Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1251
Revised: January 4, 2003
Accepted: January 8, 2003
Published online: June 15, 2003
AIM: To describe distribution of the phosphorothioated antisense oligodeoxynucleotides (PS-asODNs) conjugated to galactosylated poly-L-lysine (Gal-PLL) in mice, and to observe their effects on expression of HBV gene in the 2.2.15 cells and transgenic mice.
METHODS: According to the result of direct sequencing of PCR amplified products, a 16 mer phosphorothioate analogue of the antisense oligodeoxynucleotides (PS-asODNs) directed against the HBV U5-like region was conjugated to the hepatotropic Gal-PLL molecules. Its distribution was demonstrated using asODNs labeled with 32P at the 5’ terminus with a T4-polynucleotide Kinase. Its inhibition effect on HBV expression was observed in the transfected 2.2.15 cells and transgenic mice.
RESULTS: The Gal-PLL and asODNs could form stable complex at a molar ratio of 2:1. As shown in the HBV-transfected 2.2.15 cells, the inhibition effects of asODNs alone and asODNs conjugated to Gal-PLL, at 10 μmol/L for both, on HBsAg and HBeAg production were different, the former being 70% and 58%, respectively, and the latter being 96% and 82%, respectively. A more pronounced reduction was also observed in viral DNA load in the culture supernatant for the test with Gal-PLL-asODNs. Among many mouse organs, livers retained more asODNs molecules after administration. The preferential concentration in liver was found to be 52.14% for Gal-PLL-asODNs, as high as 2.38-fold of that for asODNs (21.9%). Both elements decreased gradually in liver, with 2.9% of the former, 5.99% of the latter retained 24 h after the administration. The injection interval, therefore, was recommended to be 24 h. In the transgenic mice, serum HBsAg decreased significantly (P < 0.01) at the 12th day after administrating Gal-PLL- asODNs, the serum HBV DNA turned negative in 4 of the 6 mice.
CONCLUSION: Antisense oligodeoxynucleotides conjugated to Gal-PLL can be concentrated in liver and intaked by hepatocytic cells. This may result in specific inhibition of expression and replication of HBV in vitro and in vivo.
- Citation: Zheng SJ, Zhong S, Zhang JJ, Chen F, Ren H, Deng CL. Distribution and anti-HBV effects of antisense oligodeoxynu-cleotides conjugated to galactosylated poly-L-lysine. World J Gastroenterol 2003; 9(6): 1251-1255
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1251.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1251
The antisense oligodeoxynucleotides (asODNs) have been demonstrated previously to be effective for inhibition of HBV gene expression and viral replication in vitro and in vivo, being potential new agents for anti-HBV therapy[1-4]. However, there are still some hinders for their potential application. First, their molecules must be water soluble and can penetrate the lipophilic
cell membrane. Second, they must be resistant enough to enzymatic degradation to allow their concentration in liver. Third, They must be bound specifically to the target HBV sequence. In the present study, a 16-mer phosphorothioate analogue of
the antisense oligonucleotides (PS-asODNs) directed against the HBV U5-like region was synthesized and then conjugated to hepa totropic galactosyted poly-L-lysine (Gal-PLL). The anti-HBV effects of asODNs and Gal-PLL-asODNs were demonstrated in the 2.2.15 cells and transgenic mice. In addition, distribution of asODNs was studied in mice.
PCR was conducted using the primer pair P1/P2 in a 50 μL system with the initial denaturation temperature at 94 °C for 4 min, followed by 30 cycles at 94 °C for 30 s, at 55 °C for 30 s and at 72 °C for 40 s. The reaction was for the pre-C and C genes of HBV (ayw1 subtype). The sequence of the PCR primers pair was as follows: P1: 5'-AAGGTCTTTGTACTAGGAGGC-3'(1761-1781); P2: 5'-TTCCCGATACAGAGCTGAGGC-3' (2000-2020). The PCR products were as long as 260 bp and were sequenced using the method given by the Pharmacia T7 kit.
