Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.595
Revised: October 15, 2002
Accepted: October 20, 2002
Published online: March 15, 2003
AIM: To determine the significance of endoscopic surveillance in the diagnosis of acute rejection after human living-donor small bowel transplantations.
METHODS: Endoscopic surveillance was performed through the ileostomy after human living-donor small bowel transplantations. The intestinal mucosa was observed and biopsies were performed for pathological observations.
RESULTS: Acute rejection was diagnosed in time by endoscopic surveillance. The endoscopic and pathological manifestations of acute rejection were described.
CONCLUSION: Endoscopic surveillance and biopsy are reliable methods to diagnose the acute rejection after human living-donor small bowel transplantations.
- Citation: Ding J, Guo CC, Li CN, Sun AH, Guo XG, Miao JY, Pan BR. Postoperative endoscopic surveillance of human living-donor small bowel transplantations. World J Gastroenterol 2003; 9(3): 595-598
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/595.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.595
Prompt and accurate diagnosis of acute rejection is a key element to ensure the success of human small intestinal transplantation. However due to the limited cases of successful human small intestinal transplantations, little is available for the prompt and accurate diagnosis of acute rejection after the operations. Here we reported the postoperative endoscopic surveillance of acute rejection in 2 cases of human living-donor small bowel transplantations performed in our hospital. We also discussed the significance of endoscopic surveillance and pathological examination of the mucosal biopsy specimen in the diagnosis of acute rejections in this paper.
Patient1, Male, 18 years old, underwent enterectomy because of small bowel necrosis after volvulus with the residual jejunum being only 49 cm in length, had more than 10 defecations in a day with the content being undigested foods, suffered from severe malnutrition, and was diagnosed as short bowel syndrome. Living-donor small bowel transplantation was performed on the patient on May 20 1999. The donor was his father. The donor and the recipient were ABO compatible (both O type) and their HLA and HLA-DR antigen were semi-compatible. The lymphocytotoxic cross matching test showed reaction < 10%. A 150 cm segment of terminal ileum 20 cm away from the ileocecal valve was removed from the donor. The distal end of graft ileum was anastomosed to the distal end of the recipient ileum. Ileostomy was performed at the terminal end of the graft ileum.
Patient 2, Male, 15 years old, underwent resection of the most part of the small bowel and part of the cecum because of necrosis due to cecum hernia trap. The residual jejunum was only 10 cm in length. The patient defecated shortly after eating. Content of the defecations was undigested food. After 1 year and a half of parenteral nutrition, the patient developed mild liver dysfunction and was diagnosed as short bowel syndrome. Living-donor small bowel transplantation was performed on the patient on Jan 6 2001. The donor was his mother (47 years old). Both the donor and the recipient have a B type in ABO blood type. Their HLA and HLA-DR were semi-compatible. Lymphocytotoxic cross matching test showed < 10% reaction. A 160 cm segment of terminal ileum 20 cm away from the ileocecal valve was removed from the donor. The distal end of graft ileum was anastomosed to the distal end of the recipient ileum. Ileostomy was performed at the terminal end of the graft ileum.
Both patients were given immunosupressors FK506, MMF and MP together with antibiotics, anticoagulant and nutrition support. Patient 1 developed acute rejection after 67 days after operation. After implosion therapy, the rejection was taken under control, and so far, the patient has survived healthily for 30 months. Patient 2 developed acute rejection after 20 days of the operation, which was controlled after implosion therapy. However acute rejection reoccurred after 80 days of the operation together with severe infection. And the patient died after 5 months of the operation.
Endoscopic surveillance through the ileostomy was started at 15 days after operation for the patient 1, and 2 days after operation for the patient 2. Endoscopy was performed when discharge from the ileostomy increased or at the case of hemorrhage or other abnormalities. Patients took no food in 12 h before the endoscopic examination, and 1000 ml 5% glucose saline was orally taken by the patients to clear the bowels. An Olympus GIF-XQ230 gastroscope was inserted through the ileostomy into the graft bowels for examination. And for a control observation, the gastroscope was extended to pass through the stoma to observe autologous small intestine or colon. Mucosal biopsies were performed at the graft bowels and the autologous small intestine or colon. Then the pathological and microbiological examinations of biopsy specimens were done.
