Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.590
Revised: October 28, 2002
Accepted: November 4, 2002
Published online: March 15, 2003
AIM: Initial report on the in situ examination of the mRNA expression of transforming growth factor betas (TGFβs), TGFβ type II receptor (TβRII) and telomerase activity in the experimental rat liver tissue during cholangiocarcinogenesis.
METHODS: Rat liver cholangiocarcinogenesis was induced by 3’-methyl 4-dimethylazobenzene (3’Me-DAB). In situ hybridization was used to examine the TGFβs) and TGFβ type II receptor (TβRII) mRNA, in situ TRAP was used to check the telomerase activity in the tissue samples.
RESULTS: There was no TGFβs, TβRII mRNA expression or telomerase activity in the control rat cholangiocytes. The expression of TGFβ1, TβRII was increased in regenerative, hyperplastic, dysplastic cholangiocytes and cholangiocarcinoma (CC) cells. The expression of TGFβ2 mRNA was observed in only a part of hyperplastic, dysplastic cholangiocytes. TGFβ3 expression was very weak, only in hyperplastic lesion. There was positive telomerase activity in the regenerative, hyperplastic, dysplastic cholangiocytes, and CC cells. Stroma fibroblasts of these lesions also showed positive TGFβs, TβRII mRNA expression and telomerase activity.
CONCLUSION: There were TGFβs, TβRII expression and telomerase activity in hyperplastic, dysplastic cholangiocytes, cholangiocarcinoma cells as well as in stroma fibroblasts during cholangiocarcinogenesis. Their expression or activity is important in cholangiocarcinogenesis andstroma formation.
-
Citation: Lu JP, Mao JQ, Li MS, Lu SL, Hu XQ, Zhu SN, Nomura S.
In situ detection of TGF betas, TGF beta receptor II mRNA and telomerase activity in rat cholangiocarcinogenesis. World J Gastroenterol 2003; 9(3): 590-594 - URL: https://www.wjgnet.com/1007-9327/full/v9/i3/590.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.590
Transforming growth factor beta (TGFβ)-TGFβ receptor (TβR) signaling system is important in growth regulating carcinogenesis and cancer progression[1,2]. Lacks of the expression of TGFβ and/or TβR, mutation of the related genes were reported in human and animal malignancies[3-5]. These abnormalities were considered to be the cause of interruption of the growth signal from the TGFβ to the cell nuclear, resulting in the uncontrolled growth of the involved cells. While there are reports on the efficacy of TGFβ which supported that TGFβ was involved in cancer invasion and metastasis[1,2,6].
Hepatocellular carcinoma (HC) and intrahepatic cholangiocarcinoma (CC) are two most common types of liver carcinoma[7,8]. Though there are reports on their expression in HC[9,10], few reports dealed with the expression of TGFβs and/or TβR in CC. Bile ducts had increased expression of TGFβ1 in inflammatory or obstructive lesions[11]. TGFβ1 was detected in small amount of cancer cells among 25 of 30 CC cases[12]. The expression and significance of TGFβs and their receptors during cholangiocarcino-genesis are poorly understood. Stroma fibrosis is one of the characteristics of CC[7,8], but the mechanism of excessive stroma formation is not clear.
Telomerase is a key enzyme in the maintenance of the telomeric DNA length[13]. Telomerase is undetectable in most normal somatic tissues. Its activity was reported in most cancer cell lines as well as cancer tissues from human and experimental animals[13,14]. mRNA of telomerase and telomerase-associated protein were detected in CC and its preneoplastic lesions[15]. It is not clear in which stage the telomerase is activated during cholangiocarcinogenesis.
Liver carcinogenesis was induced by feeding rats with 0.05% 3’-methyl 4-dimethylazobenzene (3’Me-DAB) in maize flour. The model showed progressive changes from degeneration, necrosis, to cholangiocyte hyperplasia, dysplasia and CC[16]. We found obviously increased expression of TGFβ and TGFβ type II receptor (TβRII) mRNA and telomerase activity in the proliferative, dysplastic cholangiocytes, CC cells as well as stroma fibroblasts. Here we report these findings and discuss their significance.
Male Wistar rats (n = 100, weighing 65 ± 10 g) and foods were purchased from the Shanghai Experimental Animal Center of Chinese Academy of Science. All rats received humane care.
