Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.509
Revised: June 23, 2002
Accepted: July 11, 2002
Published online: March 15, 2003
AIM: The hepatitis B surface antigen (HBsAg) is considered to be one of the best markers for the diagnosis of acute and chronic HBV infection. But in some patients, this antigen cannot be detected by routine serological assays despite the presence of virus. One of the most important explanations for the lack of detectable HBsAg is that mutations which occur within the “a” determinant of HBV S gene can alter expression of HBsAg and lead to changes of antigenicity and immunogenicity of HBsAg accordingly. As a result, these mutants cannot be detected by diagnosis assays. Thus, it is essential to find out specific and sensitive methods to test the new mutants and further investigate their distribution. This study is to establish a method to investigate the distribution of the HBsAg mutant at nt551.
METHODS: A mutation specific polymerase chain reaction (msPCR) was established for amplifying HBV DNA with a mutation at nt551. Four sets of primer pairs, P551A-PPS, P551G-PPS, P551C-PPS and P551T-PPS, with the same sequences except for one base at 3’ terminus were designed and synthesized according to the known HBV genome sequences and the popular HBV subtypes, adr and adw, in China. At the basis of regular PCR method, we explored the specific conditions for amplifying HBV DNAs with a mutation at nt551 by regulating annealing temperature and the concentration of these primers. 126 serum samples from patients of hepatitis B were collected, among which 16 were positive for HBV S DNA in the nested PCR amplification. These 16 HBV S DNAs were detected by using the msPCR method.
RESULTS: When the annealing temperature was raised to 71 °C, nt551A and nt551G were amplified specifically by P551A-PPS and P551G-PPS; At 72 °C and 5 pmole of the primers (each) in reaction of 25 μl volume, nt551C and nt551T were amplified specifically by P551C-PPS and P551T-PPS. 16 of HBV S gene fragments were characterized by using this method. 14 of them were positive for nt551A, one was positive for nt551G, and the other one was positive for nt551T. The results were confirmed by nucleotide sequencing.
CONCLUSION: The mutation specific polymerase chain reaction is a specific and sensitive method for detecting the mutations of HBV genome at nt551.
- Citation: Ma CL, Fang DX, Chen HB, Li FQ, Jin HY, Li SQ, Tan WG. A mutation specific polymerase chain reaction for detecting hepatitis B virus genome mutations at nt551. World J Gastroenterol 2003; 9(3): 509-512
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.509
Hepatitis B virus (HBV) is a hepatotrophic DNA virus, in the reverse transcription process of DNA replication, the HBV DNA template is transcribed by cellular RNA polymerase to pregenomic RNA, which is reverse transcribed to DNA by viral polymerase, and a consequence of the unique way of HBV replication is a significant tendency of mutation[1-3]. Substitution, deletion and frame-shift by insertion or deletion of short sequence were found in four open reading frames[4-7]. The diversity is also shown in different serological subtypes such as adr, adw, ayr and ayw, which have a common “a” determinant. It is well known that “a” determinant is the common antigenic epitopes of all subtypes of HBsAg. A large antigenic area of “a” determinant is now called the major hydrophilic region (MHR). Mutations within MHR of HBsAg have been considered to be associated with vaccine failure and chronic infection[1,2,6,8]. These mutations have been reported repeatedly since Carman found the first case of immune escape mutants in 1990[9]. The point mutation reported most commonly in immunized children causes a substitution of Arg for Gly at position 145 of HBsAg[1,3,8,9]. Other reports about substitutions in HBsAg such as 120, 121, 126, 129, 131, 133, 141, 144 were found repeatedly[8-12]. These findings of HBV immune escape mutants have caused attention from scientists all over the world in recent years. Immune escape of HBV mutants was best known to be associated with HBV genome itself, but the immune pressure was considered to be one of the most important factors that result in escape mutants[13-17].
The immune escape variants could influence the effect of HBV vaccine, it was argued that the components of mutant HBsAg should be considered to be added in the HBV vaccine in the future[3,13,17,18]. However, in order to achieve this aim, it is needed to confirm first the mutants that are the big problems among hepatitis B patients. At present, it is very important to find out new escape mutants and further investigate their distribution. Specific and sensitive assays are essential for investigating the distribution of mutants. To detect the mutant HBV DNA, mutation specific polymerase chain reaction (msPCR) is a potential method. Our lab had discovered a mutation at nt551 A-to-G of HBV genome, resulting in the alteration at aa133 Met to Val of HBsAg, from a four-year-old hepatitis B patient[12]. To investigate the distribution of mutants with a mutation at nt551 among the hepatitis B patients in China, a msPCR method was established according to HBV DNA sequences and the main popular subtypes, adr and adw.
