the AIs were time-and dose-dependently increased from 2.82% ± 0.73% to 7.66% ± 1.25% at 12 h (P < 0.01) and from 3.15% ± 0.88% to 10.61% ± 2.88% at 24 h (P < 0.01).
CONCLUSION: CAPE inhibits proliferation and collagen synthesis of HSC at lower concentration and induces HSC apoptosis at higher concentration.
-
Citation: Zhao WX, Zhao J, Liang CL, Zhao B, Pang RQ, Pan XH. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of hepatic stellate cells
in vitro . World J Gastroenterol 2003; 9(6): 1278-1281 - URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1278
Liver fibrosis is a progressive pathological process involving multi-cellular and molecular events that ultimately lead to deposition of excess matrix proteins in the extracellular space. It is generally accepted that HSC is the most pathogenetically relevant cell type for the development of liver fibrosis[1-5]. During liver injury and inflammation, quiescent HSCs transdifferentiate into activated-type HSCs, also termed myofibroblasts (MFBs), which result in accumulation of a cell type that provides main collagenous and non-collagenous components of the extracellular fibrotic matrix, contribute to diminished degradation of extracellular matrix by the expression of tissue inhibitors of metalloproteinases (TIMPs) and also produce an array of proinflammatory cytokines and chemokines involving the development of liver fibrosis[3-11].
It has recently been shown that the recovery from established experimental fibrosis can occur through the apoptosis of HSC, which is associated with reduction of collagen and expression of TIMPs in liver. Apoptosis, therefore, plays an important role in the resolution of fibrosis by eliminating the sources of both the neomatrix and TIMPs, and thereby facilitates net matrix degradation[12-15]. Activation and apoptosis of HSCs could play a central role in turnover of liver fibrosis.
Recent research showed that cultured HSC underwent a rapid and persistent induction of a high-mobity NF-κB DNA binding complex, and the activation of NF-κB in cultured HSC was required for activated phenotype of HSCs and might be anti-apoptotic for HSCs[16-19]. In this study, we investigated the effect of NF-κB inhibitor CAPE on the proliferation, collagen synthesis and apoptosis of HSCs.
Wistar rats, male, 450-500 g of body mass, were provided by the Center for Laboratory Animal of Kunming General Hospital. DMEM and FBS were obtained from GIBCO. CAPE and collagenase were purchased from SIGMA, 3H-TdR and 3H-proline were provided by Beijing Institute of Atomic Energy, RNA Mimi Kit and DIG DNA Labeling and Detecting Kit were respectively obtained from Gene and Boehringer Mannherm.
Isolation and culture of HSCs HSCs were isolated from normal liver by sequential in situ perfusion with collagenase, as previously described[20,21]. Briefly, the livers were perfused first with Ca2+- and Mg2+- free solution for 10 min at 37 °C, and next with 0.05% (w/v) collagenase solution for 30 min at 37 °C, the digested liver were excised, dispersed in D-Hanks, and filtered through gauze. The residual hepatocytes were removed by two low-speed centrifugation (50 g, 4 °C, 2 min). The stellate-cell enriched fraction was obtained by centrifugation with a triple-layered (9%, 11%, and 17%) Nycodenz cushion (1400 × g, 4 °C 20 min). The cells in the upper-layer were washed and seeded onto uncoated plastic tissue culture plate in DMEM supplemented with 10% FBS and grown for 14 d. Purity and viability of freshly isolated HSC were determined by Desmin immunohistochemistry and trypan-blue stain respectively.
3H-TdR and 3H-proline uptake by HSC Passaged HSCs were cultured in 24-well plates for 48 h and then treated with CAPE at concentrations of 0.0, 1.0, 2.5, 5.0, 10.0, 20.0, and 40.0 μmol/L for 24 h. CAPE was dissolved in DMSO. The cells were then labeled with 1 μci/mL 3H-TdR or 1 μci/mL 3H-proline respectively. After washed with D-Hanks, the HSCs were digested with trypsin and absorbed on glass fiber filter paper. The cells were then washed once with 10% trichloroacetic acid and three times with saline and dried overnight at 80 °C, the radioactivity (CPM) of each sample was counted using liquid-scintillation analyzer.
Expression of procollagen gene in HSC Type I and III procollagen gene sequences were enquired from GeneBank of National Center of Biotechnology Information, and primers that amplified the procollagen genes were designed with OLIGO microsoft according to the gene sequences and synthesized by Sangon Company. The primers were 5'-CGATGGATTCCCGTTCGAGTAC-3' and 5'-GTCCACAACCCTGTAGGTG-3' for type I procollagen gene, 5'-GGAAACAGCAAATTCACTTACA-3' and 5'-TCACTTGCACTGGTTGATAAGA-3' for type III procollagen gene. The RNA was extracted from the skin of newborn ICR mice by RNA mini kit, the genes were amplified by RT-PCR and procollagen gene probes were labeled with PCR and DIG-dUTP. HSCs cultured in 24-well plates were treated with 20 μmol/L CAPE for 24 h and the procollagen gene expression was detected according to the previously described procedure[22].
