Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.392
Revised: July 20, 2002
Accepted: August 9, 2002
Published online: March 15, 2003
AIM: To obtain human esophageal cancer cell EC9706 stably expressed epithelial membrane protein-1 (EMP-1) with integrated eukaryotic plasmid harboring the open reading frame (ORF) of human EMP-1, and then to study the mechanism by which EMP-1 exerts its diverse cellular action on cell proliferation and altered gene profile by exploring the effect of EMP-1.
METHODS: The authors first constructed pcDNA3.1/myc-his expression vector harboring the ORF of EMP-1 and then transfected it into human esophageal carcinoma cell line EC9706. The positive clones were analyzed by Western blot and RT-PCR. Moreover, the cell growth curve was observed and the cell cycle was checked by FACS technique. Using cDNA microarray technology, the authors compared the gene expression pattern in positive clones with control. To confirm the gene expression profile, semi-quantitative RT-PCR was carried out for 4 of the randomly picked differentially expressed genes. For those differentially expressed genes, classification was performed according to their function and cellular component.
RESULTS: Human EMP-1 gene can be stably expressed in EC9706 cell line transfected with human EMP-1. The authors found the cell growth decreased, among which S phase was arrested and G1 phase was prolonged in the transfected positive clones. By cDNA microarray analysis, 35 genes showed an over 2.0 fold change in expression level after transfection, with 28 genes being consistently up-regulated and 7 genes being down-regulated. Among the classified genes, almost half of the induced genes (13 out of 28 genes) were related to cell signaling, cell communication and particularly to adhesion.
CONCLUSION: Overexpression of human EMP-1 gene can inhibit the proliferation of EC9706 cell with S phase arrested and G1 phase prolonged. The cDNA microarray analysis suggested that EMP-1 may be one of regulators involved in cell signaling, cell communication and adhesion regulators.
- Citation: Wang HT, Kong JP, Ding F, Wang XQ, Wang MR, Liu LX, Wu M, Liu ZH. Analysis of gene expression profile induced by EMP-1 in esophageal cancer cells using cDNA Microarray. World J Gastroenterol 2003; 9(3): 392-398
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/392.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.392
EMP-1 is a member of the PMP22 family with the similarity in structure. Since EMP-1 was first found by Taylor, it has been isolated independently from human, mouse and rabbit and received many different designations, such as TMP (tumor membrane Protein), PAP (Progression Associated Protein), CL-20 and B4B[1]. All tissues expressing EMP-1 mRNA contain 2.76-kb EMP-1 transcripts. In some regions of the gastrointestinal tract, including the fundus, ileum, cecum, and colon, however, additional transcripts of approximately 1.7 kb hybridize with the EMP-1 cDNA[2] .The 2.76-kb EMP-1 cDNA contains five exons about 0.2kb, 0.12kb, 0.1kb, 0.14kb, and 2.2 kb and four introns about 15kb, 1.9kb, 0.1kb, and 0.7 kb in length respectively. EMP-1 has been mapped to chromosome 12p12 by fluorescence in situ hybridization[3]. EMP-1 is encoded by a single-copy gene with the positions of introns exactly conserved between EMP-1 and PMP22, corroborating the hypothesis that EMP-1 belongs to the PMP22 family[4]. EMP-1 transcript is expressed at high levels in heart, placenta, lung, skeletal muscle, kidney, spleen, colon prostate, ovary, testicle, small intestine and thymus in human[5].
EMP-1 was selected from a series of differential expressed genes obtained from cDNA microarray analysis of expression profiles of esophageal cancer in our previous work. EMP-1 expression was 6 fold down-regulated in esophageal cancer lower than in normal tissue. EMP-1 is highly up-regulated during squamous cell differentiation and in certain tumors, and a role in tumorigenesis has been proposed[6]. Moreover, The overexpression of PMP22 leads to an apoptotic-like phenotype in NIH3T3 growing cells[7] and delays serum-forksolin-stimulated entry of resting Schwann cells from G1 into the S+G2/M phase in Schwann cell[8]. Transient expression of EMP-1 specifically inhibited cellular proliferation by more than 50%[9]. Preliminary data suggested that EMP-1 was involved in growth control in esophageal cancer cell line EC9706. However, whether there is a similar effect of EMP-1 expression on the cell cycle of epithelial cells remains to be determined and little is known about the function of EMP-1 in growth control in esophageal cancer cell line EC9706.