Collection of serum samples 126 of serum samples were provided by Department of Laboratory Diagnosis, Nanjing Jinling Hospital. The viral markers were tested by using the enzyme immune assay (EIA) methodology. All of the samples were positive for anti-HBs and negative for HBsAg. The ALT level was considered to be abnormal to all of them.
Extraction of HBV genome DNAs DNAs was isolated and purified from 100 μL of serum samples. Proteinase K (20 mg/mL) and SDS (10%) were added into 100 μL of sera and their concentrations in reaction were 2.5 mg/mL and 0.5%, respectively. After a brief vortex, the mixture was heated at 70 °C for 3 hours. Then the same volume of phenol: chloroform was added to the mixture to extract DNA. The DNA pellet was obtained with 100% ethanol precipitation, and was washed with 70% ethanol. The dried DNA pellet was then resuspended in 20 μL of H2O and stored at -20 °C. All of 126 sera were prepared in this way.
Amplification of HBV S DNAs The amplification of HBV S gene was carried out by using the nested PCR method. In the first-round PCR, the 50 μL reaction solution including 5 μL DNA template and 40 pmole (each) of the first-round primers. The mixture was heated to 94 °C for 5 min, followed by 30 PCR cycles consisting of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 60 s in a thermal cycler. And then 1.25 μL of the first-round PCR product served as the template for the second-round of PCR amplification which consisting of the same cycles except that the annealing temperature was raised to 65 °C. Positive PCR product, a DNA band of 1.2kb as expected, was detected on agarose gel electrophoresis. After amplification, we achieved 16 HBV S DNAs.
The primers for the nested PCR were designed according to the known HBV genome sequences and the main popular subtypes, adr and adw, in China, as follows:
The primers for first-round:
P1’: 5’ACATCATCTGTGGAAGGC 3’, nt2756-nt2773, the upstream primer;
P6’: 5’TATCCCATGAAGTTAAGG 3’, nt884-nt867, the downstream primer;
The primers for second-round:
PEC: 5’CGGAATTCACCATATTCTTGGGAACAAG 3’, nt2 823-nt2 844, the upstream primer;
PPS: 5’GCTGCAGGTTTAAATGTATACCCAAAGAC 3’, nt838-nt816, the downstream primer;
An EcoRI or a Pst I sites was originally added at 5’-end respectively for cloning purpose.
Amplification of HBV DNA fragments for control To achieve the HBV DNA fragment with an A at nt551, the wild-type HBV S DNA as template was amplified by using the primer pair P551A-PPS under the condition of regular PCR. The HBsAg mutant with a G at nt551 as template was amplified by using the primer pair P551G-PPS to achieve the HBV DNA fragment with a G at nt551. The HBV DNA fragment with a C or T at nt551 was achieved by using introducing mutation in a PCR. The PCR primer sequences were as follows:
P551A: 5’TCCTGCTCAAGGAACCTCTA 3’, nt532-nt551, upstream primer;
P551G: 5’TCCTGCTCAAGGAACCTCTG 3’, nt532-nt551, upstream primer;
P551C: 5’TCCTGCTCAAGGAACCTCTC 3’, nt532-nt551, upstream primer;
P551T: 5’TCCTGCTCAAGGAACCTCTT 3’, nt532-nt551, upstream primer;
PPS: See the above.
P551C-PPS and P551T-PPS were used respectively to amplify HBV DNA fragments with a C or T at nt551, which are 314 bp long. Additionally, two upstream primers had been designed respectively by introducing mutation in order to achieve the controls of HBV DNA with a C or T at nt551. P551CM: TCCTGCTCAAGGAACCTCTCTGTTTC, nt532-nt557; P551TM: TCCTGCTCAAGGAACCTCTTTGTTTC, nt532-nt557.
Establishment of msPCR In order to amplify HBV DNA specifically, the annealing temperature of PCR was regulated according to the Tm. The reaction volume of PCR was 25 μL. The PCR reaction was carried out by using P551A-PPS, P551G-PPS, P551C-PPS and P551T-PPS as primer pairs to amplify HBV S DNA templates with an A, G, C, or T at nt551, respectively.
Application of msPCR Under the condition of the msPCR method, the 16 of samples which were positive for HBV S DNA as templates were amplified by using P551A-PPS, P551G-PPS, P551C-PPS and P551T-PPS as primer pairs respectively.