3‘Me-DAB was purchased from the Tokyo Kasai Co. Ltd. (Tokyo, Japan Cat. 0207). DIG RNA labeling kit (Cat. No. 1175025), DIG nucleic acid detecting kit (Cat. No. 1175041), and Telomerase PCR ELISA kit (Cat. No. 1854666) were bought from Roche, Germany. Mouse anti-proliferating cell nuclear antigen (PCNA), goat anti-vimentin and biotinylated secondary antibodies were purchased from DAKO. ABC Kit was the product of Vector.
Alcian blue (8GS) was the product of Chroma. Sirius red (F3B) was from Sigma. All other reagents were of analytical or molecular biology research grade from Sigma, Merck or Shanghai Analytical Chemical Co.
The rats were divided into Experimental (n = 60) and Control (n = 40) Groups randomly and fed with common compound food and tap water during the first week of adaptation. Maize flour containing 0.05% 3’Me-DAB was prescribed to the Experimental Group rats for 12 weeks to induce liver cancer. The Control Group rats were fed with maize flour only for the 12-week-period. Common compound food was given to all rats after the period. The rats were sacrificed under anesthetization from 4-week to the end of 22-week since 3’Me-DAB feeding.
The liver tissues with macroscopic lesions were sampled. Samples from half of the lesions were fixed in 4% buffered paraformaldehyde, embedded in paraffin for routine H.E, histochemical staining, immunohistochemistry and in situ hybridization. Samples from residual half of the lesions were embedded in OCT compounds, snap frozen, and cryostat section for histochemical staining and in situ TRAP reaction. H.E. alcian blue, PAS and sirius red staining were undertaken. The liver lesions were classified into not obvious, hyperplastic or cholangial proliferative, dysplastic proliferative foci and cancer[7,8,14]. The cholangial property of the cells in the lesion was confirmed by positive mucin staining with either serum albumin mRNA expression, or cytoplasmic glycogen.
Plasmids containing cDNAs of TGFβ1, 2, 3; TβRII and serum albumin (SA) were proliferated in E. Coli. The plasmids were extracted, purified and linearized with specified endonucleases (Table 1). Anti-sense and sense cRNAs were then made and labeled with digoxigenin in vitro[17].
| Probe | Vector | Endonuclease and promotor | Length of cDNA | |
| cRNA (-) | cRNA (+) | |||
| TβR II | pBluescript II KS(-) | EcoR I T3 | Hind III T7 | 485 bp |
| TGFβ1 | pBluescript II KS(-) | XhoI T3 | Hind III T7 | 400 bp |
| TGFβ2 | pGEM 3ZF(-) | Hind III T7 | EcoR I SP6 | 500 bp |
| TGFβ3 | pGEM 3ZF(-) | BamH I T7 | EcoR I SP6 | 280 bp |
| SA | pBluescript II KS(-) | HindI II T3 | EcoR I T7 | 620 bp |
Paraffin embedded tissue samples were sectioned (5 μm). The sections were deparaffinized in serial xylene and alcohol solvents, transferred into 100 mM PBS (pH7.4) and digested with proteinase K. The sections were pretreated with 4% buffered paraformaldehyde, PBS, 200 mM HCl, 100 mM TEA-HCl buffer (pH8.0), 100 mM TEA·0.25% anhydrous acetate, PBS and further dehydrated with serial alcohol. Pre-warmed hybridization solution containing digoxigenin labeled probe was dropped on the pretreated sections, covered with parafilm and incubated in wet chamber for 15 hours at 50 °C. After hybridization, the sections were washed in 5 × SSC, 2 × SSC with 50% formamide and TNE solutions. Non-hybridized probe was digested with RNAse A. Digoxigenin labeled probe was detected with alkaline phosphatase labeled anti-digoxigenin antibody and visualized with NBT-BCIP substrate[17,18]. Some of the sections were further counterstained with eosin, alcian blue, and /or hematoxylin.
Addition of SA anti-sense probe was used as positive control, sense probes were used as negative controls.
Liver tissues embedded in O.C.T. compounds were sectioned (10 μm), air-dried shortly for further processing. The in situ TRAP was performed as reported[14,19]. Briefly, the elongation and PCR mixture was dropped onto cryostat sections and incubated in wet chamber for 30 min at 30 °C. Telomerase was inactivated at 94 °C for 5 min. The elongated telomere sequence was amplified within GeneAmp in situ PCR System 1000 (Perkin-Elmer Co. Foster City, CA 94404) for 30 cycles. Each cycle included: 94 °C for 30 sec, 50 °C for 30 sec, 72 °C for 20 sec. Last cycle was followed by 72 °C for 10 min. The sections were then washed with washing buffer and fixed with 4% buffered paraformaldehyde.