To elucidate the effect of EMP-1 on EC9706 cell, the open reading frame (ORF) of human EMP-1 was cloned into pcDNA3.1/myc-his, a eukaryotic expression vector. EC9706 was transfected with the integrated plasmid containing EMP-1 to enforce expression of the exogenous EMP-1. Western blotting and RT-PCR were used to analyze positive clones. The cell growth curve was observed and the cell cycle was checked by FACS method. However, the mechanism by which EMP-1 may exert its activity remains unclear. Because the differentiation of mammalian cells is associated with changes in gene expression that is primarily controlled at the level of transcription, we tested the expression alteration with cDNA microarray technology to address the question of which genes are influenced by EMP-1 gene overexpression.
Fifteen pairs of esophageal tumors and matched adjacent normal mucosa were obtained at surgery. Samples were frozen in liquid nitrogen until RNA was extracted.
Esophageal carcinoma cell line EC9706 was established in our laboratory. The cell lines were maintained in M199 medium with 15% FBS and cultured at 37 °C in 5% CO2.
An Xhol I and Hind III fragment ORF of EMP-1 was cloned into the pcDNA3.1/myc-his vector. The correct construct sequence was confirmed by DNA sequencing.
Atlas Human Cancer cDNA Expression Array (Clontech) was used to analyze EMP-1-induced gene expression which included over 588 genes on the nylon membrane.
Paired esophageal cancer tissues and adjacent normal mucosa tissues (-100 mg) were homogenized mechanically in 1 mL of TRIzol reagent (Life Technologies, Inc.), and cell pellets were harvested to isolate the total RNA. According to the manufacturer’s protocol (Life Technologies, Inc.). First-strand cDNA was synthesized from 5 μg of total RNA using Superscript II reverse transcriptase (Life Technologies, Inc.) and OligodT12-18 primers following the company’s protocol. The same amount of cDNAs was subsequently used for PCR amplification. PCR reaction was performed in 25 μL buffer containing 1 μL cDNA, dATP, dCTP, dGTP, dTTP each 0.2 mmol/L, 1 × PCR buffer, 1.5 mmol/L MgCl2 and 1.5 U Taq DNA polymerase (GIBCO). The primers for GAPDH are 5’-ACC ACA GTC CAT GCC ATC AC-3’ and 5’-TCC ACC ACC CTG TTG CTG TA-3’; EMP-1 5’-GGA TCA GGG CTC CTA GGC TCA-3’ and 5’-GGT GGC TTG CCC TCA ACA TT-3’; the primer for the ORF of EMP-1 is 5’-ATC TTT GTG GTC CAC ATC GCT-3’ and 5’-CTT CTC CAT GGT GAA GAG CT-3’; Primers for four random selected genes were respectively designed for human CDK inhibitor p19INK4d, 5’-TCC ATG ACG CAG CCC GCA CT-3’ and 5’-TCT CTG CAG TGC CAG CTC CA-3’; Human tissue-type plasminogen activator (t-PA), 5’-AGT GCA TTT TCC CAG ATA CT-3’ and 5’-TTT GTG GTC CTG TTT CCA AAG-3’; Human interleukin-6, 5’-AGG CAC TGG CAG AAA ACA AC-3’ and 5’-TCC AAG AAA TGA TCT GGC TC-3’; Human alpha-1 type XI collagen (COL11A1), 5’-TCC TGT TTG TTT TCT TGG CT-3’ and 5’-TTA TGA TTT TCA AAG CTT TTG T-3’. The amplification was performed with one denaturing cycle of 5 min at 95 °C, then 94 °C for 40s, 55 °C for 40s, 72 °C for 40 s for 25-27 cycles, and one final extension of 7 min at 72°C. RT-PCR products were analyzed on 1.2% agarose gel. The number of cycles and melting temperature was adjusted depending on the genes amplified.