2.2.15 cell line, human hepatoblastoma Hep G2 cell line stably transfected by HBV genome[5-7], were cultured in PRMI1640 medium at 37 °C under 5% CO2 , pH: 7.2-7.4, which contained 15% fetal calf serum, 2 mmol/L glutamine, 0.1 mu/L
penicillin and streptomycin, and 380 mg/L G418 (Sigma).
A targeting water soluble DNA carrier was prepared by coupling galactose and poly-L-lysine using the method of Schwartz
and Gray[8], with their molar ratio being 10:1. A 16-mer phosphorothioated antisense oligodeoxynucleotide (5'-CATGCCCCAAAGCCAC-3'), corresponding to the nucleotides 1980-1905, and complementary to preC/C regions of HBV genome (ayw1 subtype), was synthesized. As a reference molecule, another 16-mer phosphorothioated oligodeoxynucleotide (5'-AGTCACTCAGTCAGTC-3'), unrelated to the HBV sequence, was prepared. By agarose gel(2.5%) electrophoresis, conjunctive ratio of Gal-PLL to PS-asODNs was identified[9].
The 2.2.15 cells were incubated for 60 h, and then were transferred to the medium containing asODNs, HBV-specific Gal-PLL-asODNs, HBV-unrelated Gal-PLL-ODNs, or the medium without any oligodeoxynucleotides. The concentration of ODNs in the media was 10 μmol/L. 72 h later, the 2.2.15 cells were transferred to the ordinary medium for 72 h, then 200 ul of supernatant was used for the ELISA reaction for HBsAg and HBeAg, HBV DNA was detected by dot hybridization.
Kunming mice, (weighing 20 g in average) were used to describe the distribution of asODNs. HBV transgenic mice, were provided by 458 Hospital of PLA in Guangzhou, China, whose expression of HBsAg (126-930 ng/ml) was well documented
The 32P-labelling of asODNs was at 5’ terminus with T4-polynucleotide kinase (Amersham) in the reaction mixture containing asODNs 6.6 ug/20 μL, [γ-32P] ATP 90 μL (10 mCi/ml), 10 × buffer 18 μL, T4-polynucleotide kinase 9 μL and deionized water
43 μL for 1.5 h. The reaction was stopped by adding 9 μL 0.5M EDTA (pH8.0). The product was extracted by chloroform and separated using Sephadex-G50. Using the same method, the reaction was carried out twice.
A total number of 24 Kunming mice were equally divided randomly into two groups, one for asODNs and the other for Gal-PLL-asODNs. The labeled asODNs were equally divided into 25 pieces, each piece for 200 μL, with the radioactivity of 9.42 × 106 cpm. Twelve pieces of asODNs were mixed with Gal-PLL at a molar ratio of 1:2, and were incubated at room temperature for 60 min. Twenty-four pieces of asODNs were administered intravenously via tail vein to 24 mice, respectively.
At 2 min, 30 min, 1 h, 4 h, 13 h, 24 h following intravenous administration, 2 animals were sacrificed for each group at each time point, their blood and organs were collected for determination of total radioactivity using liquid scintillation counter.
Twelve mice, strongly positive for HBsAg and carrying HBV DNA in serum were equally divided into Gal-PLL-asODNs and control groups. Gal-PLL was then mixed with asODNs in a molar ratio of 2:1 at room temperature. The mixture was then administered intravenously via the tail vein in a dose of 15 μg/g. weight/d of asODNs for successive 12 d. The same volume of saline was used for the control mice by the same means. Blood samples were collected at the venous plexus behind orbital cavity before, or 12 d after the treatment. Sera were separated by incubating at 37 °C for 30 min and contrifuged, and stored at -20 °C for use. Being diluted at 1:100 with normal saline, the sera were subjected to the ELISA for HBsAg and nested PCR (TakaRa Biotechnology Co.) for viral DNA, with its product being 230 bp in size.