Apoptosis assay Passaged HSCs were cultured for 48 h and then cultured in 5% FBS DMEM and treated with CAPE at predetermined concentrations of 0.0, 20.0, and 40.0 μmol/L for 12 h and 24 h, the cells were fixed in formaldehyde solution and stained with TUNEL reaction solution containing DIG-dUTP and terminal deoxynucleotide transferase for 60 min at 37 °C. DIG was detected by anti-DIG-AP conjugate and colorimetric substrate NBT/BCIT. Positive cells were counted under microscope.
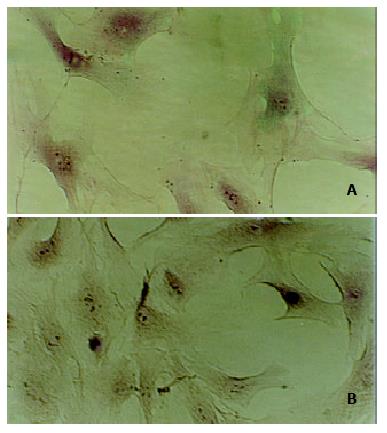
Freshly isolated HSCs were round-shaped with many yellow-coloured droplets in cytoplasm, after 2-3 d in culture on uncoated plastic surface, the cells had spread and showed a typical "star"-like configuration. More than 80% of freshly isolated cells were desmin-positive, and the cell viability was about 90% according to trypan-blue staining.(Figure 1)
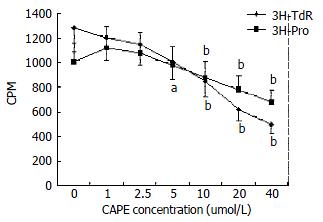
We observed the effect of CAPE on 3H-TdR and 3H-proline incorporation by cultured HSCs. As shown in Figure 2, CAPE significantly and dose-dependently suppressed the incorporation of 3H-TdR and 3H-proline by HSCs. The median inhibitory concentrations were 5 and 10 μmol/L respectively for 3H-TdR and 3H-proline. These concentrations were lower, especially for the inhibition of HSCs proliferation, indicating that CAPE was a potent inhibitor for proliferation, and collagen synthesis.
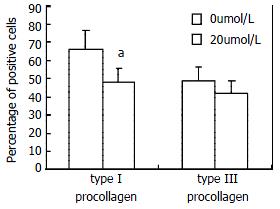
Type I and III collagens are the main components of extracellular matrix in liver fibrosis. In situ hybridization analysis of type I and III procollagen genes showed that positive HSCs were reduced by CAPE at the concentration of 20 μmol/L. And the reduction of type I procollagen gene expression was statistically significant, indicating that CAPE suppressed the procollagen gene expression.(Figure 3)
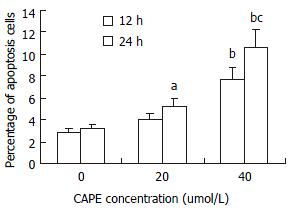
Apoptosis of HSC was demonstrated by TUNEL, the nucleus of apoptotic HSCs were stained with violet blue (Figure 4). After treatment with CAPE, AIs were time- and dose-dependently increased from 2.82% ± 0.73% to 7.66% ± 1.25% at 12h and from 3.15% ± 0.88% to 10.61% ± 2.88% at 24 h. The data indicated that CAPE induced HSC apoptosis at higher concentrations (Figure 5).
HSCs, previously termed as fat or vitamin A-storing cells or Ito cells, localized in close proximity to sinusoidal endothelial cell and hepatocyte in the space of Disse, are the most pathogenetically relevant cell type for development of liver fibrosis[2-5]. Activated HSCs in liver tissue provide virtually most main components of ECM, contribute to diminished degradation of ECM by expressing TIMPs, and produce an array of proinflammatory cytokines and chemokines involving the development of liver fibrosis[3-11]. It is generally accepted that HSCs are important target cells for the treatment of liver fibrosis[5,23].
In our study, we observed that the NF-κB inhibitor CAPE inhibited the proliferation and collagen synthesis of HSCs. CAPE dose-dependently suppressed the incorporation of 3H-TdR and 3H-proline by HSCs. This indicated CAPE inhibited the proliferation of HSCs. Because the reduction of either total cell number or collagen synthesis may contribute to the reduction of 3H-proline incorportion by HSCs, the expressions of type I and type III procollagen genes were further explored in HSCs. Our data showed that the procollagen gene expressions were reduced by CAPE, and the reduction of 3H-proline uptake partially indicated that CAPE inhibited collagen synthesis, in addition to the reduction of HSC number.