According to the manufacturer’s instructions (Invitrogen), the day before transfection, seed 3 × 106 EC9706 cells in 3 mL of the 15% serum complete growth medium. Combine diluted DNA with diluted LipofectamineTM Reagent. For each transfection, add 0.8 mL of medium without serum to the tube containing the complexes. Incubate the cells with the complexes for 5 hours at 37 °C in a CO2 incubator. Following incubation, add 3 mL of growth medium containing 30% serum without removing the transfection mixture. Add G418 after 3 days and select monoclonal cell to a fresh bottle and keep on culturing with G418 (1 mg/mL). The positive clones together with the negative parental vector without EMP-1 and EC9706 Cells (3 × 105 in 2 mL) were seeded and incubated at 37 °C in 5% CO2 with G418 (1 mg/mL), Cells were harvested and then quantitated every 24 hours.
Flow cytometry was performed to assess the cell cycle alteration. Trypsinized adherent and floating cells were collected, washed twice with cold PBS, and resuspended in PBS containing 0.1% Triton X-100 and 0.1% RNase for 5 min at room temperature. The samples were then stained with propdium iodide (0.1 mg/mL), filtered through a 300-μm-pore-size nylon mesh, and analyzed in a cell sorter (Coulter, epics® elite ESP)[10].
Cells were rinsed twice with PBS, and protein preparation was performed according to the manufacturer’s instruction (Santa Cruz). Protein concentration in homogenates was determined using a BSA-Protein assay (Hyclone) employing bovine serum albumin as the standard. The extracted proteins (20 µg) were subjected to 15% polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate under reducing conditions, and then transferred onto poly (vinylidene difluoride) (PVDF) membranes (Hybond-P). Mouse monoclonal anti-human hexad-HIS antibodies (Santa Cruz; final dilution 1:1000) were used as the primary antibody, and horseradish peroxidaselabeled anti-mouse immunoglobulin (Santa Cruz; final dilution 1:1500) was used as the secondary reagent. Detection was performed using an ECL system (Amersham-Pharmacia).
Total RNA was extracted by TRIzol from positive clone 2 cells and the parental vector without cDNA insert pcDNA3.1myc-his (-) C cells as negative control. Poly (A) RNA was purified using an Oligotex-dT30 mRNA purification kit (TAKARA). One µg of highly purified poly (A) RNA from the negative control and and cell clone 2 was performed for cDNA microarray analysis. The mRNA was reverse transcribed with 33P-dATP (Amersham). The paired reactions were purified with a TE-30 column (Clontech, Palo Alto, CA). The radioactive labeled probe was then applied to the array for hybridization at 68 °C for 12 hours. After hybridization, the membrane was washed with buffer of decreasing ionic strength. The X-ray film was scanned at a resolution of 16-bit. AtlasImage software (version1.01a, Clontech) was used for image analysis. The area surrounding each element image was used to calculate a local background, which was then subtracted from the total element signal. Background subtracted element signals are used to calculate intensity ratios. The average of the resulting total intensity signal gives a ratio that is used to balance or normalize the signals. Then semi-quantitative RT-PCR analysis of 4 randomly selected genes confirms the microarray results.
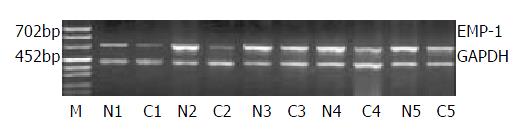
RT-PCR was used to detect the EMP-1 gene expression in the pair of esophageal cancer (C) and normal mucosa (N). We checked 15 pairs of esophageal cancer and its adjacent normal tissue and found the expression of EMP-1 was higher in normal tissue than that in cancer tissue in 14 pairs, and lower in only 1 pair (Figure 1).
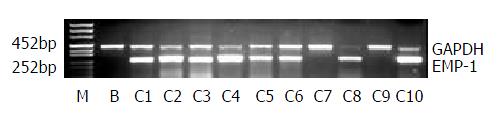
Since the expression of EMP-1 should be up-regulated after transfection, RT-PCR was used to analyze the positive clones and the parental vector as negative control. The clones 1, 2, 3, 4, 5, 6, 8 and 10 showed a comparable increased expression of EMP-1 (Figure 2).
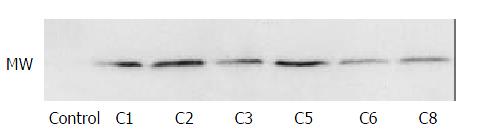
Since the vector carrying 6xHis peptide as a selective marker, it can be detected by anti-His monoclonal antibody with Western blot. Hexad-His peptide has been expressed in host cell clone 1, clone 2, clone 3, clone 5, clone 6 and clone 8, however, EMP-1 protein was not identified in clone 4 which was highly expressed in transcriptional level for unknown reasons (Figure 3).