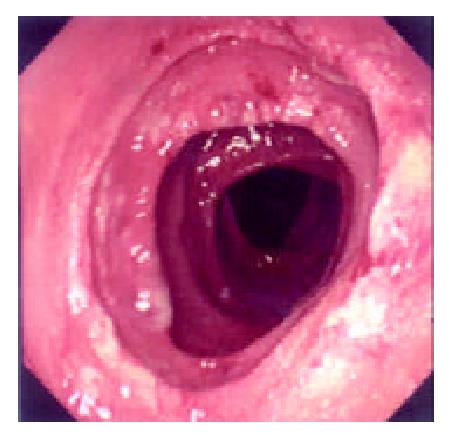
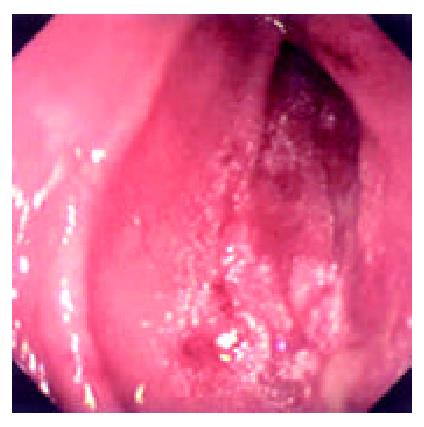
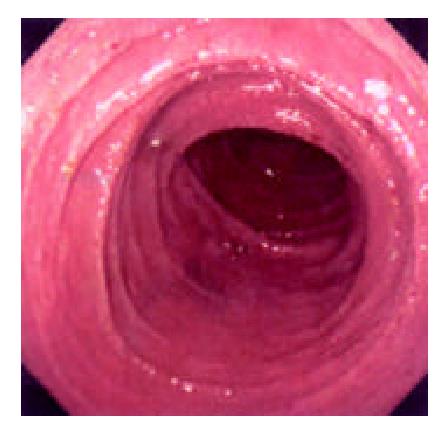
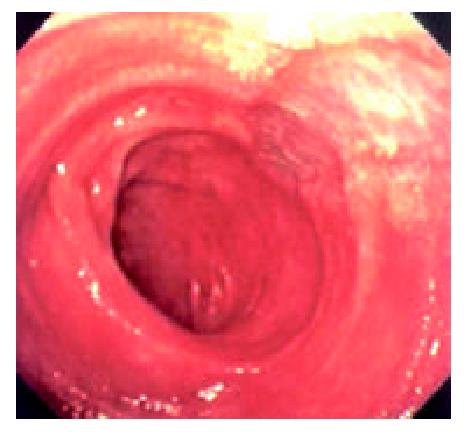
During the 48 days after the operation, 8 times of endoscopic examinations were performed on patient 1. Biopsies were performed at the same time for pathological examinations. No signs of rejection or infection were seen. After 60 days of the operation, discharge from the ileostomy was increased, and it went up to 1200 ml at the 66th day. At the 66th day, endoscopy examination was performed, and it was found that the segments of graft bowel 20-35 cm, 40-60 cm and 70-90 cm away from the ileostomy had hyperemia and edema in the mucosa displaying a strong reflection; light yellow mucus was seen on the mucosal surface; patches of mucosal hemorrhage and erosion were also observed; besides, there were several oval-shaped (0.3-0.6 cm in diameter) and linear ulcers (paralleling with the mucosal folds) in the graft bowel; the ulcers were covered with white coat (Figure 1, Figure 2, Figure 3, Figure 4). The intestinal wall was quite fragile and easy to bleed. There was much hemorrhage in the biopsy sites after the biopsy. The vermiculation of the graft became weak. The residual autologous small bowel showed no signs of hyperemia, edema, erosion or ulceration. Pathological examination of the biopsy specimen revealed that there were local erosions in the mucosa; some parts of the epithelia showed atrophy in a shape of short column; the goblet cells decreased in size or just disappeared; edema was found universally in the lamina propria; there were infiltrations of neutrophils, plasma cells and lymphocytes in the lamina propria and the muscularis mucosa; there was an increase of the number of neutrophils in the blood vessels. Microbiological examination found no abnormalities. The residual autologous bowel was normal as confirmed by pathological examination in the biopsy specimen. The patient was diagnosed as acute rejection and was treated accordingly. After 3 days of treatment, endoscopic examination was performed, and it was found that the hyperemia and edema abated a lot, the erosions healed up; the ulcers decreased in size and depth and were partly scarred over (Figure 5). Follow-up by endoscopic and pathological examinations detected no lesions thereafter.
