INTRODUCTION
Radiofrequency ablation (RFA) was developed in 1990s and thereafter evolved rapidly as a minimally invasive technique for the treatment of primary and secondary malignant tumors in solid organs[1-25]. The fundamental principle is that alternating electric current operated in the range of radiofrequency (460-500 kHz) can produce a focal thermal injury in living tissue, the tip of the electrode conducts the current, causing local ionic agitation and subsequent frictional heat (90-110 °C). Temperatures over 60 °C produce uniform tissue coagulative necrosis, a 2-5 cm spherical thermal injury can be produced with each ablation. The potential benefits of these techniques over conventional surgical options include tumor ablation in nonsurgical candidates with low morbidity, and being used on an outpatient basis[1-15].
One of the most manifestations of liver cirrhosis with portal hypertension was hypersplenism secondary to splenic congestion with intrasplenic sequestration and destruction of erythrocytes, leucocytes and platelets resulting in anemia, leucopenia and thrombocytopenia[26]. Splenectomy is a routine therapy for hypersplenism and can correct the abnormal hematologic parameters[26,27]. However, with the awareness of the role and importance of the spleen in the immune system, splenic conservative methods have gained prominence in the treatment of benign conditions of the spleen[27-29]. Several minimally invasive treatment modalities such as transcatheter selective splenic arterial embolization[30], absolute alcohol or ethanolamine oleate intrasplenic injection[31,32] have been investigated clinically, but the clinical applications were restricted due to associated complications.
In order to establish an effective therapy for hypersplenism with splenomegaly, animal model with splenomegaly was induced by ligation of splenic vein (LSV), the computerized tomographic (CT) and pathologic changes of the thermal lesions in spleen after RFA were observed, and the feasibility and safety of RFA for secondary splenomagely and hypersplenism and its potential clinical prospective were investigated.
MATERIALS AND METHODS
Animals and experimental design
Sixteen healthy adult mongrel dogs (12 to 17 kg) were randomly divided into two groups. LSV plus Laparotomy were performed in Group I (n = 4), and LSV plus RFA were done in Group II (n = 12).
After fasting overnight, the animals were anesthetized generally with sodium pentobarbital (25 mg/kg) and maintained with supplemental doses of sodium pentobarbital. The great saphenous vein was cannulated for transfusion with 500 ml of acetate Ringer’s solution. Midline incision was used to access the spleen, splenic veins in both groups were ligated at the confluent to portal vein, and then the main branches of splenic vein were further ligated separately at the splenic hilus in order to minimize collateral formation. The animals were cared in cages at 24 ± 2 °C and fed with standard diet and water ad libitum after operation. At the end of the third week, under general anesthesia again, the animals in group I were performed laparotomy only; and both laparotomy and RFA of spleen were done in group II.
Ablation procedures
RFA was performed with the RF 2000 generator system (Radio Therapeutics Corp., CA, USA), which consists of a generator generating [pup to 90 W in power, a leVeen monopolor array needle electrode (3.5 cm maximum array diameter), and tow electrode pads (grounding pads) placed on the animal’s shaved thighs. The leVeen needle electrode is a 15-gauge, 15-20 cm in length insulated cannula containing 10 individual hook-shaped retractable electrode arms that deployed in situ after placement of the needle electrode in splenic parenchyma. The probe was inserted perpendicularly into the splenic parenchyma in lateral region of superior or inferior pole of spleen, and the spleen was elevated to avoid to contact with adjacent organs and skin. A 90 W monopolar radiofrequency generator (RF 2000, Radiotherapeutics) was used as the energy source. Power output was initially set at 30 W and manually titrated 10 W per 60 sec until the maximum power. Thereafter, impedance could rise with automatic power adjustment until power output was terminated. The electrode 0.5 cm retrograded in situ and above session repeated. The needle track was cauterized on needle removal.
During RFA procedure, the temperatures on the sites of thermal lesions, hilum of spleen and the tail of pancreas were measured frequently by thermosensors, and cold saline was used to lower the temperature on the hilum of spleen, and tail of pancreas. Five-hundred ml of acetate Ringer’s solution and 0.8 M IU of penicillin were given intravenously.
Observation of animals
The complications during RFA, such as hemorrhage, thermal injury of other organs, the appetites, eating and activities of animals, morbidity and mortality after RFA were carefully documented.