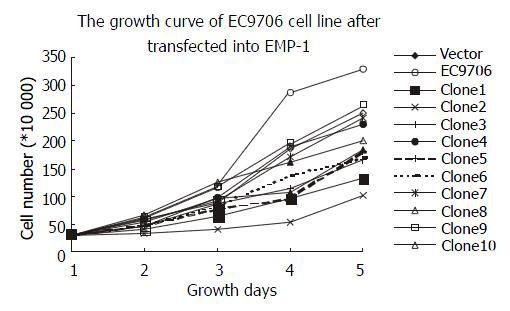
The cell proliferation rate had been observed and the cells displayed a decreased proliferation rate in clone 1, clone 2, clone 3, clone 5, clone 6, clone 8 and clone 10, especially in clone 2. The proliferation rates of clone 4, clone 7, and clone 9 are close to that of negative control. The expression of EMP-1 in clone 1, clone 2, clone 3, clone 6 and clone 8 was up-regulated in both RNA and protein levels by RT-PCR and Western blot, respectively, and a reduced ability to proliferate was as shown in these clones (Figure 4).
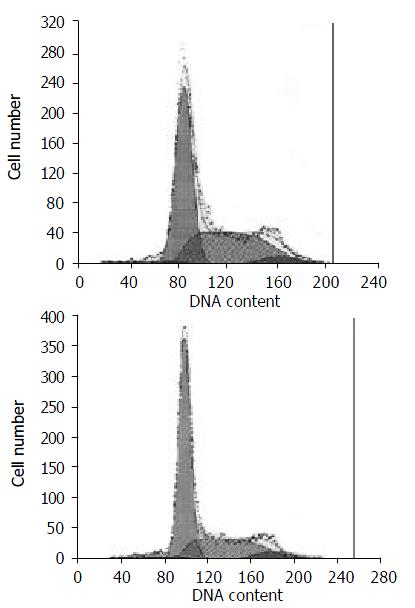
Five of ten clones showed similar results after flow cytometry analysis. The percentages of cells in G1 and S phases of clone 1, clone 2, clone 3, clone 6 and clone 8 were 66.6%, 17.9%; 64.0%, 30.2%; 63.8%, 29.1%; 60.9%, 34.6%; and 69.5%, 24.2%; respectively. The percentage of cells in G1 and S phases of negative control were 52.2% and 41.7%, respectively. Comparing the cells trasfected with cDNA insert and the negative control, we found that S phase was arrested and G1 phase was prolonged. Then cell clone 2 was used as a representative because its proliferation has been inhibited obviously (Figure 5).
By cDNA microarray analysis, 35 genes showed an over 2.0-fold change in expression level after transfection, with 28 genes being consistently up-regulated and 7 genes being down-regulated in clone 2 cells (Figure 6).
To confirm the gene expression profile, semi-quantitative RT-PCR was carried out for 4 randomly selected differentially expressed genes. As a result, the expression of these 4 genes was similar with the microarray analysis (Figure 7).
In further analysis of the data from this study provided an insight into the alteration in the EMP-1 induced genes. Because these 588 genes are cancer associated genes and include almost all aspects involved in carcinogenesis and development of tumor and can be the representative of tumor, these data provide an independent and relatively unbiased estimate of the EMP-1 induced expression profile. In general, genes that expression were was more than 2.0-fold were informative and valuable (Table 1).