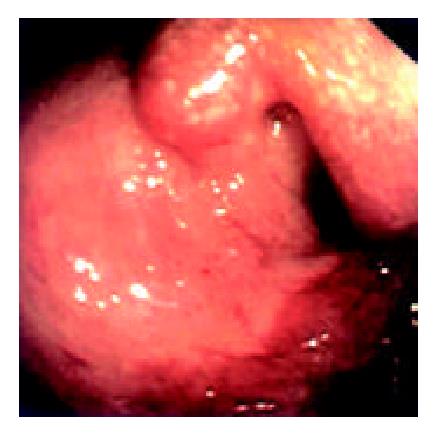
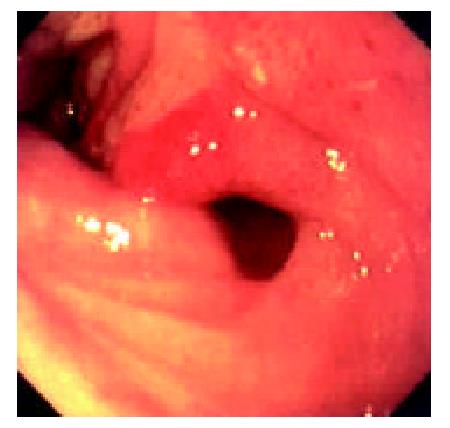
Endoscopic examinations were performed during the time from the 2nd day to the 17th day after the transplantation. No signs of rejection were detected. However, on day 20 the discharge from the ileostomy increased up to 1000 ml. Endoscopy was performed on day 22. It was found that: there was severe mucosal hyperemia and edema in the distal end of the graft bowel; patches of hemorrhage and erosions could be seen; a 0.6 × 1.2 cm oval-shaped longitudinal ulcer was seen 30 cm away from the ileostomy in the graft bowel. The ulcer was shallow and had a flat base with gray coat covering it. The autologous bowel was normal. On day 26, endoscopy was performed again and found that the ulcer was enlarged and deepened with edge raised. Circular broken plica was seen (Figure 6). Pathological exmination of the specimen from the lesions suggested that there was acute rejection. No significant changes were seen in the microbiological examination. The autologous bowel showed normal pathological features. After 9 days of anti-rejection treatment, the ulcer became smaller and shallower with no coat on it and showed partial healing (Figure 8). On day 80 after transplantation, the discharge from the ileostomy increased again. Endoscopy was performed and found that there were multiple lesions of hyperemia and edema in the graft bowel (showing strong reflection under the gastroscope); a great deal of white yellow mucus was seen on the lesions; the bowel lumen at the lesion sites became narrower; and the vermiculation of the graft bowel became weak; in the graft bowel, there were spots or patches of hemorrhage, superficial erosions, and deep round or oval ulcers varying from 0.8-2.0 cm in diameter; the edges of the ulcers showed severe hyperemia and edema. Pathological examination showed that there were ulcerations in the graft bowel; obvious edema could be seen in the lamina propria; the lamina propria and the muscularis mucosa had severe infiltration of neutriphils and lymphocytes. The patient was therefore diagnosed as severe acute rejection. Anti-rejection therapy had no effect on the patient. Follow-up by endoscopies witnessed the enlargement and deepening of the previous ulcers and the formation of new ulcers on the premise of mucosal edema and erosion (Figure 9). Ulcerative hemorrhage was also found.