CAPE, a structural relative of flavonoids that is an active component of propolis from honeybee hives, has been shown to inhibit the growth of different types of cells including endothelial cells, keratinocyte and tumor cells, which involves in nuclear factor, protein kinase C and cytokine signal transduction[24-29]. CAPE inhibited the proliferation and collagen synthesis of HSCs, properly by reducing the reactive oxygen intermediates, this is consistent with the antioxidant property of CAPE[30,31]. Previous studies showed that reactive oxidant species induced HSCs activation and collagen gene expression in vivo and in vitro[32-35]. The antioxidant phenolic compounds reduced the proliferation and collagen synthesis by suppressing inositol phosphate metabolism, tyrosine and protein kinase activation[33,36]. Since CAPE is a structural relative of flavonoids, CAPE probably inhibits the proliferation and collagen synthesis of HSCs similarly. Other mechanisms might include inhibition of the expression of COX2 and activation of NF-κB by CAPE, which are associated with the phenotype of activated HSCs[37,38].
Activated HSCs undergo auto-apoptosis in serum-deprived DMEM and in experimental fibrosis induced by CCl4. P75 and Fas have already been identified as molecules associated with the apoptosis of HSCs, which were expressed in HSCs. Apoptosis of HSCs can be induced by nerve growth factor and soluble Fas-ligand[39,40]. The recovery from established experimental fibrosis is relevant to the apoptosis of HSCs, which contributes to the reduction of neomatrix synthesis and expression of TIMPs. Recent reports showed the persistent activation of NF-κB was induced in activated but not in quiescent HSCs, which is required for activated phenotype of HSCs and may be antiapoptotic for HSCs[16-18]. CAPE is a potent and specific inhibitor of NF-κB, CAPE inhibits the activation of NF-κB induced by TNF and other inflammatory agents including phorbol ester, ceramide, hydrogen peroxide, etc[41]. Our data showed the AIs of HSCs were increased by CAPE, the inhibition of NF-κB activation by CAPE probably played a key role in HSC apoptosis. CAPE inhibited the activation of NF-κB and the expression of proinflammatory cytokines TNF-α and IL-1β; CAPE also induced apoptosis in macrophages[42], similar results were observed in leucocytes as well[43]. However, CAPE induced apoptosis of tumor cells by regulating the expression of caspas-3, bcl-2, bax and P53[44-46]. For HSCs, the mechanism of apoptosis induced by CAPE needs further investigation.
CAPE has been shown to be a pharmacologically safe compound with known antiinflammatory, antimitogenic, anticarcinogenic, antioxidant, and immunomodulatory effects[37,47]. Therefore, CAPE might have a therapeutic role in liver fibrosis by inhibiting the proliferation or inducing the apoptosis of HSCs.
| 1. | Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Benyon RC, Iredale JP. Is liver fibrosis reversible. Gut. 2000;46:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Rockey DC. The cell and molecular biology of hepatic fibrogenesis. Clinical and therapeutic implications. Clin Liver Dis. 2000;4:319-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest. Liver Physiol. 2000;279:G245-G249. [PubMed] |
| 5. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 397] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Yao XX, Tang YW, Yao DM, Xiu HM. Effects of Yigan Decoction on proliferation and apoptosis of hepatic stellate cells. World J Gastroenterol. 2002;8:511-514. [PubMed] |
| 7. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Gressner AM. The up-and-down of hepatic stellate cells in tissue injury: apoptosis restores cellular homeostasis. Gastroenterology. 2001;120:1285-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Wang JY, Zhang QS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol. 2001;7:115-119. [PubMed] |
| 10. | Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899-d1914. [PubMed] |
| 11. | Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 374] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 15. | Liu XJ, Yang L, Mao YQ, Wang Q, Huang MH, Wang YP, Wu HB. Effects of the tyrosine protein kinase inhibitor genistein on the proliferation, activation of cultured rat hepatic stellate cells. World J Gastroenterol. 2002;8:739-745. [PubMed] |
| 16. | Hellerbrand C, Jobin C, Licato LL, Sartor RB, Brenner DA. Cytokines induce NF-kappaB in activated but not in quiescent rat hepatic stellate cells. Am J Physiol. 1998;275:G269-G278. [PubMed] |
