Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.1051
Revised: October 23, 2002
Accepted: November 4, 2002
Published online: May 15, 2003
AIM: To compare the effects of Roux-en-Y and jejunum interposition reconstruction procedures after total gastrectomy on intestinal motility.
METHODS: Fifty male Sprague-Dawley rats were randomly divided into 5 groups: the control group (C), the laparotomy group (L), the jejunal transection group (JT) where the jejunum was transected 10 cm distal from the Treitz ligament and anastomosed, the Roux-en-Y group (RY) and the jejunal interposition group (JI) after total gastrectomy. To evaluate intestinal transit, the animals were given 0.1 ml Evans Blue solution through an orogastric tube. The rats were executed by CO2 inhalation 30 minutes later and the intestinal transmit was determined as the distance between the site of esophageojejunal anastomosis and the most distal site of small intestine colored with blue.
RESULTS: One month after operation, the body weight of rats among JI and RY were almost identical (274.6 ± 9.5 vs 270.4 ± 10.6, P > 0.05), but were significantly lighter than those of JT and L group. Four months after the operation, the body weight in the JI group increased compared to the preoperative level (345.2 ± 15.7 g vs 299.5 ± 8.3 g, P < 0.01). However, the body weight of RY group decreased compared to preoperative (255.1 ± 11.3 g vs 295.0 ± 12.0 g, P < 0.01). The difference was more significant at six months postoperative. Small bowel transmit time in RY was slower than that in JI group and C group (P < 0.01).
CONCLUSION: Changes of body weight and intestinal motility in JI group are less influenced than in RY group.
- Citation: Qin XY, Lei Y, Liu FL. Effects of two methods of reconstruction of digestive tract after total gastrectomy on gastrointestinal motility in rats. World J Gastroenterol 2003; 9(5): 1051-1053
- URL: https://www.wjgnet.com/1007-9327/full/v9/i5/1051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.1051
Total gastrectomy is one of the popular operative procedures for gastric malignancy, but there still are debates on the recommended method of gastrointestinal reconstruction after total gastrectomy. The choice of suitable method is crucial to the patients’ quality of life. To a great extent, surgeons choose the reconstruction methods according to personal experiences. We compared the prognosis of the different reconstruction methods and explored their effects on gastrointestinal motility in rats.
Fifty male Sprague-Dawley rats (weight 280-310 g) were randomly divided into 5 groups, each of which contained 10 subjects. Five groups included controlled group (C), laparotomy group (L), jejunal transection group (JT) receiving transection of jejunum at 10 cm distal to the Treitz ligament and anastomosis in situ in each rat, jejunal interposition group (JI) receiving jejunal interposition with the segment of 5 cm length after total gastrectomy, and esophageojejunal Roux-en-Y anastomosis group (RY). All rats in RY underwent anastomosis of esophagus and jejunum after total gastrectomy with the R-Y loop of 10 cm. Rats resumed to normal diet on day 3 post-surgery.
Body weight of each rat was measured before operation and 1, 4, 6 months after operation, respectively. After fasting for 12 hours (free access to water), rat underwent corresponding operation. After six months, weight and the time of intestinal transition were recorded. Each rat was given 0.1 ml Evans Blue solution (50 mg/ml, Sigma, St Louis, USA) through orogastric tube to stomach directly or esophageojejunal junction in the JI and RY groups.
Statistical analysis was performed with statistical software SPSS 10.0. The results were expressed as mean ± SD and comparisons among groups were performed by Student’s t Test, ANOVA test and q test. All tests were two sided. P values < 0.01 were considered significant.
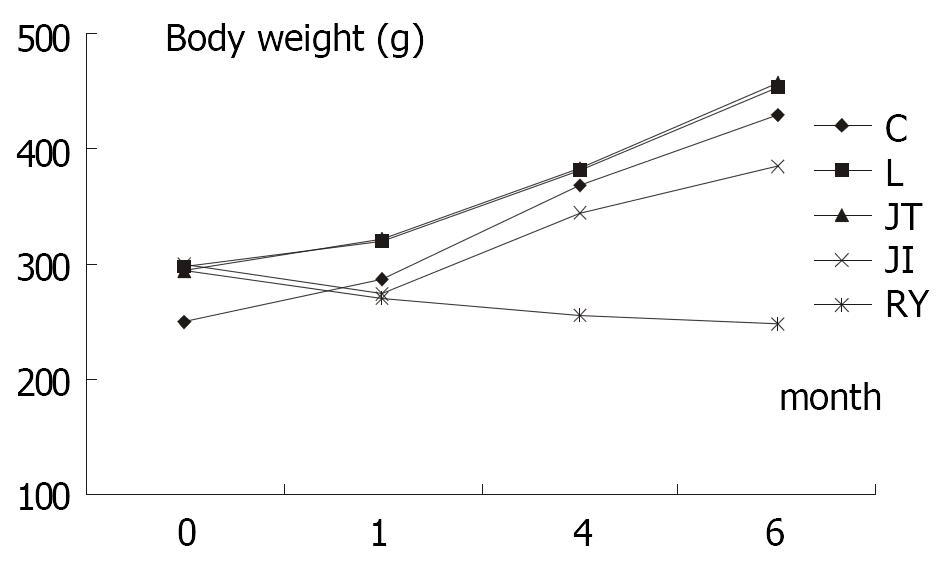
No significant difference in body weight of rats before operation was detected among L, JT, JI and RY group, though the weight of C group was significantly lower than other groups (P < 0.01) (Table 1); One month after operation (F > F(0.01,49), P < 0.01), body weight in L and JT was significantly higher than that in JI and RY, while no significant differences between L and JT group, as well as between RY and JI (P > 0.01) were not observed; At fourth month postoperatively, weight in C, L and JT group were similar, while these of JI and RY were significantly lower than those in other groups. Furthermore, the weight of RY was much lower than that of JI (P < 0.01); At 6 months after operation, rats in group C, L and JT gained weight significantly (P < 0.01). Although the weight of rats in group JI was not as much as that of group C, L and JT (P < 0.01), it still increased significantly compared to preoperative weight (385.0 ± 21.2 g vs 299.5 ± 8.3, P < 0.01). Nevertheless, the rats in group RY lost weight compared to preoperative weight (247.7 ± 13.4 g vs 295.0 ± 12.0 g, P < 0.01), and they were significantly lighter than those of other groups at 6 months after operation, including JI group (P < 0.01).