HBV S DNA sequencing In order to confirm the validity of the msPCR, the purified PCR products of HBV S gene fragments from selected samples according to the results of msPCR, NO.2, NO.5, NO.8 and NO.57 were sequenced by using the primer PPS.
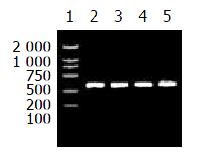
The HBV S DNA with an A, G, C or T at nt551 was amplified respectively for control. This result is shown in Figure 1.
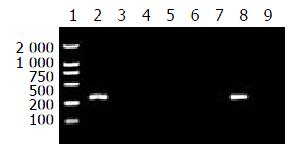
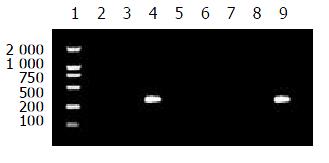
When the annealing temperature was set from 65 °C to 70 °C, the results of amplification were not specific to the four primer pairs. In other words, the templates were amplified by no-matching primers. Then the annealing temperature was raised to 71 °C, the specific amplified results were found for P551A-PPS and P551G-PPS; When the annealing temperature was raised to 72 °C and the concentration of the primers (each) were at 5 pmole in reaction of 25 μL volume, P551C-PPS and P551T-PPS amplified HBV DNAs with C or T at nt551 specifically. The specific results to establish msPCR method are shown in Figures 2 and 3.
16 samples were tested by using the msPCR method. This result is shown in Table 1.
| Primerpairs | HBV DNA samples | |||||||||||||||
| 2 | 5 | 8 | 13 | 17 | 31 | 32 | 33 | 34 | 46 | 47 | 50 | 53 | 57 | 67 | 85 | |
| P551A-PPS | - | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + |
| P551G-PPS | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - |
| P551C-PPS | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| P551T-PPS | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
According to the results of msPCR, four DNA samples, No.2, No.5, No.8 and No.57 were selected to sequence. The sequencing results showed that No.2 was a T at nt551, both No.5 and No.8 were an A at nt551, and No.57 was a G at nt551, confirming the results of the msPCR. The sequencing results are shown in Figure 4.
In the recent years, mutant HBsAg have caused great academic interest[19,20], and many analyses and researches have been made for the emergence of HBV mutants with mutations in the “a” determinant of HBsAg which result in immune escape[13-16,21]. According to these research results, it is very important to further investigate mutant distributions and clarify mutations in HBV S gene which could cause the changes of antigenicity and immunogenicity of HBsAg[3,12,22-24]. It is well known that the mutants of HBsAg were able to cause infection and horizontal transmission despite the presence of anti-HBs[25-29]. The increasing use of HBV vaccine has had overwhelming positive influence on the prevention of hepatitis B viral infection, but have no effective impact to those mutants[30]. The mutations in the coding region of “a” determinant of HBsAg could not be detected in some routine assays[31,32].
This study was to obtain a specific and sensitive method for monitoring the HBsAg mutant with a mutation at nt551. The method of msPCR is different from immnoassays that are based on the antigen-antibody reaction[33,34]. To detect mutant HBsAg, the unique specific monoclonal antibodies are required. But these kinds of antibodies are limited or not available commercially. Because HBV is a double-stranded DNA virus, its genome is fairly stable in the blood and tissue, the PCR amplification of HBV DNA is relatively easy[35]. The msPCR is actually a method to detect the specific site mutation. This method was firstly developed to detect site mutation of allele-special genes of β-globin genome DNA for sickle cell anemia[36]. And then it was used in virological studies. This mechanism was used in our study.
The msPCR was established at the basis of the known HBV DNA. The primers were only one-base different from each other at 3’ terminus, thus the non-specific DNA should not be amplified at regular value of Tm. However, we considered the variability of annealing temperature and set it as high as possible. When it was 71 °C and 72 °C, the ideal result was found respectively for different primer pairs. The process confirmed that the annealing temperature is the key factor to establish msPCR method of nt551. Additionally, the concentration of the primers is also an important factor for this msPCR. In short, different primers amplify HBV DNA specifically with different conditions.