| 17. | Saile B, Matthes N, El Armouche H, Neubauer K, Ramadori G. The bcl, NFkappaB and p53/p21WAF1 systems are involved in spontaneous apoptosis and in the anti-apoptotic effect of TGF-beta or TNF-alpha on activated hepatic stellate cells. Eur J Cell Biol. 2001;80:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Elsharkawy AM, Wright MC, Hay RT, Arthur MJ, Hughes T, Bahr MJ, Degitz K, Mann DA. Persistent activation of nuclear factor-kappaB in cultured rat hepatic stellate cells involves the induction of potentially novel Rel-like factors and prolonged changes in the expression of IkappaB family proteins. Hepatology. 1999;30:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069-11076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 347] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Kawada N, Klein H, Decker K. Eicosanoid-mediated contractility of hepatic stellate cells. Biochem J. 1992;285:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M, Ito S. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun. 2001;286:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Zhao W, Liang C, Chen Z, Pang R, Zhao B, Chen Z. [Luteolin inhibits proliferation and collagen synthesis of hepatic stellate cells]. Zhonghua Ganzangbing Zazhi. 2002;10:204-206. [PubMed] |
| 23. | Beljaars L, Meijer DK, Poelstra K. Targeting hepatic stellate cells for cell-specific treatment of liver fibrosis. Front Biosci. 2002;7:e214-e222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Song YS, Park EH, Jung KJ, Jin C. Inhibition of angiogenesis by propolis. Arch Pharm Res. 2002;25:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Maffia P, Ianaro A, Pisano B, Borrelli F, Capasso F, Pinto A, Ialenti A. Beneficial effects of caffeic acid phenethyl ester in a rat model of vascular injury. Br J Pharmacol. 2002;136:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Zheng ZS, Xue GZ, Grunberger D, Prystowsky JH. Caffeic acid phenethyl ester inhibits proliferation of human keratinocytes and interferes with the EGF regulation of ornithine decarboxylase. Oncol Res. 1995;7:445-452. [PubMed] |
| 28. | Usia T, Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. Constituents of Chinese propolis and their antiproliferative activities. J Nat Prod. 2002;65:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Huleihel M, Ishano V. Effect of propolis extract on malignant cell transformation by moloney murine sarcoma virus. Arch Virol. 2001;146:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Uz E, Söğüt S, Sahin S, Var A, Ozyurt H, Güleç M, Akyol O. The protective role of caffeic acid phenethyl ester (CAPE) on testicular tissue after testicular torsion and detorsion. World J Urol. 2002;20:264-270. [PubMed] |
| 31. | Son S, Lewis BA. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J Agric Food Chem. 2002;50:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 32. | Greenwel P, Domínguez-Rosales JA, Mavi G, Rivas-Estilla AM, Rojkind M. Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology. 2000;31:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 294] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Svegliati-Baroni G, Saccomanno S, van Goor H, Jansen P, Benedetti A, Moshage H. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver. 2001;21:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 458] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Nagaoka T, Banskota AH, Tezuka Y, Saiki I, Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem. 2002;10:3351-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Gallois C, Habib A, Tao J, Moulin S, Maclouf J, Mallat A, Lotersztajn S. Role of NF-kappaB in the antiproliferative effect of endothelin-1 and tumor necrosis factor-alpha in human hepatic stellate cells. Involvement of cyclooxygenase-2. J Biol Chem. 1998;273:23183-23190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Cheng J, Imanishi H, Liu W, Iwasaki A, Ueki N, Nakamura H, Hada T. Inhibition of the expression of alpha-smooth muscle actin in human hepatic stellate cell line, LI90, by a selective cyclooxygenase 2 inhibitor, NS-398. Biochem Biophys Res Commun. 2002;297:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Trim N, Morgan S, Evans M, Issa R, Fine D, Afford S, Wilkins B, Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000;156:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Gong W, Pecci A, Roth S, Lahme B, Beato M, Gressner AM. Transformation-dependent susceptibility of rat hepatic stellate cells to apoptosis induced by soluble Fas ligand. Hepatology. 1998;28:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090-9095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 930] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 42. | Lu B, Wang L, Medan D, Toledo D, Huang C, Chen F, Shi X, Rojanasakul Y. Regulation of Fas (CD95)-induced apoptosis by nuclear factor-kappaB and tumor necrosis factor-alpha in macrophages. Am J Physiol Cell Physiol. 2002;283:C831-C838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Orban Z, Mitsiades N, Burke TR, Tsokos M, Chrousos GP. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Nomura M, Kaji A, Ma W, Miyamoto K, Dong Z. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol Carcinog. 2001;31:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem. 2001;49:5615-5619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Isla MI, Nieva Moreno MI, Sampietro AR, Vattuone MA. Antioxidant activity of Argentine propolis extracts. J Ethnopharmacol. 2001;76:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Edited by Yuan HT