The sections were further treated with digoxigenin labeled probes, peroxidase labeled anti-digoxigenin antibody and coloration substrate to show the products of amplification. The reaction products were directly photographed before the addition of stop solution. Negative controls included: elongation after inactivation of telomerase, no probe, no antibody or substrate only control.
Paraffin sections were routinely deparaffinized and transferred to PBS. PCNA affinity to antibody was recovered by microwave oven treatment of the sections in 10mM TAE. Immunohistochemical detection of PCNA and vimentin was performed following routine procedure[20].
Alcian blue and sirius red staining were undertaken on paraffin sections. PAS staining was carried out on paraffin as well as frozen sections.
The experiment was undertaken on at least 6 rats from different period of carcinogenesis with lesions of regeneration, hyperplasia, dysplasia and carcinoma foci separately. The experiments on the same sample were duplicated to ensure the results.
The Control Group rats showed no obvious pathologic changes. There was no detectable expression of TGFβ1, 2, 3, TβRII mRNA in the cholangiocytes and bile duct cells from the control rat liver. There was a zonal expression of SA in hepatocytes, stronger at zone 1 and weaker at zone 3. Neither telomerase activity, nor PCNA reaction was detected in the cholangiocytes and bile duct cells. The stellate cells of the sinus were positive to vimentin.
There were successive histologial changes in the liver tissue samples in Experimental Group rats: from degeneration and necrosis, regeneration and proliferation, hyperplasia and dysplasia, to carcinoma.
At the early stage, there were massive degeneration and necrosis of the liver tissue samples. No TGFβ1, 2, 3, TβRII expression or telomerase were detected in the degenerative and necrotic liver tissue samples.
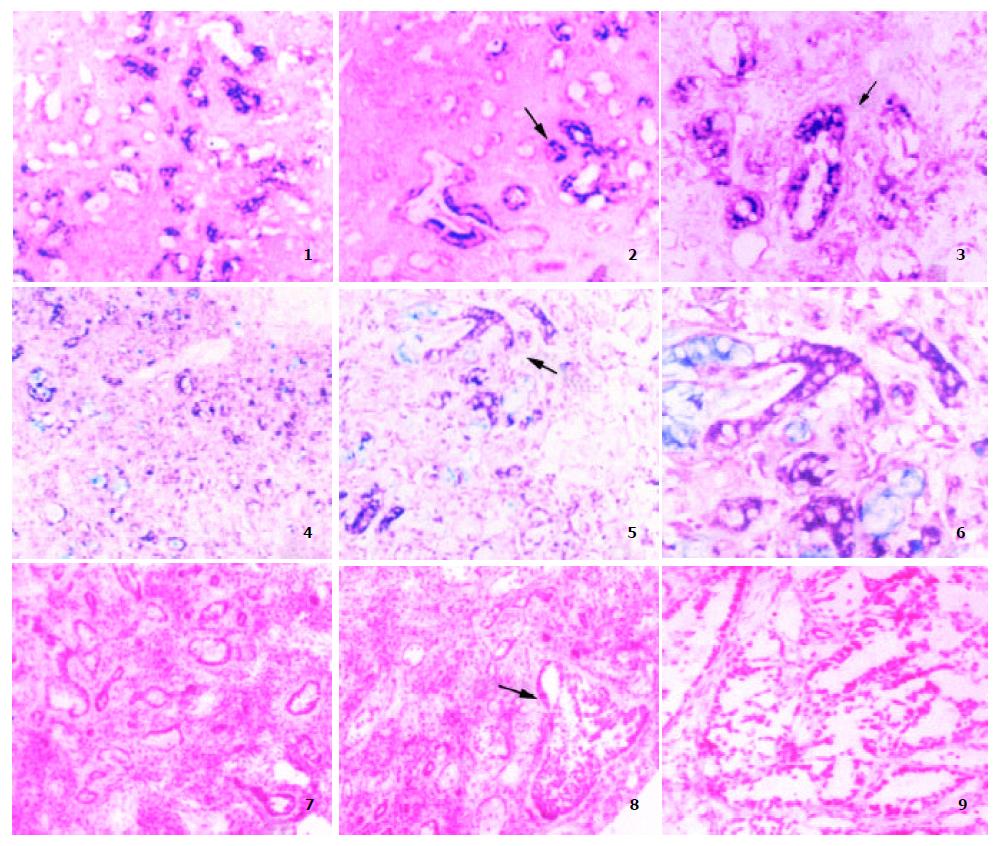
Later, regeneration and proliferation of cholangiocytes and hepatocytes were observed. Early in the regenerative and proliferative lesion, there were epithelial cells with edematous stroma. The epithelial cells were scattered in small clusters or forming cell cords, sometimes with lumen in the cords. When the cells differentiate toward cholangiocytes, the cytoplasm of the cells became basophilic without SA or glycogen. There was mucus accumulation in the cytoplasm or in some of the lumens. These cells showed positive TGFβ1 mRNA expression (Figure 1(1-2)). Telomerase activity and PCNA positive nucleus appeared in these epithelial cells.