The aim of this study was to detect the mutation of the known HBV S gene at nt551. All of 126 serum samples were collected from hepatitis B patients in hospital. After the nested PCR amplification, 16 samples were positive for HBV S gene. The msPCR detection showed that 14 of them were an A at nt551, one was a G at nt551, and the other was a T at nt551. The reliability of msPCR was confirmed by sequencing analysis of four samples. A special attention should be paid to No.2. It is a T at nt551, resulting in a Met (ATG) to Leu (TTG) change at aa133 of HBsAg. Whether this mutation caused the change of antigenicity need further identification. In addition to these mutations in HBsAg “a” determinant, there were several other mutations in S gene. These results further confirmed the diversity and popularity of HBV S gene mutation.
In conclusion, this msPCR is a sensitive and specific approach for the detection of mutations in HBV S gene, and will have tremendous potential in screening HBsAg mutants and further investigating their distribution in patients of hepatitis B.
| 1. | Koyanagi T, Nakamuta M, Sakai H, Sugimoto R, Enjoji M, Koto K, Iwamoto H, Kumazawa T, Mukaide M, Nawata H. Analysis of HBs antigen negative variant of hepatitis B virus: unique substitutions, Glu129 to Asp and Gly145 to Ala in the surface antigen gene. Med Sci Monit. 2000;6:1165-1169. [PubMed] |
| 2. | Brunetto MR, Rodriguez UA, Bonino F. Hepatitis B virus mutants. Intervirology. 1999;42:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Kfoury Baz EM, Zheng J, Mazuruk K, Van Le A, Peterson DL. Characterization of a novel hepatitis B virus mutant: demonstration of mutation-induced hepatitis B virus surface antigen group specific "a" determinant conformation change and its application in diagnostic assays. Transfus Med. 2001;11:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Dong J, Cheng J, Wang Q, Huangfu J, Shi S, Zhang G, Hong Y, Li L, Si C. [The study on heterogeneity of hepatitis B virus DNA]. Zhonghua Yixue Zazhi. 2002;82:81-85. [PubMed] |
| 5. | Kreutz C. Molecular, immunological and clinical properties of mutated hepatitis B viruses. J Cell Mol Med. 2002;6:113-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Zhong S, Chan JY, Yeo W, Tam JS, Johnson PJ. Hepatitis B envelope protein mutants in human hepatocellular carcinoma tissues. J Viral Hepat. 1999;6:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Weinberger KM, Zoulek G, Bauer T, Böhm S, Jilg W. A novel deletion mutant of hepatitis B virus surface antigen. J Med Virol. 1999;58:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Roznovsky L, Harrison TJ, Fang ZL, Ling R, Lochman I, Orsagova I, Pliskova L. Unusual hepatitis B surface antigen variation in a child immunised against hepatitis B. J Med Virol. 2000;61:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 775] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 10. | Yang X, Lei J, Zhang Y, Luo H, Huang L, Zheng Y, Tang X, Li L. [A novel stop codon mutation in S gene: the molecular basis of a patient with cryptogenic cirrhosis]. Zhonghua Yixue Zazhi. 2002;82:400-402. [PubMed] |
| 11. | Zhu Q, Lu Q, Xiong S, Yu H, Duan S. Hepatitis B virus S gene mutants in infants infected despite immunoprophylaxis. Chin Med J (Engl). 2001;114:352-354. [PubMed] |
| 12. | Fang DX, Li FQ, Tan WG, Chen HB, Jin HY, Li SQ, Lin HJ, Zhou ZX. Transient expression and antigenic characterization of HBsAg of HBV nt551 A to G mutant. World J Gastroenterol. 1999;5:73-74. [PubMed] |
| 13. | Santantonio T, Gunther S, Sterneck M, Rendina M, Messner M, Launois B, Francavilla A, Pastore G, Will H. Liver graft infection by HBV S-gene mutants in transplant patients receiving long-term HBIg prophylaxis. Hepatogastroenterology. 1999;46:1848-1854. [PubMed] |
| 14. | Rodriguez-Frias F, Buti M, Jardi R, Vargas V, Quer J, Cotrina M, Martell M, Esteban R, Guardia J. Genetic alterations in the S gene of hepatitis B virus in patients with acute hepatitis B, chronic hepatitis B and hepatitis B liver cirrhosis before and after liver transplantation. Liver. 1999;19:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | He C, Nomura F, Itoga S, Isobe K, Nakai T. Prevalence of vaccine-induced escape mutants of hepatitis B virus in the adult population in China: a prospective study in 176 restaurant employees. J Gastroenterol Hepatol. 2001;16:1373-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Cooreman MP, Leroux-Roels G, Paulij WP. Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen. J Biomed Sci. 2001;8:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Komatsu H, Fujisawa T, Sogo T, Isozaki A, Inui A, Sekine I, Kobata M, Ogawa Y. Acute self-limiting hepatitis B after immunoprophylaxis failure in an infant. J Med Virol. 