CT imaging and histopathology on splenic lesions
Splenic sizes in group I after LSV were documented with CT scans (Siemens Medical System, Germany) at 3, 6, 9 weeks after laparotomy. CT imaging (plain scan plus enhancement) performed for the dogs in group II after RFA on day 1, and at 1, 2, 4, 6 and 9 weeks after RFA to study the thermal injured lesions in spleens. Two dogs in group II were killed immediately after CT scan, and all dogs were sacrificed at the end of the 9th week. Before sacrificing the dogs, spleens were removed, specimen of liver and pancreas were harvested and fixed in 10% formaldehyde, and routine histopathological studies were processed and examined under light microscopy.
RESULTS
Animals
The RFA procedure took 30 to 58 minutes, the temperatures on 0.5 cm in depth of thermal lesion in spleens were over 100 °C as reaching to the maximum impedance. The temperatures on the hilum of spleen were 45-50 °C, the temperatures was kept below 42 °C with iced cold saline. There was a temperature gradient rising from lateral to center of splenic hilum due to blood inflow. Thus, choosing puncture site on lateral region of superior or inferior pole of spleen could enlarge the size of thermal lesions, and approximately 40%-50% of spleen could be damaged on each episode of RFA. Minor or mild bleeding from the puncture site of spleen immediately after inserting the electrode may occur and would be stopped automatically in 5 minutes due to thermal coagulation if the electrode was kept still. The animals could well tolerate the RFA, distressing in 5 dogs due to high tension of spleen capsule caused by thermal injury was resolved after administration of tranquilizer. The dogs fasted on the day of RFA were lethargic or anorexic. All dogs were fed normally after RFA. Restlessness, vomiting and anorexia, significant body weights change and complications of surgery such as gastrointestinal perforation, peritonitis, intra-abdominal hemorrhage, rupture of spleen or hydrothorax and death were not observed at all.
CT imaging of spleens
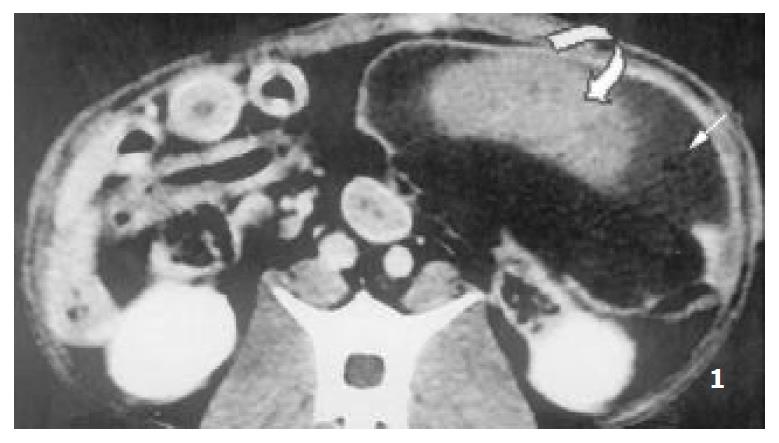
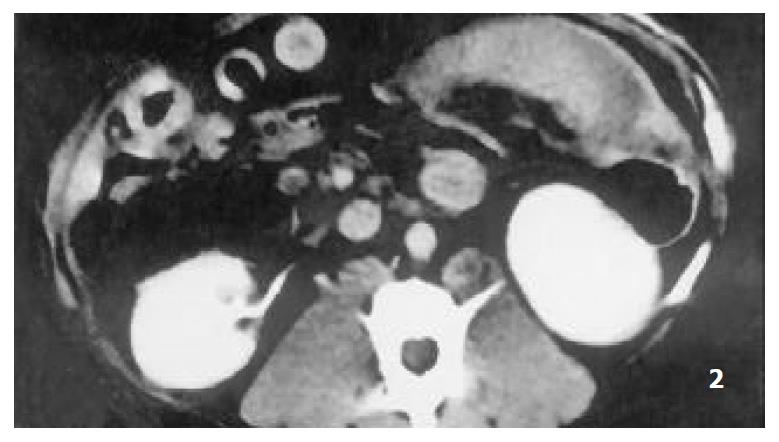
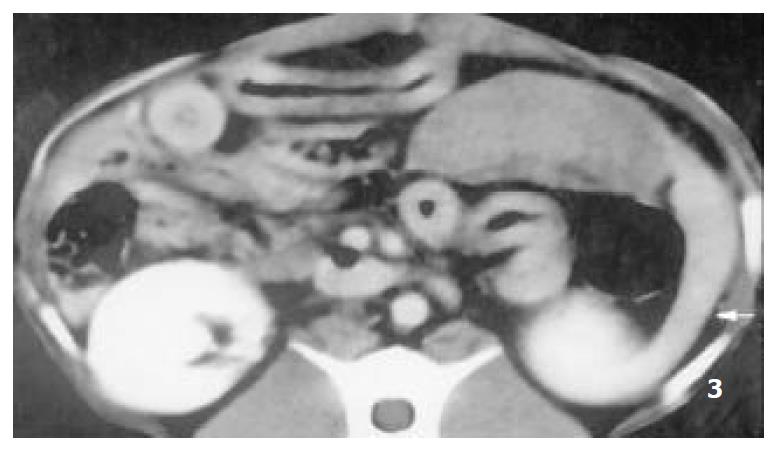
Splenomegaly in group I could persisted over 2 months. The enhanced CT revealed that each RF thermal lesion could destroy one lobe or multiple splenic segments, and the splenic capsule was intact except small part of hilum. The ablated lesion included two zones: (a) hyperintense zone of coagulative necrosis; (b) more extensive peripheral hypointense infarcted zone - the latter was called “bystander effect”. No perisplenic or splenic abcess was formed (Figure 1). The infarcted zone would be absorbed in 4 to 6 weeks after RFA, and subsequently disappeared in two months, and the size of remnant spleen shrunk significantly (Figure 2, Figure 3).