The proliferation of cholangiocytes continued but the edema of the stroma reduced with time, while vimentin positive fibroblast proliferation appeared in stroma followed by deposition of collagen. At this stage, the cholangiocytes and fibroblasts expressed high level of TGFβ1, 2 and TβRII mRNA (Figure 1(3-4)). These cells were also positive to PCNA and telomerase reactions (Figure 1(7)). TGFβ3 can also be detected transiently in some cholangiocytes. The lesion developed into cholangiocyte hyperplasia with stroma fibrosis (Figure 1(4, 7)).
Later, the cholangiocytes in some areas disappeared with the maturation of fibroblasts to fibrocytes and increased deposition of collagen forming a "burnt-out" picture.
In other areas, the cholangiocytes kept growing with atypical cell morphology, forming irregular cellular clusters, and abortive tubular or glandular structures, indicating cholangiocyte dysplasia. Some of them may accompany with CC. The dysplasia was first found in the liver tissues after 12 weeks of 3’Me-DAB treatment. Small foci of CC appeared in the liver lesion at the 16th to 20th week of experiment.
There was mucin in the cytoplasm of the dysplastic cholangiocytes, CC cells or in the lumen formed in the cell clusters. The expressions of TGFβ1 and TβRII mRNA in the dysplastic cholangiocytes and CC cells differed greatly from negative to strong positive among different cells and different cell clusters (Figure 1(2, 5, 6)). TGFβ2 mRNA expression was also uneven in the dysplastic lesions (Figure 1(3)). TGFβ3 expression was undetectable. Most of the dysplastic cholangiocytes and cancer cells showed telomerase activity (Figure 1(8-9)). Strong PCNA positive reaction was observed in the hyperplastic, dysplastic cholangiocytes and CC. The stroma was abundant with proliferative fibroblasts (PCNA and vimentin positive) and collagen deposition. The fibroblasts had positive TGFβ1, TbRII mRNA expression and telomerase activity.
The liver tissues from our carcinogenesis model had lesions from cholangiocyte hyperplasia, dysplasia to CC with positive mucin staining with neither albumin mRNA, nor glucagon in the cytoplasm.
TGFβ is well known for its effects on fibroblasts which can induce formation of stroma[1,2,21]. But there is no report on the expression of TβR during experimental cholangiocarcinogenesis. We observed the expression of TGFβ1 and TβR II expression in the fibroblasts of regenerative, dysplastic cholangiocyte lesions and in CC. There was increased fibrous stroma formation around the fibroblasts and fibrocytes. These results supported the function of TGFβ-TβR II system in the excessive stroma formation in these lesions.
Present experiment showed that there was no TGFβ1, 2, 3 and TβRII expression in normal bile duct cells. TGFβ1, 2, 3 and TβRII mRNA expression was detected in the repairing and proliferative cholangiocytes. In the dysplastic cholangiocytes and CC cells, their expression varied from negative to strong positive. TGFβ1 protein was also detected in experimental rat and human CC cells[21-23]. TGFβ1 can suppress the proliferation of epithelium, prevent epithelial carcinogenesis[1,2]. On the other hand, there are reports that TGFβ can not inhibit the cancer growth or even accelerate the cancer invasion[2,6]. TGFβ can suppress the growth of the normal bile duct cell but not the CC cells[12]. Transgenic mouse with TGFβ1 over expression accelerates hepatocarcinogenesis[24]. Dominant-negative TβR II mice had accelerated carcinogenesis[25]. Our results showed that TGFβ and TβRII expression accompanied with the cholangiocarcinogenesis procedure.