The difficulty of small bowel transplantation lies mainly in the high immunogenicity of the organ. The small bowel is rich in lymphocytes and dendritic cells (DC), especially in the Peyer’s patches, lamina propria and the mesenteric nodes. The DC cells have been reported of great importance in the host’s rejection to liver graft[1]. All of the mentioned features of small bowel present a formidable challenge to small bowel transplantation. The major impediment to success of small bowel transplantation is the rejection, which will lead to failure if it is severe. Even though there have been some improvements in the technique of small bowel transplantation, both in human[2] and animal model[3], the rejection remains the major cause of failure in transplantation. Therefore, prompt and accurate diagnosis and treatment of rejection is the crux for successful transplantation. Pathological examinations of the mucosal biopsy specimen of the graft serve as the most important and fundamental method in current clinical diagnosis of rejection, for it can well show the characteristics of the rejection and its degree. The biopsy specimen can be openly taken through the stoma or under the endoscopic surveillance. However, typical lesions can not be easily taken by open biopsy, and erosions and ulcerations are often induced near the stoma due to repeated biopsies. Endoscopic surveillance has a good view of the mucosa of the graft bowel and biopsy specimen can be taken precisely at the lesion sites. Now it has been accepted as the most reliable conventional method for surveillance.
Living small bowel transplantation has high tissue compatiblity and can reduce the frequency of rejection or its severity. Novel immunosuppressor agents such as FK506 can effectively suppress rejection after transplantation. Living-donor small bowel transplantation was first reported by Deltz et al[4]. Since then, there have been several reports of living small bowel transplantations reported, and most of the transplantations in 1990’s were successful[5]. Compared with cadaveric bowel transplantation, living-donor small bowel transplantation has a lower rate of rejection and infection[6]. But acute rejection is not uncommon in living small bowel transplantation[7,8], and if the rejection is severe, it can cause loss of the graft or death of the patients. So, the surveillance, prevention and treatment of the rejection should be paid attention to all the time after the small bowel transplantation. Some indices of enteral function and biochemistry and immunology have been used in immune surveillance after small bowel transplantation in experimental animal, but most of them are under investigation and show no values of clinical practice. The recognition and diagnosis of the rejection are mainly depended on clinical observation, endoscopy and pathological examination. Rejection has no clinical characteristics for diagnosis; the biopsy specimen is not always a mirror of the situation of rejection; and for definite diagnosis of rejection, the specimen should cover all the layers of the intestinal wall, but the biopsy can easily cause severe complications such as perforation. Therefore, endoscopic examination is the most important method for posttransplant surveillance and diagnosis of rejection.
Most of the living-donor small bowel transplantations were staged operations, and there was an ileostomy often left for postoperative observation. The graft bowel and the discharge could be observed directly through the ileostomy. And the stoma also provided a passage for the endoscopic surveillance and mucosal biopsy. The endoscopy plays an important role in the assessment of the graft bowel, and in 1999, Kato et al[9] reported the first case of using zoom videoendoscope to evaluate graft bowel mucosa in human intestine transplantation. This method was further proved to be effective to determine the severity of acute cellular rejection and to be able to minimize the times of biopsies[10]. In this report, with a gastroscopy, we successfully performed endoscopic examinations and mucosa biopsies for 39 times through the stoma in these 2 patients. No complications were observed, which suggested that endoscopy and biopsy are safe and convenient methods for the surveillance of the graft bowel. As for the timing of the endoscopic surveillance, the frequency of endoscopy should be generally 1 or 2 times every week during the first 3 months after the operation. Rejections and other complications of small bowel transplantation such as hemorrhage and thrombosis in mesentry occur most often during the initially several days after the transplantation. So we consider that it is proper to perform endoscopic examination everyday in the first 3 days, and afterwards, the frequency of endoscopy be reduced to once every 2 or 3 days and the intervals between two endoscopies can be prolonged gradually in the following 2 or 3 weeks. Emergency endoscopic examinations should be taken in case of intestinal bleeding and increased discharge from the stoma, etc. As results shown by the study of Sigurdsson and his colleagues, the endoscopy was sensitive enough to diagnose only 63% of the rejections[8]. We think it necessary to perform biopsies at the same time. As the rejections have a high anatomic variability in the graft bowel[11], we recommend that the specimen should be taken at multiple sites in the graft bowel, and residual autologous small should bowel be sampled for the control. If necessary, the specimen should undergo microbiological examination to exclude lesions caused by infection.
Acute rejections in human small bowel transplantation often occur early after operation, especially during the first 30 days[12], but also may happen late beyond the first year after transplantation. Generally, clinical features of rejection show as fever, nausea, vomiting, abdominal pain, diarrhea and increased discharge from the stoma. Under endoscopic observation, mucosal hyperemia and edema, fragile intestine wall, erosion, ulceration and hypoperistalsis can be found. Uleration always suggests the onset of acute rejection. Pathological changes vary with the severity of the rejection. At the early stage, microvillus may become blunt, the goblet cell may disappear, and there may be infiltration of inflammatory cells. And then, there may be crypt inflammation, increased apoptotic cells, and in cases of severe rejection, they can be mucosa hemorrhage, patchy mucosal exfoliation and formation of small abscesses.