| Genes up-regulated by EMP-1 | ||||
| Coordinate | Gene Name | Ratio | Category | Sub Category |
| A3i | Cyclin-dependent kinase 4 inhibitor 2D | 2.04 | Cell division | Cell-cycle regulators |
| A7d | Cytokeratin 1 | 2.26 | Cell structure | Intermediate Filament Proteins |
| B3i | Caspase 9 | 4.11 | Cell division | Cell-apoptosis |
| B7b | Chromatin assembly factor 1 p48 subunit | 2.93 | Cell division | Chromosome structure |
| B7j | Abelson-related gene | 4.49 | Tumor Suppressors | Oncogenes & Suppressors |
| C2e | Chromatin assembly factor 1 p48 subunit | 2.80 | Cell division | Apoptosis-Associated Proteins |
| C2j | Abelson-related gene | 2.47 | Cell signaling | Intracellular transducers |
| C3e | Chromatin assembly factor 1 p48 subunit | 5.15 | Cell division | DNA synthesis/replication |
| C4b | Abelson-related gene | 3.09 | Cell Adhesion | Extracellular Communication Proteins |
| C4k | Chromatin assembly factor 1 p48 subunit | 4.13 | Cell signaling | Intracellular Transducers |
| C7m | Abelson-related gene | 5.47 | Cell division | Death Receptors |
| D3e | Chromatin assembly factor 1 p48 subunit | 2.36 | Cell structure | Extracellular Matrix Proteins |
| D4j | Abelson-related gene | 2.27 | Cell Adhesion | Matrix Adhesion Receptors |
| D4k | Chromatin assembly factor 1 p48 subunit | 2.45 | Cell Adhesion | Cell-Cell Adhesion Receptors |
| D5g | Ezrin; cytovillin 2; villin 2 (VIL2) | 3.06 | Tumor Suppressors | Oncogenes & Tumor Suppressors |
| D6l | Caveolin 1 | 3.29 | Metabolism | GTP/GDP Exchangers |
| E1h | Matrix metalloproteinase 11 (MMP11) | 4.64 | Cell structure | Extracellular matrix Proteins |
| E1m | Matrix metalloproteinase 16 (MMP16) | 3.06 | Protein modification | Metalloproteinases |
| E2b | Tissue inhibitor of metalloproteinase 1 | 3.82 | Protein modification | Protease Inhibitors |
| E2h | Tissue-type plasminogen activator | 4.39 | Protein modification | Serine Proteases |
| E5b | RHO GDP-dissociation inihibitor 1 | 2.42 | Metabolism | GTP/GDP Exchangers |
| E5h | Cadherin 5 (CDH5); | 2.64 | Cell Adhesion | Cell-Cell Adhesion Receptors |
| F1h | Bone morphogenetic protein 2A (BMP2A) | 12.45 | Cell communication | Cytokines |
| F3j | Early growth response protein 1 | 16.06 | RNA synthesis | Transcription Activators & Repressors |
| F4l | Interleukin 6 (IL6) | 4.28 | Cell communication | Interleukins & Interferons |
| F5e | Interleukin 13 (IL13) | 2.62 | Cell communication | Interleukins & Interferons |
| F5k | Interferon gamma (IFN-gamma; IFNG) | 6.04 | Cell communication | Interleukins & Interferons |
| F5l | Leukocyte interferon-inducible peptide | 2.73 | Unclassified | Functionally Unclassified Proteins |
| A1j | Cell division cycle 25 homolog A | 0.35 | Cell division | Cell-cycle regulators |
| 2m | Cyclin D2 (CCND2) | 0.34 | Cell division | Cell-cycle regulators |
| A2n | G1/S-specific cyclin D3 (CCND3) | 0.30 | Cell division | Cell-cycle regulators |
| A3k | Polo-like kinase (PLK) | 0.47 | Cell division | Cell Cycle-Regulating Kinases |
| D1m | Collagen VI alpha 2 subunit (COL6A2) | 0.32 | Cell communication | Extracellular Matrix Proteins |
| D2b | Collagen XI alpha 1 subunit (COL11A1) | 0.17 | Cell communication | Extracellular Matrix Proteins |
| D7e | Vascular endothelial growth factor C | 0.11 | Cell communication | Growth Factors, |
In our previous work on the expression profile of esophageal cancer, we found that the expression of EMP-1 was 6 fold down-regulated in esophageal cancer than that in its adjacent normal mucosa. This study was designed to determine the diverse cellular action of EMP-1 by enforcing its expression in EC9706. Human EMP-1 gene can be stably expressed by EC9706 cell line transfacted with human EMP-1. As a result, the cell proliferation was inhibited, S phase was arrested and G1 phase was prolonged after EMP-1 overexpresssion in EC9706 cells, which is similar to the action of PMP22 in Schwann cells[8]. We then analyzed EMP-1 induced gene expression using AtlasTM human cancer cDNA expression microarray composed of 588 genes, 35 genes showed an over 2.0- fold change in expression level, in which 28 genes were consistently up-regulated and 7 genes were down-regulated. Among the classified genes, almost half of the induced genes (13 out of 28 genes) were related to cell signaling, communication and adhesion, which indicated that EMP-1 may be one of the cell signaling, communication and adhesion regulators (Table 2).