| C | L | JT | JI | RY | |
| Preoperative | 249.5 ± 10.4 | 297.5 ± 11.1 | 294.7 ± 11.9 | 299.5 ± 8.3 | 295.0 ± 12.0 |
| Postoperative 1 month | 287.4 ± 12.3 | 320.4 ± 9.3 | 322.3 ± 10.6 | 274.6 ± 9.5 | 270.4 ± 10.6 |
| Postoperative 4 month | 368.3 ± 13.5 | 381.7 ± 15.5 | 384.0 ± 19.3 | 345.2 ± 15.7 | 255.1 ± 11.3 |
| Postoperative 6 month | 430.0 ± 30.9 | 453.0 ± 32.2 | 457.0 ± 29.8 | 385.0 ± 21.3 | 247.7 ± 13.4 |
Transit time of intestine (TTI) was 24.8 ± 10.3 cm/30 min in group RY, which was significantly longer than that of group C (54.7 ± 6.7 cm/30 min) (P < 0.01). No significant differences in TTI were found among group JI, JT, L and C (Table 2).
Since Karl Schlatter first successful performed total gastrectomy in 1897, it has been mainly indicated for Zollinger-Ellison syndrome and for potentially curable gastric carcinoma[1]. Formerly, it was quite common to apply esophagus-jejunum anastomosis after total gastrectomy. Although this procedure has merit of simple, the operative complication of reflux esophagitis restricts its application[2]. To avoid the complication, many surgeons adopt Roux-en-Y anastomosis of esophagus and jejunum to reconstruct digestive tract. At first, the results were quite promising; later, many patients complained about fullness, abdominal pain, nausea, even food vomiting. Many studies indicated that the gastrointestinal motility of patients underwent Roux-en-Y anastomosis of esophagus and jejunum was obviously weakened. Such symptoms were nominated as "Roux stasis syndrome"[3-5]. Nowadays, many researches advocate jejunal pouch with Roux-en-Y reconstruction as a modified method[6].
Recently, some researchers advocate gastric substitution by interposition of jejunum. As the procedure is more accordant with physiology, it can eliminate the shortcomings of Roux-en-Y esophagojejunostomy induced by chyme bypass of duodenum[7,8]. Moreover, interposition of jejunum makes preparatory assimilation possible by mixing of chyme with bile and pancreatic fluid in duodenum. Also, such procedure has fewer disturbances on gastrointestinal motility. Our results indicated that the rats in group JI gained weight six months after operation and had similar gastrointestinal motility as control group, which means the nutrition and growth of rats were not impaired. But Miholic J measured the emptying of gastric substitute after total gastrectomy and compared the differences between jejunal interposition and Roux-y esophagojejunostomy and contended that the concept of a gastric substitute pouch was not supported[9].
Our study illuminated that the rats lost weight in group RY and group JI at one month after total gastrectomy, but rats in group C, L and JT steadily gained weight during the same time. At four months after operation, the growth curves of group RY and group JI were markedly different. After initial fall in weight of 8% in the gastrectomized rats (274.6 ± 9.5 vs 299.5 ± 8.3), a sizable rise was observed in the rats of group JI. As for RY group, the rats constantly lost weight till 6 month after gastrectomy. Our results revealed that procedure of jejunal interposition may affect the nutrition status of rats and the effect may last less than four month, which was consistent with results of other researches[10,11]. But rats among RY group still lost weight at six month after operation, and it indicated the Roux-en-Y esophagojejunostomy might influence the nutrition status of rats for at least 6 months, much longer than jejunal interposition. So it seems that the jejunal interposition is superior to Roux-en-Y esophagojejunostomy after total gastrectomy.