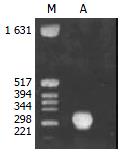
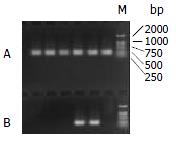
Figure 1 shows that the band of PCR product was between the size of 221-298 bp. The sequence of the 132 bp product
was:5'-CCAGCACCATGCAACTTTTTCACCTCTGCC-TAATCATCTCTTGTTCATGTCCTACTGTTCAAGCCTCCAA-GCTGTGCCTTGG
GTGGCTTTGGGGCATGGACATC-GACCCTTATAAAGAATTTGGAGCTACTG-3'. This was in accordance with the corresponding sequence of HBV genome (ayw1 subtype) which contained the preC region (1816-1902), U5-like sequence (1857-1918)
and partial poly-A addition signal sequence (1919-1962)[12,13].
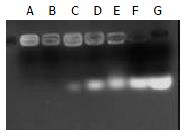
Gal-PLL at different concentrations was incubated with asODNs, and the proper molar ratio for their binding was assessed by agarose gel electrophoresis. The binding was detectable at the molar ratio of 1:1, and complete at the molar ratio of 2:1 or more (Figure 2).
As shown in Table 1, after incubation of 2.2.15 cells with asODNs for 8.5 d, the inhibition rates of HBsAg, HbeAg were 70% and 50%, respectively, while those with Gal-PLL-asODNs were 96% and 82%, respectively. Only slightly negative effects were observed for the reference agent Gal-PLL-ODNs on expression of HBsAg (19%) and HBeAg (10%). The Gal-PLL-asODNs treatment was shown to markedly reduce the amount of HBVDNA in the 2.2.15 cells and the culture medium, compared with free asODNs and conjugated HBV-unrelated Gal-PLL-ODNs.
| Groups | HBsAg | Inhibitory rate (%) | HBeAg | Inhibitory rate (%) |
| Cell control | 9.4 ± 0.16 | 15.10 ± 0.15 | ||
| PS-asODNs | 4.30 ± 0.25 | 70 | 7.60 ± 1.10 | 58 |
| Gal-PLL:PS-asODNs | 2.40 ± 0.26 | 96 | 4.50 ± 0.42 | 82 |
| Gal-PLL:PS-ODNs | 8.10 ± 0.13 | 19 | 13.80 ± 0.76 | 10 |
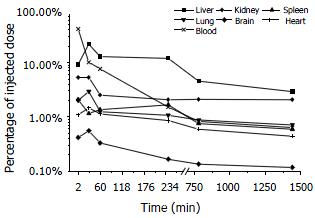
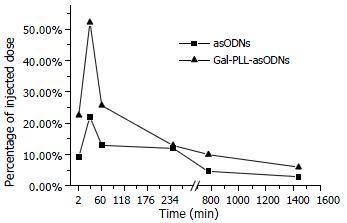
Plasma level of asODNs declined rapidly after intravenous administration, with its half-life being approximately 1-2 min, and 7.6% of asODNs was left in circulation one hour later. Among organs examined, liver retained more asODNs (21.9%) 30 min after the administration, and the retained molecules decreased gradually, with 2.93% of them detected 24 h later. At the time point of 30 min, the retained radioactivity listed in the order of intensity was as following: liver > kidney > lung > heart > spleen > brain (Figure 3).
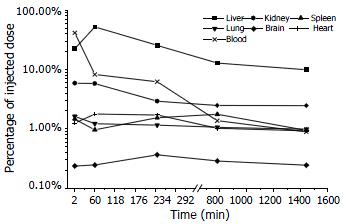
The elimination process of Gal-PLL-asODNs from bloodstream was similar to that of asODNs, with its half-life being 1-2 min. Less Gal-PLL-asODNs was shown to be retained in the circulation at the time points of 30 min (8.26%), 60 min (6.24%) than asODNs (10.17% for 30 min, 7.65% for 60 min) at the corresponding time points. Up to 52.14% of Gal-PLL-asODNs was shown to be in liver at the time point of 30 min, and only 5.99% of the activity was retained in liver 24 h after its administration. Kidney was the second radioactivity concentrating organ, retaining 5.89% of injected Gal-PLL-asODNs at the time point of 2 min. The amount in the brain was the smallest (Figure 4). In comparison to that for asODNs, more radioactivity for Gal-PLL-asODNs was retained in liver at each time points (Figure 5). Evidently, Gal-PLL had prompted hepatic intake of asODNs. It might enhance the preferential concentration of agent in liver, with a factor of 8.98-fold to kidney, 42.66-fold to lung, 54.24-fold to spleen. However, there were only few asODNs in liver for both groups 24 h later (5.99% and 2.93%, respectively).