| Major category | Sub category | Up-regulated | Down-regulated |
| Cell division | General | 1 | |
| DNA synthesis/replication | 1 | ||
| Apoptosis | 2 | ||
| Cell cycle | 1 | 4 | |
| Chromosome structure | 1 | ||
| Total | 6 | 4 | |
| Cell signaling | Cell adhesion | ||
| or cell | Channels/transport protein | ||
| communication | Effectors/modulators | ||
| and adhesion | Extracellular Communication | 1 | |
| Hormones/growth factors | 4 | 1 | |
| Intracellular transducers | 2 | ||
| Protein modification | 3 | ||
| Receptors | 3 | 2 | |
| Total | 13 | 3 | |
| Cell structure/ | General | ||
| motility | Cytoskeletal | 3 | |
| Extracellular matrix Protein | 2 | ||
| Microtubule-associated proteins/motors | 1 | ||
| Total | 5 | ||
| Cell/organism | General | ||
| defense | Homeostasis | ||
| Immunology | 1 | ||
| Total | 1 | ||
| Gene/protein | RNA synthesis | 1 | |
| expression | protein synthesis | ||
| Total | 1 | ||
| Metabolism | General | ||
| Amino acid | |||
| Cofactors | |||
| Energy/TCA cycle | 2 | ||
| Lipid | |||
| Nucleotide | |||
| Protein modification | |||
| Sugar/glycolysis | |||
| Transport | |||
| Total | 2 | ||
| Unclassified | 1 | ||
| Total | 1 |
cDNA microarray analysis offers the opportunity to monitor changes in gene expression across the entire set of expressed genes in cells. These 588 known genes are classified on the basis of the biological function of the encoded protein, using a modified version of a previously established classification scheme[11,12]. The classification scheme was composed of six major functional categories and several minor functional categories within the major categories. As shown in Table 2, 34 genes were classified as known function genes, and one gene was categorized as unclassified.
Interestingly, the largest categories (13 out of 28 genes) of EMP-1-induced genes are those involved in cell signaling (Table 1), cell adhesion and cell-cell communication, which included integrin beta 7 (ITGB7), integrin beta 8 (ITGB8) and cadherin 5 (CDH5). These results indicated that EMP-1 might be related with cell adhesion and cell-cell communication.
Cadherin-5 and the alpha E beta 7 integrin mediated heterotypic adhesive interactions between epithelial cells and intraepithelial lymphocytes in vitro[13]. Integrin beta 7 (ITGB7) and integrin beta 8 (ITGB8) are heterodimeric (alpha/beta) transmembrane receptors for extracellular matrix (ECM) ligands. The beta subunit of integrins are considered important for regulation of stimulated cell adhesion and adhesion-dependent signal transduction. Through interactions with molecular partners at cell junctions, they provide a connection between the ECM and the cytoskeleton and regulate many aspects of cell behaviors. Integrins play an important role in lymphocyte adhesion to cellular and extracellular components of their microenvironment[14]. Cadherin 5 was also induced by EMP-1 in our microarray analysis. It is generally accepted that cadherins are a group of cell adhesion molecules located at intercellular junctions, and they play an important role in embryogenesis and morphogenesis in animals and humans due to their adhesive and cell-signaling functions. Disturbances of the expression or function of cadherins and their associated proteins are crucial for the initiation and development of many pathological states. Cadherin 5 is an epithelium-specific cadherin that is required for the development and maintenance of the normal function of all epithelial cells in tissues. The loss or down-regulation of cadherin 5 is a key event in the process of tumour invasion and metastasis[15] Cadherin 5 has the major role in intercellular adhesion in esophageal mucosa[16]. Appropriate cadherin expression reflects the differentiation of squamous cell carcinoma[17]. These results support the putative function of EMP-1, which was a potential maker of differentiation[18] and was related to cell proliferation, differentiation, regulation and cell death[3].