Figure 1 Enhanced CT demonstrated multiple segmental ab-lated lesion at the end of 2nd week after RFA, the splenic cap-sule was continuous, the thermal lesion included 2 zones, namely hyperintense zone of coagulative necrosis and periph-eral hypointense infarcted zone; no perisplenic or splenic abcess was seen.
Figure 2 The lesion of infarcted zone was mostly absorbed at the end of 6th week after RFA.
Figure 3 The lesion of infarcted zone was absorbed absolutely at the end of 9th week after RFA, however, the lesion of coagu-lative necrosis hardly altered, the remnant spleen shrinked sig-nificantly (arrow).
Histopathology
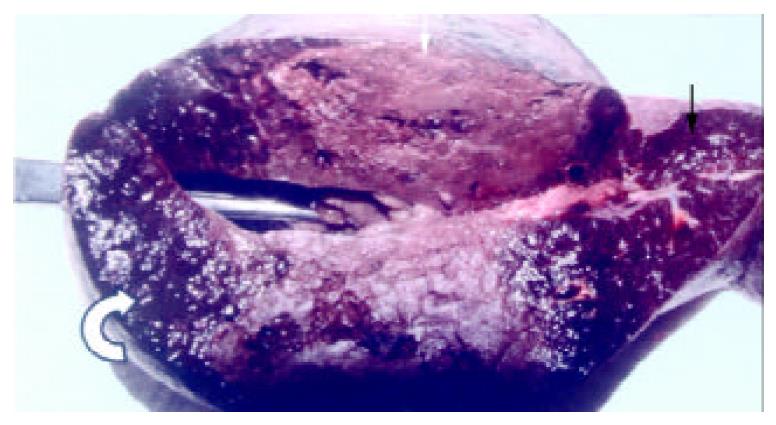
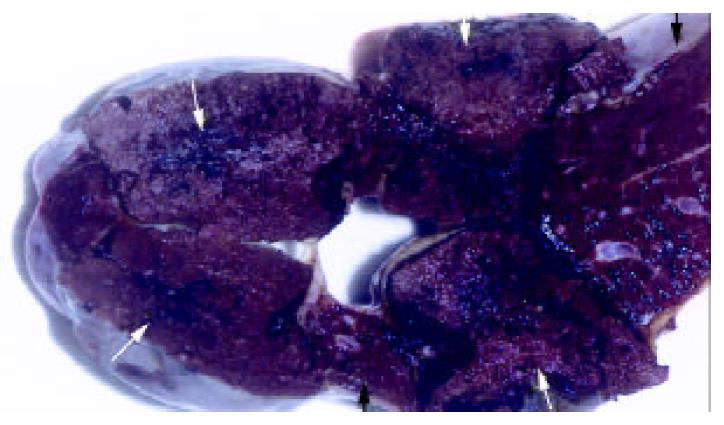
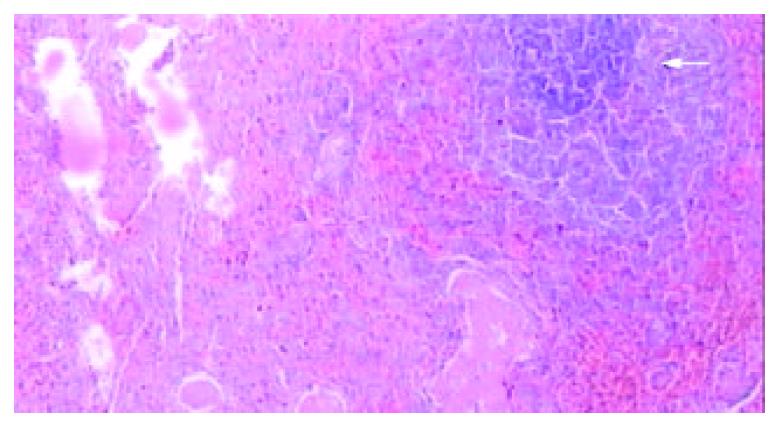
Splenomegaly in gross appearance was existed in 2 months after ligation of splenic veins. The areas of ablated lesions had dot swollen dark red appearance, the spleens shrunk significantly and approached to the normal size at the end of ninth week after RFA. The macroscopic and microscopic of liver and pancreas were normal. The omentum was adherent to the ablated surface of the spleen in most cases. The appearances of cut surface of the ablated lesions matched with the CT imaging, including the zone of soil-yellow dry necrosis and zone of thrombotic infarction (Figure 4), and the lesions became dry necrosis after the thrombotic infarction was absorbed (Figure 5). No obvious intrasplenic hemorrhage except the puncture tract was seen with microscopic examination (Figure 