2002;66:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Heijtink RA, van Bergen P, van Roosmalen MH, Sünnen CM, Paulij WP, Schalm SW, Osterhaus AD. Anti-HBs after hepatitis B immunization with plasma-derived and recombinant DNA-derived vaccines: binding to mutant HBsAg. Vaccine. 2001;19:3671-3680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | François G, Kew M, Van Damme P, Mphahlele MJ, Meheus A. Mutant hepatitis B viruses: a matter of academic interest only or a problem with far-reaching implications. Vaccine. 2001;19:3799-3815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Burda MR, Günther S, Dandri M, Will H, Petersen J. Structural and functional heterogeneity of naturally occurring hepatitis B virus variants. Antiviral Res. 2001;52:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Coleman PF, Chen YC, Mushahwar IK. Immunoassay detection of hepatitis B surface antigen mutants. J Med Virol. 1999;59:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Torresi J, Earnest-Silveira L, Civitico G, Walters TE, Lewin SR, Fyfe J, Locarnini SA, Manns M, Trautwein C, Bock TC. Restoration of replication phenotype of lamivudine-resistant hepatitis B virus mutants by compensatory changes in the "fingers" subdomain of the viral polymerase selected as a consequence of mutations in the overlapping S gene. Virology. 2002;299:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Cooreman MP, van Roosmalen MH, te Morsche R, Sünnen CM, de Ven EM, Jansen JB, Tytgat GN, de Wit PL, Paulij WP. Characterization of the reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to G145R and other naturally occurring "a" loop escape mutations. Hepatology. 1999;30:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Wu L, Yuan ZH, Liu F, Waters JA, Wen YM. Comparing the immunogenicity of hepatitis B virus S gene variants by DNA immunization. Viral Immunol. 2001;14:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Owiredu WK, Kramvis A, Kew MC. Molecular analysis of hepatitis B virus genomes isolated from black African patients with fulminant hepatitis B. J Med Virol. 2001;65:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Banerjee K, Guptan RC, Bisht R, Sarin SK, Khandekar P. Identification of a novel surface mutant of hepatitis B virus in a seronegative chronic liver disease patient. Virus Res. 1999;65:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Chen WN, Oon CJ. Hepatitis B virus surface antigen (HBsAg) mutants in Singapore adults and vaccinated children with high anti-hepatitis B virus antibody levels but negative for HBsAg. J Clin Microbiol. 2000;38:2793-2794. [PubMed] |
| 28. | Oon CJ, Chen WN, Goo KS, Goh KT. Intra-familial evidence of horizontal transmission of hepatitis B virus surface antigen mutant G145R. J Infect. 2000;41:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Chen WN, Oon CJ, Koh S. Horizontal transmission of a hepatitis B virus surface antigen mutant. J Clin Microbiol. 2000;38:938-939. [PubMed] |
| 30. | Schories M, Peters T, Rasenack J. Isolation, characterization and biological significance of hepatitis B virus mutants from serum of a patient with immunologically negative HBV infection. J Hepatol. 2000;33:799-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Weinberger KM, Bauer T, Böhm S, Jilg W. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J Gen Virol. 2000;81:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Chen WN, Oon CJ, Moh MC. Detection of hepatitis B virus surface antigen mutants in paraffin-embedded hepatocellular carcinoma tissues. Virus Genes. 2000;20:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Ijaz S, Torre F, Tedder RS, Williams R, Naoumov NV. Novel immunoassay for the detection of hepatitis B surface 'escape' mutants and its application in liver transplant recipients. J Med Virol. 2001;63:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Jolivet-Reynaud C, Lésenéchal M, O'Donnell B, Becquart L, Foussadier A, Forge F, Battail-Poirot N, Lacoux X, Carman W, Jolivet M. Localization of hepatitis B surface antigen epitopes present on variants and specifically recognised by anti-hepatitis B surface antigen monoclonal antibodies. J Med Virol. 2001;65:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Worman HJ, Feng L, Mamiya N. Molecular biology and the diagnosis and treatment of liver diseases. World J Gastroenterol. 1998;4:185-191. [PubMed] |
| 36. | Wu DY, Ugozzoli L, Pal BK, Wallace RB. Allele-specific enzymatic amplification of beta-globin genomic DNA for diagnosis of sickle cell anemia. Proc Natl Acad Sci USA. 1989;86:2757-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 404] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
Edited by Zhao M