After treatment for 12 d, serum HBsAg decreased significantly in the Gal-PLL-PSODNs treated group (P < 0.01); in contrast, no apparent change was detected after treatment with HBV-unrelated Gal-PLL-ODNs (Table 2). In serum HBsAg level, as shown in Figure 6, serum HBV DNA turned negative in 4 of the 6 (66.7%) Gal-PLL-asODNs treated transgenic mice, but it remained unchanged in the control mice.
The 2.2.15 cells were shown to carry HBVDNA, being a useful model for screening of HBV-specific asODNs. Exonucleases and endonucleases were identified in serum, cells, and other body fluid, both being able to degrade ODNs. Nucleoside modifications may enhance the resistance of ODNs to nuclease digestion. Among them, phosphorothioate oligodeoxynucleotides were found to be more stable in cell culture medium, cells, the cell extraction, serum, tissue and urine. PSODNs, therefore, were used as a drug in this study.
Foreign DNA can be introduced into cells by transfection using several protocols, including electroporation, liposome and calcium phosphate-mediated procedure. In addition, some DNA delivery systems have been designed for binding foreign gene to target cells[14,15]. The DNA-carrier system consisting of an asialoglycoprotein (asialoorosomucoid, ASOR) covalently linked to poly-L-lysine has been used as a hepatotropic ligand to bring a foreign gene preferentially to hepatocytes via asialoglycoprotein receptors[16-23].
For this purpose, Gal-PLL was prepared in this laboratory previously. Its selective affinity to hepatocytes was demonstrated in vitro, proposed to be mediated by asialoglycoprotein receptors on hepatocytes membrane[24-30]. In this study, the optimized molar ratio 2:1 between Gal-PLL and ODNs was estimated to be 2:1, being in agreement with the reported data (17). Gal-PLL was also suggested to be useful as a liver-targeting plasmid DNA carrier in vivo[27,28,30,31]. This was approved in this study.
The distribution of different ODNs in vivo may vary with their length and sequence[32], and this shoud be clarified before gene therapy. More than a half of asODNs conjugated to Gal-PLL was shown to be retained in liver, being 2.38-fold of that to free asODNs. In order to avoid the possible interference by plasma HBV DNA in HBV infected animal, normal Kunming mice were used to explore the distribution of asODNs in vivo.
According to the data presented, Gal-PLL appears to be a more favorable hepatotropic ligand for gene delivery in vivo regarding its convenient preparation and stability, as compared to other compounds such as the well characterized tetra-antennary cluster galactoside L3 G4[33].
Considering the dynamic of asODNs, free or conjugated to Gal-PLL, asODNs in liver were 2.97% and 5.99%, respectively, 24 hrs after administration, the recommended drug injection interval was 24 h.
In the same condition of experiment, the inhibition rates of HBsAg and HBeAg by asODNs were 70% and 58%, respectively, while those of HBsAg and HBeAg by Gal-PLL-asODNs were 96% and 82%, These data indicate that asODNs conjugated to Gal-PLL can block HBV gene expression more efficiently in vitro.