6). The RF energy caused focal tissue coagulative necrosis, massive vessels in the spleen were damaged thermally and occluded, consequently broad microthrombus leaded to the peripheral thrombotic infarction, which is called as bystander effect corresponding to the CT finding. Thrombotic infarction zone had absorbed by macrophages gradually in 4-6 weeks, where fibroblasts proliferation and local fibrosis were demonstrated. Even though some part of spleen appeared normal and viable, there had obvious thickened intima, occluded vessels, extensive fibrous protein deposition, and the absence of congestive splenic sinusoid, which we called as splenic carnification, the most important pathological basis of the shrunken spleen (Figure 7, Figure 8, Figure 9, Figure 10).
Figure 4 The appearance of the spleen the day after RFA, showed the lesion included the zone of soid-yellow dry necrosis (white arrow) and dark-red zone of thrombotic infarction (curve arrow), and the bright red normal spleen (black arrow); each ablation created a lesion with maximum diameter of 9 cm.
Figure 5 The lesion of infarcted zone was absorbed absolutely at the end of 9th week after RFA, however, the lesion of coagu-lative necrosis hardly altered (arrow).
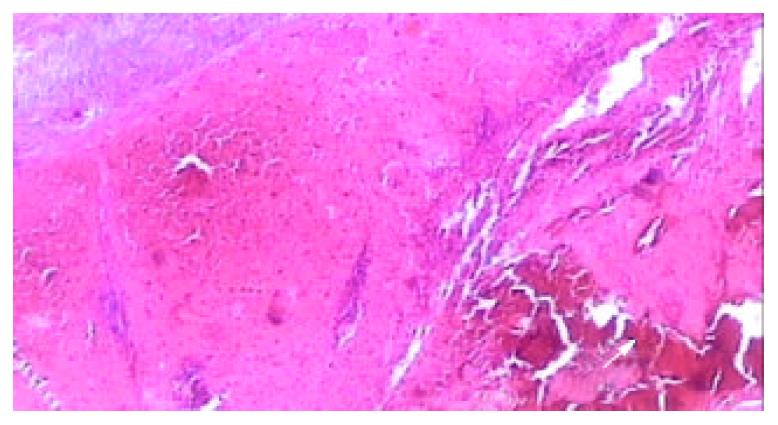
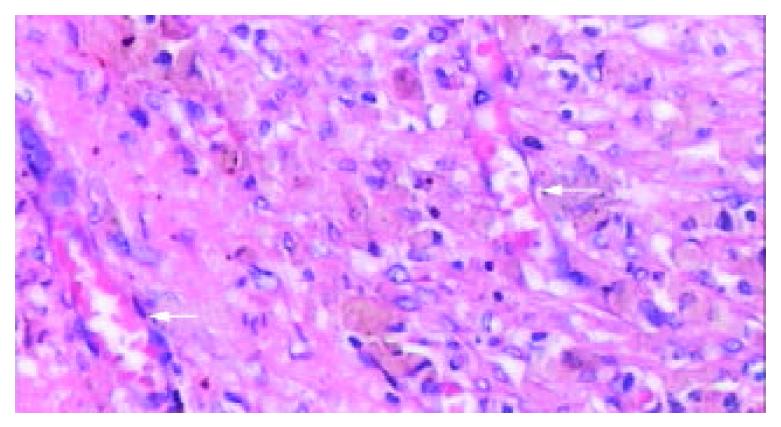
Figure 6 Light microscopic appearance of the coagulative ne-crosis at the end of 2nd week after RFA, the intrasplenic hemor-rhage at the probe insertion site could see (arrow), the splenic capsule thickened (HE.