In addition, the second largest categories of EMP-1-induced genes were those involved in cell division, Such as cyclin-dependent kinase 4 inhibitor 2D, DNA damage-inducible transcript 3, and retinoic acid receptor beta. The up-regulated cyclin-dependent kinase 4 inhibitor 2D and the down-regulated genes in this categories including cell division cycle 25 homolog A[19] and cyclin D2 (CCND2) and cyclin D3 (CCND3)[20] affect the cell cycle collaboratively. These support our finding that the inhibition of cell proliferation after overexpression of EMP-1 associates with the induction of S phase arrested and the prolonged G1 phase with flow cytometric assay.
Retinoic acid receptors beta (RAR-β) is another up-regulated gene induced by EMP-1. It is well established that retinoids can modulate epithelial cell growth, differentiation, and apoptosis in vitro and in vivo by binding to specific nuclear retinoid receptors, which include RAR-β[21]. Retinoids can prevent abnormal squamous cell differentiation in nonkeratinizing tissues physiologically. Retinoids can also reverse squamous cell metaplasia, which develops during vitamin A deficiency[22]. The expression of RAR-beta varied along with the differentiation level of esophageal cancer. RAR-beta mRNA was expressed in 62.7%(42/67), 55.1%(43/78) and 29.2%(7/24) of well, moderated and poorly differentiated SSCs, respectively[23]. Retinoic acid, which inhibits squamous cell differentiation with RAR-beta involved in, represses EMP-1 expression in normal human bronchial epithelial cells[4]. EMP-1 is one of down-regulation of a cluster of squamous cell differentiation marker genes[18] and RAR-β may be involved in this process.
Some genes associated with the intracellular signaling pathway, such as dishevelled homolog 1-like protein and ras associated with diabetes protein 1, were induced by EMP-1 overexpression (Table 1).
A study addressing the relationship between EMP-1 and these cell adhesion and cell-cell communication proteins has not, to our knowledge, previously been reported. The presumed protein structure of EMP-1 also supports its function in cell signaling, cell adhesion and communication. The predicted EMP-1 polypeptide of 157 amino acid residues has a calculated molecular weight of approximately 18 kDa, and bioinformatics analysis reveals that amino acid residues 1-28, 64-89, 95-117, and 134-157 represent four hydrophobic, potentially membrane-spanning domains. Computer-predicted structural domains of EMP-1 are partially mirrored by the exon/intron structure of EMP-1. Most interestingly, exon 4, which covers the potential second transmembrane domain, a small intracellular loop, and half of the third transmembrane domain, encodes the most highly conserved regions between the EMP-1 and PMP22 proteins indicating some shared functional significance for this module in the PMP22 family. Because of the small size of the intracellular loops, it appears unlikely that they are involved in specific interaction with intracellular proteins and therefore in intracellular signaling. In contrast, the extracellular loops would be able to interact with other molecules and could have functional significance. Furthermore, the first hydrophilic region of PMP22/EMPs, between the first and second hydrophobic domains, forms an extracellular loop that contains one or more consensus sequences that can be N-linked glycosylated in vitro, a structure which has been implicated in cell-cell recognition and adhesion processes[4,24].
In conclusion, the study of the gene expression changes sustains cellular finding that the inhibition of cell proliferation after enforcing expression of EMP-1 associates with the induction of S phase shorten and the prolonged G1 phase. The expression profile and localization of EMP-1 suggests a role in cell signaling, adhesion and communication. This is the first demonstration of global gene expression analysis of esophageal cancer cell line EC9706 transfectant with EMP-1, and these results provides a new insight in the study of the relationship between EMP-1 and esophageal cancer.