HBV transgenic mice were proved to be the suitable animal model for screening anti-HBV drugs[10,11,34,35,36]. In the present study, a pronounced inhibition was observed by Gal-PLL-asODNs treatment. In addition, serum HBVDNA turned negative in 4 of the 6 transgenic mice. These data show the potential application significance of Gal-PLL-asODNs as an anti-HBV agent.
| 1. | Jensen KD, Kopecková P, Kopecek J. Antisense oligonucleotides delivered to the lysosome escape and actively inhibit the hepatitis B virus. Bioconjug Chem. 2002;13:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Liu S, Sun W, Cao Y. [Study on anti-HBV effects by antisense oligodeoxynucleotides in vitro]. Zhonghua Yufang Yixue Zazhi. 2001;35:338-340. [PubMed] |
| 3. | Robaczewska M, Guerret S, Remy JS, Chemin I, Offensperger WB, Chevallier M, Behr JP, Podhajska AJ, Blum HE, Trepo C. Inhibition of hepadnaviral replication by polyethylenimine-based intravenous delivery of antisense phosphodiester oligodeoxynucleotides to the liver. Gene Ther. 2001;8:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Soni PN, Brown D, Saffie R, Savage K, Moore D, Gregoriadis G, Dusheiko GM. Biodistribution, stability, and antiviral efficacy of liposome-entrapped phosphorothioate antisense oligodeoxynucleotides in ducks for the treatment of chronic duck hepatitis B virus infection. Hepatology. 1998;28:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987;84:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 948] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 6. | Acs G, Sells MA, Purcell RH, Price P, Engle R, Shapiro M, Popper H. Hepatitis B virus produced by transfected Hep G2 cells causes hepatitis in chimpanzees. Proc Natl Acad Sci U S A. 1987;84:4641-4644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Sells MA, Zelent AZ, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988;62:2836-2844. [PubMed] |
| 8. | Schwartz BA, Gray GR. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977;181:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Zhong S, Zhang D, Wen S. [Inhibition of hepatitis B viral gene expression and replication in vitro by targeted antisense oligonucleotides]. Zhonghua Yixue Zazhi. 1995;75:392-395, 444. [PubMed] |
| 10. | Xiong Y, Jia Y, Wang H, Liu G, Ren H, Zhuo Z, Zhang D. Hepatitis B virus transgenic mice for the model of anti-hepatitis B virus drug study. Zhonghua Ganzangbing Zazhi. 2001;9:19-21. [PubMed] |
| 11. | Xiong YL, Jia YZ, Wang HM, Liu GZ, Zhang YJ. High-level hepatitis B virus expression in transgenic mice. Chuanranbing Xinxi. 2000;13:164-165. |
| 12. | Miller RH, Kaneko S, Chung CT, Girones R, Purcell RH. Compact organization of the hepatitis B virus genome. Hepatology. 1989;9:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 792] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Wu CH, Wilson JM, Wu GY. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J Biol Chem. 1989;264:16985-16987. [PubMed] |
| 15. | Lu XM, Fischman AJ, Jyawook SL, Hendricks K, Tompkins RG, Yarmush ML. Antisense DNA delivery in vivo: liver targeting by receptor-mediated uptake. J Nucl Med. 1994;35:269-275. [PubMed] |
| 16. | Nakazono K, Ito Y, Wu CH, Wu GY. Inhibition of hepatitis B virus replication by targeted pretreatment of complexed antisense DNA in vitro. Hepatology. 1996;23:1297-1303. [PubMed] [DOI] [Full Text] |
| 17. | Wu GY, Walton CM, Wu CH. Targeted polynucleotides for inhibition of hepatitis B and C viruses. Croat Med J. 2001;42:463-466. [PubMed] |
| 18. | Wu GY, Wu CH. Specific inhibition of hepatitis B viral gene expression in vitro by targeted antisense oligonucleotides. J Biol Chem. 1992;267:12436-12439. [PubMed] |