Cancer progress is related to the reaction between cancer cells and its stroma[26]. Treatment of Ras-transformed mammary epithelial cells with TGF-beta results in resistant to growth inhibition by TGF-beta. These cells start to secrete TGF-beta, leading to maintenance of the invasive phenotype. The action is dependent on epithelial-stromal interaction[27]. Our results showed that there was TGFβ1 and TβRII expression in the dysplastic cholangiocytes, CC cells and stroma fibroblasts. Thus, the paracrine and autocrine functions of TGFβ1 are important in supporting the process of cholangiocarcinogenesis.
The expression of TGFβ2 mRNA was only detected in part of hyperplastic, dysplastic cholangiocytes. TGFβ3 mRNA was only weakly positive in some hyperplastic cholangiocytes. There is few reports on the expression of TGFβ2, 3 mRNA in the process of cholangiocarcinogenesis. Their role may be transient.
Phase of telomerase activation during cholangiocarcinogenesis is not specified. Present experiment showed that normal bile duct cells were telomerase negative. There was telomerase activity in the regenerative, hyperplastic, and dysplastic cholangiocytes as well as CC cells. The activation of telomerase occurred in the early stage of cholangio-carcinogenesis. There were also reports on the positive hTR and TP1 mRNA expression in intrahepatic biliary dysplasia[28]. Increased telomerase activity was reported in dysplastic hepatocytes during hepatocellular carcinogenesis[29].
The expression of TERT can induce resistance to TGFβ growth inhibition[30]. This may be another reason for the hyperplastic, dysplastic cholangiocytes and CC cells escaping from TGFβ- TβR growth suppression in our cholangiocarcinogensis model.
The telomerase activity is a marker of immortalized or malignant cells[13,14,31]. In present experiment, telomerase was positive in the proliferating cells no matter they were parenchyma or stroma cells. The phenomenon was observed in other liver proliferative lesions[14,32]. So that telomerase activation was also a good marker of cell in proliferation.
In summary, this is the first report on the in situ detection of TGFβ1, 2, 3, TβRII mRNA and telomerase activity during rat cholangiocarcinogenesis. There is TGFβ1, 2, 3, TβRII mRNA and telomerase activity in the hyperplastic, dysplastic cholangiocytes, CC cells as well as stroma fibroblasts. There is gradual increase of the fibrous stroma (fibrosis) during the development of CC. It is considered that the expression of TGFβ1, 2, 3, TβRII and telomerase activation has important implication in cholangiocarcino-genesis and cancer stroma formation.
| 1. | Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2875] [Cited by in RCA: 2940] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 2. | Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1791] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 3. | Francis-Thickpenny KM, Richardson DM, van Ee CC, Love DR, Winship IM, Baguley BC, Chenevix-Trench G, Shelling AN. Analysis of the TGF beta functional pathway in epithelial ovarian carcinoma. Br J Cancer. 2001;85:687-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Jonson T, Albrechtsson E, Axelson J, Heidenblad M, Gorunova L, Johansson B, Höglund M. Altered expression of TGFB receptors and mitogenic effects of TGFB in pancreatic carcinomas. Int J Oncol. 2001;19:71-81. [PubMed] |
| 5. | Paterson IC, Matthews JB, Huntley S, Robinson CM, Fahey M, Parkinson EK, Prime SS. Decreased expression of TGF-beta cell surface receptors during progression of human oral squamous cell carcinoma. J Pathol. 2001;193:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Xiong B, Yuan HY, Hu MB, Zhang F, Wei ZZ, Gong LL, Yang GL. Transforming growth factor-beta1 in invasion and metastasis in colorectal cancer. World J Gastroenterol. 2002;8:674-678. [PubMed] |
| 7. | Ishak KG, Anthony PP, Sobin LH. Histological Typing of Tumours of the Liver. WHO International Histological Classifi-cation of Tumours. Berlin: Springer Verlag. 1994;. [DOI] [Full Text] |
| 8. | Barwick WK, Rosai J. Liver. In Rosai J ed. Ackerman's Surgical Patholgy 7th ed. Mosby CO. St Louis. 1989;675-735. |