× 40).
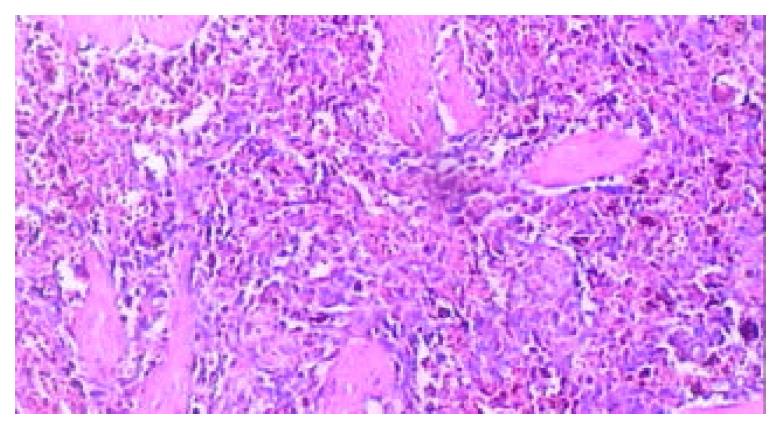
Figure 7 Microscopic examination at the junction of the ne-crotic region and infarcted region at the end of 2nd week after RFA, massive fibroblasts and inflammatory cells aggregated, the microthrombus dissolved (HE.
× 200).
Figure 8 Microscopic examination of the thrombotic infarc-tion at the end of 4th week after RFA, the microthrombus dissolved, and extensive macrophages with hemosiderin depo-sition presented (HE.
× 100).
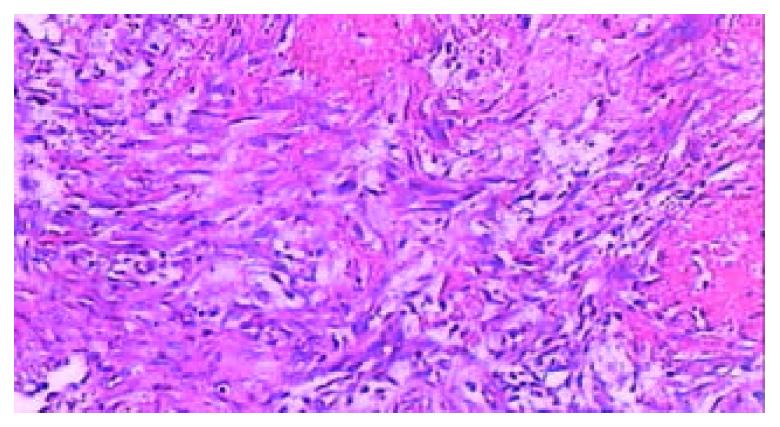
Figure 9 Microscopic appearance of “plenic carnification”of the normal viable splenic tissue distant form ablative lesion at the end of 9th week after RFA, the tissue structure consolidated, larger vessels occluded, extensive fibrous protein deposited, and the congestive splenic sinusoid disappeared; however, the struc-ture of splenic lymphoid nodule was intact (arrow) (HE.
× 40).
Figure 10 The typical appearance of poorly vascularized splenic tissue due to “plenic carnification” after RFA.
The splenic sinusoid disappeared, tissue structure consolidated, granular hemosiderin deposition and sparsely neovascularized vessels (arrow) presented clearly (HE. × 200).
DISCUSSION
Hypersplenism is one of the common manifestations, 70%-80% patients with cirrhotic hypertension present with various degrees of splenomegaly and hypersplenism. The immune function in patients with hypersplenism would be reduced due to leucopenia, thrombocytopenia and erythropenia. Splenectomy is the traditional treatment for hypersplenism. However, patients with cirrhotic splenomegaly and hypersplenism often have many complications, which are the contraindication of splenectomy. Furthermore, endoscopic ligation or sclerosing of varices for esophageal varices bleeding due to cirrhotic portal hypertension was advocated as first line treatment in western countries, splenectomy plus devascularization are commonly abandoned[33-38]. With the awareness of the role and importance of the spleen in the immune system, it is recommended to reserve the splenic tissue and function as much as in the treatment of benign conditions of the spleen. Thereby, new safe, effective and minimal invasive modalities should be established.