| 19. | Martinez-Fong D, Mullersman JE, Purchio AF, Armendariz-Borunda J, Martinez-Hernandez A. Nonenzymatic glycosylation of poly-L-lysine: a new tool for targeted gene delivery. Hepatology. 1994;20:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Guo J, Zhou YX, Yao ZQ, Wang SQ, Weng SM, Wang BC. Specific delivery to liver cells by asialoglycoprotein modified antisense oligodeoxynucleotides in vitro and in vivo. Zhonghua Chuanranbing Zazhi. 1997;15:16-19. |
| 21. | Dini L, Falasca L, Lentini A, Mattioli P, Piacentini M, Piredda L, Autuori F. Galactose-specific receptor modulation related to the onset of apoptosis in rat liver. Eur J Cell Biol. 1993;61:329-337. [PubMed] |
| 22. | Anderson WF. Human gene therapy. Science. 1992;256:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 450] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Walton CM, Wu CH, Wu GY. A DNA delivery system containing listeriolysin O results in enhanced hepatocyte-directed gene expression. World J Gastroenterol. 1999;5:465-469. [PubMed] |
| 24. | Zhang J, Chen F, Zhong S, Tang K, Shi X, Wang M, Peng J. Anti-HBV effect of targeted antisense RNA against HBV C gene. Zhonghua Ganzangbing Zazhi. 2000;8:169-170. [PubMed] |
| 25. | Zhong S, Wen S, Zhang D. [Inhibition of HBV gene expression by antisense oligonucleotides using galactosylated poly (L-lysine) as a hepatotropic carrier]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2001;15:150-153. [PubMed] |
| 26. | Zhoug S, Wen SM, Zhang DF, Wang QL, Wang SQ, Ren H. Sequencing of PCR amplified HBV DNA pre-c and c regions in the 2.2.15 cells and antiviral action by targeted antisense oligonucleotide directed against sequence. World J Gastroenterol. 1998;4:434-436. [PubMed] |
| 27. | Yang CQ, Wang JY, He BM, Liu JJ, Guo JS. Glyco-poly-l-lysine is better than liposomal delivery of exogenous genes to rat of liver. World J Gastroenterol. 2000;6:526-531. [PubMed] |
| 28. | Yang CQ, Wang JY, Fang GT, Liu JJ, Guo JS. A comparison between intravenous and peritoneal route on liver targeted uptake and expression of plasmid delivered by Glyco-poly-l-lysine. World J Gastroenterol. 2000;6:508-512. [PubMed] |
| 29. | Chen YP, Zhang L, Lu QS, Feng XR, Luo KX. Lactosamination of liposomes and hepatotropic targeting research. World J Gastroenterol. 2000;6:593-596. [PubMed] |
| 30. | Yang C, Wang J, Wen S, Liu J, Guo J. [Comparative studies of different carriers and introducing routes on the effects of liver targeted uptake of exogenous gene]. Zhonghua Ganzangbing Zazhi. 2000;8:227-229. [PubMed] |
| 31. | Mani SA, Harish S, Vathsala PG, Rangarajan PN, Padmanaban G. Receptor-mediated gene delivery approach demonstrates the role of 5'-proximal DNA region in conferring phenobarbitone responsiveness to CYP2B2 gene in rat liver in vivo. Biochem Biophys Res Commun. 2000;268:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Biessen EA, Vietsch H, Kuiper J, Bijsterbosch MK, Berkel TJ. Liver uptake of phosphodiester oligodeoxynucleotides is mediated by scavenger receptors. Mol Pharmacol. 1998;53:262-269. [PubMed] |
| 33. | Biessen EA, Vietsch H, Rump ET, Fluiter K, Kuiper J, Bijsterbosch MK, van Berkel TJ. Targeted delivery of oligodeoxynucleotides to parenchymal liver cells in vivo. Biochem J. 1999;340:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Morrey JD, Korba BE, Sidwell RW. Transgenic mice as a chemotherapeutic model for hepatitis B virus infection. Antivir Ther. 1998;3:59-68. [PubMed] |
| 35. | Morrey JD, Bailey KW, Korba BE, Sidwell RW. Utilization of transgenic mice replicating high levels of hepatitis B virus for antiviral evaluation of lamivudine. Antiviral Res. 1999;42:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Julander JG, Sidwell RW, Morrey JD. Characterizing antiviral activity of adefovir dipivoxil in transgenic mice expressing hepatitis B virus. Antiviral Res. 2002;55:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Edited by SuQ