| 9. | Rossmanith W, Schulte-Hermann R. Biology of transforming growth factor beta in hepatocarcinogenesis. Microsc Res Tech. 2001;52:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Sasaki Y, Tsujiuchi T, Murata N, Tsutsumi M, Konishi Y. Alterations of the transforming growth factor-beta signaling pathway in hepatocellular carcinomas induced endogenously and exogenously in rats. Jpn J Cancer Res. 2001;92:16-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Su WC, Shiesh SC, Liu HS, Chen CY, Chow NH, Lin XZ. Expression of oncogene products HER2/Neu and Ras and fibrosis-related growth factors bFGF, TGF-beta, and PDGF in bile from biliary malignancies and inflammatory disorders. Dig Dis Sci. 2001;46:1387-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Yokomuro S, Tsuji H, Lunz JG, Sakamoto T, Ezure T, Murase N, Demetris AJ. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology. 2000;32:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Lu JP, Zhu SN, Song BP, Mao JQ, Li MS, Hayashi K. In situ de-tection of telomerase activity in hepatic carcinogenesis of rats. J Shanghai Med Univ. 1999;26:413-416. |
| 15. | Ozaki S, Harada K, Sanzen T, Watanabe K, Tsui W, Nakanuma Y. In situ nucleic acid detection of human telomerase in intrahepatic cholangiocarcinoma and its preneoplastic lesion. Hepatology. 1999;30:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Yuan ST, Hu XQ, Lu JP, KeiKi H, Zhai WR, Zhang YE. Changes of integrin expression in rat hepatocarcinogenesis induced by 3'-Me-DAB. World J Gastroenterol. 2000;6:231-233. [PubMed] |
| 17. | Wada K, Nomura S, Morii E, Kitamura Y, Nishizawa Y, Miyake A, Terada N. Changes in levels of mRNAs of transforming growth factor (TGF)-beta1, -beta2, -beta3, TGF-beta type II receptor and sulfated glycoprotein-2 during apoptosis of mouse uterine epithelium. J Steroid Biochem Mol Biol. 1996;59:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Lu JP. Non-isotopic in situ hybridization technique. Nanhai Publication. 1996;Haikou, China. |
| 19. | Lu JP, Zhu SN, Song BP, Mao JQ, Lu SL. In situ telomerase re-peat amplification protocol (TRAP) detecting telomerase activ-ity in human hepatocellular carcinoma. Acta Hitochem Cytochem. 1999;32:477-480. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Lu JP, Hayashi K. Transferrin receptor distribution and iron deposition in the hepatic lobule of iron-overloaded rats. Pathol Int. 1995;45:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Mao JQ, Lu JP, Zhu SN, Li MS, Lu SL, Hayashi K, Nomura S. The significance of TGFβ- and its receptor on the difference of stroma formation in experimental rat hepatocellular carcinoma and cholangiocarcinoma. Chinese Hepatol. 2001;6:23-25. |
| 22. | Nakatsukasa H, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Expression of transforming growth factor-beta 1 during chemical hepatocarcinogenesis in the rat. Lab Invest. 1991;65:511-517. [PubMed] |
| 23. | Vogelbruch M, Wellmann A, Maschek H, Schäfer MK, Flemming P, Georgii A. Transforming growth factor beta 1 in human liver tumors. Verh Dtsch Ges Pathol. 1995;79:132-136. [PubMed] |
| 24. | Factor VM, Kao CY, Santoni-Rugiu E, Woitach JT, Jensen MR, Thorgeirsson SS. Constitutive expression of mature transforming growth factor beta1 in the liver accelerates hepatocarcinogenesis in transgenic mice. Cancer Res. 1997;57:2089-2095. [PubMed] |
| 25. | Kanzler S, Meyer E, Lohse AW, Schirmacher P, Henninger J, Galle PR, Blessing M. Hepatocellular expression of a dominant-negative mutant TGF-beta type II receptor accelerates chemically induced hepatocarcinogenesis. Oncogene. 2001;20:5015-5024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Lu JP, Hayashi K. Myofibroblasts and stroma difference between cholangiocarcinoma and hepatocellular carcinoma. Arch Histopathol D. 1996;3:55-62. |
| 27. | Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 527] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Hytiroglou P, Kotoula V, Thung SN, Tsokos M, Fiel MI, Papadimitriou CS. Telomerase activity in precancerous hepatic nodules. Cancer. 1998;82:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Stampfer MR, Garbe J, Levine G, Lichtsteiner S, Vasserot AP, Yaswen P. Expression of the telomerase catalytic subunit, hTERT, induces resistance to transforming growth factor beta growth inhibition in p16INK4A(-) human mammary epithelial cells. Proc Natl Acad Sci USA. 2001;98:4498-4503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] |
| 32. | Ogami M, Ikura Y, Nishiguchi S, Kuroki T, Ueda M, Sakurai M. Quantitative analysis and in situ localization of human telomerase RNA in chronic liver disease and hepatocellular carcinoma. Lab Invest. 1999;79:15-26. [PubMed] |
Edited by Xu XQ