When the discharge from the stoma increased in the 2 patients taking small bowel transplantation in our hospital, the manifestations mentioned above were observed by endocopic surveillance. Combining the endocopic and pathological findings together, the acute rejection was diagnosed. After the pulse therapy, the lesions of acute rejection abated or disappeared, which confirmed the diagnosis of acute rejection. Endoscopic surveillance is significant for diagnosing rejection and determining the outcomes of corresponding treatment. According to our experience, when there are mucosal hyperemia, edema, hemorrhage and erosions, they should be regarded them as precautions for rejection; if there are ulcers, it often means the onsets of rejections, in addition to the pathological findings, a prompt diagnosis is warranted; improved situations of the patients and healing ulcers suggests the validity of anti-rejection therapy, while no amelioration, enlargement and deepening of ulcers, hemorrhage or formation of new ulcers are indicators of invalidity of anti-rejection treatment and progress of rejection.
So far, there are no standard criteria for the diagnosis of rejection after human small transplantation, and little is known about the endoscopic characteristics of rejection and pathological changes. But more detailed standard of endosopic surveillance and pathological examinations will be set with more cases of human small bowel transplantation performed.
| 1. | Xu MQ, Yao ZX. Functional changes of dendritic cells derived from allogeneic partial liver graft undergoing acute rejection in rats. World J Gastroenterol. 2003;9:141-147. [PubMed] |
| 2. | Zhang WJ, Liu DG, Ye QF, Sha B, Zhen FJ, Guo H, Xia SS. Combined small bowel and reduced auxiliary liver transplantation: case report. World J Gastroenterol. 2002;8:956-960. [PubMed] |
| 3. | Wu XT, Li JS, Zhao XF, Zhuang W, Feng XL. Modified techniques of heterotopic total small intestinal transplantation in rats. World J Gastroenterol. 2002;8:758-762. [PubMed] |
| 4. | Deltz E, Schroeder P, Gundlach M, Hansmann ML, Leimenstoll G. Successful clinical small-bowel transplantation. Transplant Proc. 1990;22:2501. [PubMed] |
| 5. | Margreiter R. Living-donor pancreas and small-bowel transplantation. Langenbecks Arch Surg. 1999;384:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Cicalese L, Rastellini C, Sileri P, Abcarian H, Benedetti E. Segmental living related small bowel transplantation in adults. J Gastrointest Surg. 2001;5:168-172; discussion 173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Uemoto S, Fujimoto Y, Inomata Y, Egawa H, Asonuma K, Pollard S, Tanaka K. Living-related small bowel transplantation: the first case in Japan. Pediatr Transplant. 1998;2:40-44. [PubMed] |
| 8. | Sigurdsson L, Reyes J, Putnam PE, del Rosario JF, Di Lorenzo C, Orenstein SR, Todo S, Kocoshis SA. Endoscopies in pediatric small intestinal transplant recipients: five years experience. Am J Gastroenterol. 1998;93:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kato T, O'Brien CB, Nishida S, Hoppe H, Gasser M, Berho M, Rodriguez MJ, Ruiz P, Tzakis AG. The first case report of the use of a zoom videoendoscope for the evaluation of small bowel graft mucosa in a human after intestinal transplantation. Gastrointest Endosc. 1999;50:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Sasaki T, Hasegawa T, Nakai H, Kimura T, Okada A, Musiake S, Doi R. Zoom endoscopic evaluation of rejection in living-related small bowel transplantation. Transplantation. 2002;73:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Sigurdsson L, Reyes J, Todo S, Putnam PE, Kocoshis SA. Anatomic variability of rejection in intestinal allografts after pediatric intestinal transplantation. J Pediatr Gastroenterol Nutr. 1998;27:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sudan DL, Kaufman S, Horslen S, Fox I, Shaw BW, Langnas A. Incidence, timing, and histologic grade of acute rejection in small bowel transplant recipients. Transplant Proc. 2000;32:1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Edited by Xu XQ