| 1. | Gnirke AU, Weidle UH. Investigation of prevalence and regulation of expression of progression associated protein (PAP). Anticancer Res. 1998;18:4363-4369. [PubMed] |
| 2. | Taylor V, Welcher AA, Program AE, Suter U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem. 1995;270:28824-28833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Medvedev A, Ruzanov P, Marvin KW, Jetten AM. cDNA cloning, genomic structure, and chromosome mapping of the human epithelial membrane protein CL-20 gene (EMP1), a member of the PMP22 family. Genomics. 1997;41:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lobsiger CS, Magyar JP, Taylor V, Wulf P, Welcher AA, Program AE, Suter U. Identification and characterization of a cDNA and the structural gene encoding the mouse epithelial membrane protein-1. Genomics. 1996;36:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Taylor V, Suter U. Epithelial membrane protein-2 and epithelial membrane protein-3: two novel members of the peripheral myelin protein 22 gene family. Gene. 1996;175:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Jetten AM, Suter U. The peripheral myelin protein 22 and epithelial membrane protein family. Prog Nucleic Acid Res Mol Biol. 2000;64:97-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Fabbretti E, Edomi P, Brancolini C, Schneider C. Apoptotic phenotype induced by overexpression of wild-type gas3/PMP22: its relation to the demyelinating peripheral neuropathy CMT1A. Genes Dev. 1995;9:1846-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Zoidl G, Blass-Kampmann S, D'Urso D, Schmalenbach C, Müller HW. Retroviral-mediated gene transfer of the peripheral myelin protein PMP22 in Schwann cells: modulation of cell growth. EMBO J. 1995;14:1122-1128. [PubMed] |
| 9. | Ruegg CL, Wu HY, Fagnoni FF, Engleman EG, Laus R. B4B, a novel growth-arrest gene, is expressed by a subset of progenitor/pre-B lymphocytes negative for cytoplasmic mu-chain. J Immunol. 1996;157:72-80. [PubMed] |
| 10. | Kadowaki Y, Fujiwara T, Fukazawa T, Shao J, Yasuda T, Itoshima T, Kagawa S, Hudson LG, Roth JA, Tanaka N. Induction of differentiation-dependent apoptosis in human esophageal squamous cell carcinoma by adenovirus-mediated p21sdi1 gene transfer. Clin Cancer Res. 1999;5:4233-4241. [PubMed] |
| 11. | Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH, Kirkness EF, Weinstock KG, Gocayne JD, White O. Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature. 1995;377:3-174. [PubMed] |
| 12. | Naiki T, Nagaki M, Shidoji Y, Kojima H, Imose M, Kato T, Ohishi N, Yagi K, Moriwaki H. Analysis of gene expression profile induced by hepatocyte nuclear factor 4alpha in hepatoma cells using an oligonucleotide microarray. J Biol Chem. 2002;277:14011-14019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 904] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Poinat P, De Arcangelis A, Sookhareea S, Zhu X, Hedgecock EM, Labouesse M, Georges-Labouesse E. A conserved interaction between beta1 integrin/PAT-3 and Nck-interacting kinase/MIG-15 that mediates commissural axon navigation in C. elegans. Curr Biol. 2002;12:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Nachtigal P, Gojová A, Semecký V. The role of epithelial and vascular-endothelial cadherin in the differentiation and maintenance of tissue integrity. Acta Medica (Hradec Kralove). 2001;44:83-87. [PubMed] |
| 16. | Bailey T, Biddlestone L, Shepherd N, Barr H, Warner P, Jankowski J. Altered cadherin and catenin complexes in the Barrett's esophagus-dysplasia-adenocarcinoma sequence: correlation with disease progression and dedifferentiation. Am J Pathol. 1998;152:135-144. [PubMed] |
| 17. | Sanders DS, Bruton R, Darnton SJ, Casson AG, Hanson I, Williams HK, Jankowski J. Sequential changes in cadherin-catenin expression associated with the progression and heterogeneity of primary oesophageal squamous carcinoma. Int J Cancer. 1998;79:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889-895. [PubMed] |
| 19. | Wang Z, Wang M, Lazo JS, Carr BI. Identification of epidermal growth factor receptor as a target of Cdc25A protein phosphatase. J Biol Chem. 2002;277:19470-19475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Büschges R, Weber RG, Actor B, Lichter P, Collins VP, Reifenberger G. Amplification and expression of cyclin D genes (CCND1, CCND2 and CCND3) in human malignant gliomas. Brain Pathol. 1999;9:435-442; discussion 432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924-2933. [PubMed] |
| 22. | Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940-954. [PubMed] |
| 23. | Xu M, Jin YL, Fu J, Huang H, Chen SZ, Qu P, Tian HM, Liu ZY, Zhang W. The abnormal expression of retinoic acid receptor-beta, p 53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J Gastroenterol. 2002;8:200-202. [PubMed] |
| 24. | Schachner M, Martini R. Glycans and the modulation of neural-recognition molecule function. Trends Neurosci. 1995;18:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
Edited by Zhang JZ