Radiofrequency ablation (RFA) technique is a safe and effective minimally invasive modality and has been clinically used in treatment of many malignant tumors and some benign tumors in solid organs[1-25]. Until now, only two cases in treatment of colorectal and renal splenic metastasis using RFA were reported[39,40], no data of experimental or clinical study regarding the safety and efficacy of RFA for hypersplenism is available. The safety and effectiveness may be the cardinal considerations that impede the attempts. The limited necrotic volume identical to the lesion in liver for hepatocellular carcinoma with 3-5 cm maximal diameter could scarcely reduce the size of splenomegaly, consequently could be futile to ameliorate cytopenia for hypersplenism. However, theoretically, RFA could be used for hypersplenism due to portal hypertension: (a) spleen is a solid organ, thermal energy can cause focal tissue coagulative necrosis, and the RF heat energy has the role of electric coagulation[39,40]; (b) certain time of RF thermal energy can lead to endothelial cells degeneration of vessels and sinus, thrombosis, and occlusion of vessels and splenic sinusoid, therefore produces effect of ablating larger lesion[17]; (c) puncture of spleen with needle is relative safe, the morbidity rate of biopsy reported using 18-22 gauge needles for splenic lesions including splenomegaly is 0%-5.2%, without mortality[41-43]; (d) the inflammatory reaction and neo-vascularization at the site of RFA lesion could create extensive collateral circulation between portal-cava vein systems, and reduce blood inflow to portal vein due to smaller spleen, sequentially depress the portal hypertension[28]. Clinically, we performed RFA for splenomegaly intraoperatively before splenectomy, only minor or mild bleeding occurred but stopped within few minutes (data not shown), and we found this procedure was practicable.
For these reasons, we carried out the study on RFA for splenomegaly and hypersplenism in canine models (the blood counts of peripheral samples in splenomegaly models also showed thrombocytopenia, erythropenia without leucopenia, which is similar to the result of Sahin et al[44], and the hematologic abnormalities could be corrected by RFA; data not shown), results of preliminary study confirmed part of above presumptions.
First, RFA procedure for spleen is relative safe. Although bleeding occurred immediately following inserting of electrodes into the spleen, it ceased soon due to thermal coagulation. In order to avoid the potential risks, several important steps should be followed, the puncture site must be kept away from large vessels at hilum of spleen, the electrode should be fixed to reduce bleeding; the spleen should be elevated to avoid to contact with adjacent organs and skin; cool saline should be used on the surface of the hilum of spleen, tail of pancreas to reduce energy deposition and avoid thermal damage of pancreas. In our preliminary experiment, dog’s thighs were burned due to insufficient shave of thigh and thus incomplete attachment of electrode pads; and another dog died from severe burning of internal organs, because spleen was not isolated from skin and intra-abdominal viscera. However, no morbidity and mortality occurred after procedures above in experiment were followed strictly. In addition, puncture of needle at lower pole of spleen is preferable to reduce the pulmonary complications and referred pain. If this procedure were used clinically, exposure and protection of spleen would be helpful in avoiding complications.
Second, the area of RF thermal ablated lesion is so large that compasses one lobe or multiple segments of spleen. The area of coagulative necrosis zone in spleen is similar to that of RF lesion in liver[12,25], but the RFA in spleen also has “bystander effect” - the RF energy causes multiple segmental thrombotic infarction, enables for the management of hypersplenism effectively. The RF energy triggers occlusion of broad intrasplenic microthrombi and vessels, and absorption of area with thrombotic infarction. Even in the remaining normal splenic tissue, obvious occluded vessels, extensive fibrous protein deposition with no congestion of splenic sinusoid are still existed. The changes of “splenic carnification” are responsible for the shrinking of the remnant spleen, which could explain precisely why splenic RFA ameliorated the cytopenia.
In summary, it is feasible and safe to perform RFA in spleen to treat secondary splenomegaly and hypersplenism in a canine model, and yet its clinical application is worthy of further study. The peculiar radiological and pathological splenic changes after RFA could be used as clinical indicators for therapeutic effect and the follow-up in the therapy of hypersplenism. With the development of technique, such as RFA with the minimally invasive procedures, it would provide promising effect on selected patients with hypersplenism[17].