Rats in RY group revealed delayed transit time of intestine at six months after the operation, just as Pellegrini reported[12], which also supports that gastrointestinal motility was impaired after Roux-en-Y esophagojejunostomy. Meanwhile, rats in group C, L, JT and JI revealed similar intestinal transit time, which was significantly different from that in group RY. The result was consistent with the growth curve of body weight of rats (Figure 1). Thus slowed intestinal transit may be one main etiological factor in postgastrectomy malnutrition. But some studies did not found abnormal intestinal transit after total gastrectomy[13], more conclusive research is required.
Although no significant differences in TTI in other 4 groups were observed at six months after operation, previous study has revealed that different operative procedure and anesthesia do affect the gastrointestinal motility[14]. Although we did not measure the TTI soon after operation, our results showed that TTI could resume to preoperative level after several months for some surgical procedures. In addition to abdominal operation, prior study indicated that gastric emptying was markedly delayed after cardiac surgery[15]. Some researchers advocate that motilin may be one of possible reason for such changes[16], which is a widely distributed neuropeptide and has been located in digestive tract and brain[17]. Specific receptor of motilin has been isolated and located on chromosome[18], and is proved at mocosa[19]. And new neoropepetide has been identified and proved to be related with gastrointestinal activity[20].
In conclusion, the procedure of jejunal interposition has fewer disturbances on intestinal transit than Roux-en-Y, and the gastrointestinal motility and nutrition status of rats of group JI are much better than those of rats of group RY.
| 1. | Schrock TR, Way LW. Total gastrectomy. Am J Surg. 1978;135:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Morrow D, Passaro ER. Alkaline reflux esophagitis after total gastrectomy. Am J Surg. 1976;132:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Gustavsson S, Ilstrup DM, Morrison P, Kelly KA. Roux-Y stasis syndrome after gastrectomy. Am J Surg. 1988;155:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Schirmer BD. Gastric atony and the Roux syndrome. Gastroenterol Clin North Am. 1994;23:327-343. [PubMed] |
| 5. | Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am. 1992;72:445-465. [PubMed] |
| 6. | Iivonen MK, Mattila JJ, Nordback IH, Matikainen MJ. Long-term follow-up of patients with jejunal pouch reconstruction after total gastrectomy. A randomized prospective study. Scand J Gastroenterol. 2000;35:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Armbrecht U, Lundell L, Stockbruegger RW. Nutrient malassimilation after total gastrectomy and possible intervention. Digestion. 1987;37 Suppl 1:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Olbe L, Lundell L. Intestinal function after total gastrectomy and possible consequences of gastric replacement. World J Surg. 1987;11:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Miholic J, Meyer HJ, Kotzerke J, Balks J, Aebert H, Jähne J, Weimann A, Pichlmayr R. Emptying of the gastric substitute after total gastrectomy. Jejunal interposition versus Roux-y esophagojejunostomy. Ann Surg. 1989;210:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Morsiani E, Tassinati E, Rescazzi R, Benea G. Rapid intestinal transit and postgastrectomy malnutrition. An experimental study in growing rats. Ital J Surg Sci. 1985;15:139-144. [PubMed] |
| 11. | Miholic J, Meyer HJ, Müller MJ, Weimann A, Pichlmayr R. Nutritional consequences of total gastrectomy: the relationship between mode of reconstruction, postprandial symptoms, and body composition. Surgery. 1990;108:488-494. [PubMed] |
| 12. | Pellegrini CA, Deveney CW, Patti MG, Lewin M, Way LW. Intestinal transit of food after total gastrectomy and Roux-Y esophagojejunostomy. Am J Surg. 1986;151:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Sategna-Guidetti C, Bianco L. Malnutrition and malabsorption after total gastrectomy. A pathophysiologic approach. J Clin Gastroenterol. 1989;11:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Le Blanc-Louvry I, Coquerel A, Koning E, Maillot C, Ducrotté P. Operative stress response is reduced after laparoscopic compared to open cholecystectomy: the relationship with postoperative pain and ileus. Dig Dis Sci. 2000;45:1703-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Goldhill DR, Whelpton R, Winyard JA, Wilkinson KA. Gastric emptying in patients the day after cardiac surgery. Anaesthesia. 1995;50:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Schwarz A, Büchler M, Usinger K, Rieger H, Glasbrenner B, Friess H, Kunz R, Beger HG. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: prospective randomized clinical study. World J Surg. 1996;20:60-66; discussion 66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Huang YX, Xu CF, Liao H, Wang QL. Genesis and distribution of motilin in human fetus. China Natl J New Gastroenterol. 1995;1:37-40. |
| 18. | McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LH. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics. 1997;46:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 218] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Alcalde AI, Plaza MA, Marco R. Study of the binding of motilin to the membranes of enterocytes from rabbit jejunum. Peptides. 1996;17:1237-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Wang H, Zhang YQ, Ding YQ, Zhang JS. Localization of neurokinin B receptor in mouse gastrointestinal tract. World J Gastroenterol. 2002;8:172-175. [PubMed] |
Edited by Ren SY