INTRODUCTION
Pancreatic cancer is the fourth leading malignancy in terms of incidence in Western countries. Chemotherapy and radiotherapy could only produce minimal benefit. Extensive surgery is not boundless, either[1]. Pancreatic cancer has been investigated in many institutes[2-5], a great improvement of prognosis will only depend on clarifying molecular-biology of this grim tumor[6]. Obstructive jaundice is the most common symptom of pancreatic cancer, especially in developing countries and regions. Pancreatic cancer patients are always complicated with profound jaundice. About 70% of pancreatic cancer patients already have obstructive jaundice at the time of diagnosis. Obstructive jaundice is a very life threatening pathophysiological disorder because elevation of bile acids in serum may attack many vital organs. Only two days after bile duct ligation, bile acids in serum have been reported to increase 30 fold compared to that of sham operated animals[7]. Bile acids are amphiphilic compounds that behave as detergents in aqueous solution. The hydrophobicity-hydrophibicity determine the cytotoxicity of bile acids by destroying bio-membrane specifically. No specific cytotoxicities of bile acids have been proved in hepatocytes, erythrocytes[8] and intestinal mucous epithelium[9] culture systems. However, proliferation of colonic epithelium stimulated by bile acids has been reported to occur with or without obvious cell surface damage[10,11]. TUDC has been reported recently to enhance intrahepatic bile duct carcinogenesis in the hamster model. Although obstructive jaundice is very closely related to pancreatic cancer and both the pathophysiology of obstructive jaundice and biology of pancreatic cancer have already been investigated respectively, we know little of interaction between bile acids and biology of pancreatic cancer. In treatment of pancreatic cancer, the main purpose of surgery is to eliminate jaundice, and sometimes it is also a way to access extensive resection. If the biological effect of bile acids in serum on pancreatic cancer is clarified, it will be very helpful for treating pancreatic cancer with jaundice. In this study, three typical pancreatic cancer cell lines and six kinds of common bile acids were used to explore the direct effects of bile acids on proliferation and morphological changes of pancreatic cancer cells.
MATERIALS AND METHODS
Cell culture and treatment
Human pancreatic cancer cell lines PANC-1, MIA PaCa-2 (purchased from American Type Culture Collection) and hamster pancreatic cancer cell line PGHAM-1(induced by BOP in the laboratory of First Department of Surgery, Nippon Medical School[12]) were used in this study. PANC-1, MIA PaCa-2 were primarily cultured in a 75 cm2 flask with the cell concentration of about 2.0 × 103/ml in RPMI 1640 (GIBCO BRL, New York) medium. PGHAM-1 was incubated in Dulbeccon’s modified eagle medium (MEM: GIBCO BRL, New York) in the same way. Both media were supplemented with 10% fetal bovine serum (FBS), 100 u/ml penicillin-streptomycin and 100 g/ml kanamycin and 100 g/ml amphotericin B (GIBCO, New York). Four days later, PANC-1, MIA PaCa-2 and PGHAM-1 cells were harvested and re-suspended in media supplemented with or without certain concentrations of bile acids including cholic acid (CA), deoxycholic acid (DCA), lithocholic acid (LCA), taurochenodeoxycholic acid (TCDC), taurodeoxycholic acid (TDCA) and glycocholic acid (GCA) (Sigma Chemical Co. USA). PANC-1, MIA PaCa-2 and PGHAM-1 cells in each group were treated and continuously cultured according to the protocols that the concentration of bile acid in each medium ranged from 10 µmol/L to 60 µmol/L. Cell density in the suspension was adjusted to 6.0 × 103/ml. In the 96-well plates, 100 μl of cell suspension was added to each well, 6 wells for each group. The cells were incubated for 72 h and the viability of cells was measured by MTT assay. In another 96-well plate, PANC-1 cell suspension at very low concentration of bile acids (from 2 µmol/L to 8 µmol/L) was incubated for 144 h and tested by MTT assay. The medium was changed every 2 days at the same concentration of bile acids. PANC-1 cell suspension was incubated in normal or 40 µmol/L of bile acids (DCA, CA, GCA and TDCA) media in 25 cm2 flasks for 72 h for cell cycle analysis. For scanning and transmission electron microscopic (SEM and TEM) investigations, 4 ml of PANC-1 cell suspension was incubated in normal or 20 µmol/L or 40 µmol/L DCA modified media in 25 cm2 flasks for 48 h.
MTT assay
After 48 h or 96 h incubation, the viability of cells in each group was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Chemicon International Inc. Temecula, CA, USA) assay[13]. Each group had six samples. With this method, 10 µl of (5 mg/ml) MTT was added to each cell suspension and the cells were incubated for 4 h. The purple formazan product formed by the action of mitochondrial enzymes in living cells was solubilized by the addition of acidic isopropanol. The absorbency of each cell suspension was measured using a microplate reader (Model 3550, Bio-Rad, CA, USA) at a test wavelength of 560 nm and a reference wavelength of 655 nm. The inhibitory rates were calculated according to the following formula: inhibitory rate = (1-tested MTT viability/mean of control MTT viability) × 100%.
Flow cytometry
The cell cycles of PANC-1 cells incubated in 40 μM of bile acids added media for 24 h were measured by the following method. Cells were washed, permeabilised and exposed for 30 min at 4 °C to 800 µl of DNA-staining solution in 0.1% Nonidet P-40 (Sigma-milan, Italy) and 25 ug/ml propidium iodide (Sigma-Aldrich). The cellular DNA content was analysed by fluorescent activated cell sorter (FACS) can (Bencton Dickinson Immunocytometry Systems, San Jose, CA, USA) using cell Quest software system for histograms of cell frequency versus propidium iodide fluorescence intensity.
SEM and TEM
PANC-1 cells in 25 cm² flasks were harvested by trypsinization after 24 h and 48 h of incubation. The cells were fixed with 2.5% glutaraldehyde and 1% osmium tetroxide (TAAB Laboratories Equipment Ltd. Berkshire, UK). The dehydration was carried out with an ethanol series. SEM specimens were dried using a critical point dryer (Hitachi Hcp-2), and the sputter was coated with Pt+Cd using an ion sputtering device (Hitachi Oie-102). The cell surface alteration was observed under a SEM device (Hitachi S-570). The dehydrated TEM samples were mixed with proplylene oxide and Epok 812, which were embedded and proymerilizated. The semithin sections were stained with touluidine blue. Ultrathin sections were made using the ultra-microtome (Porter-Blum MTZ-B) and then were stained with uranyl acetate and lead critrate. The inner ultrastructure of the cells was investigated using the TEM Device (Hitachi H-500).
Statistical analyses
The digital data in this study were represented by -x±s and assessed by the analysis of variance (ANOVA). Student’s t-test (SPSS/PC7.5) was also used for cross comparison between different groups and different incubation times. Probabilities of less than 0.05 were considered as significant.
RESULTS
Inhibitory effects of bile acids on cultured pancreatic cancer cell lines
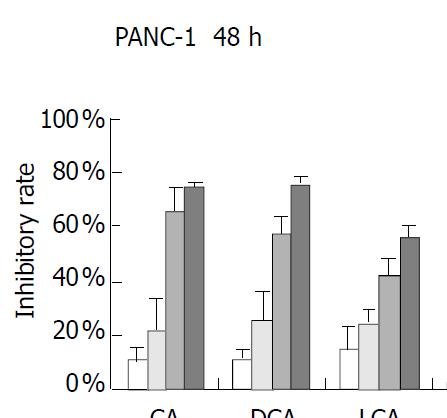
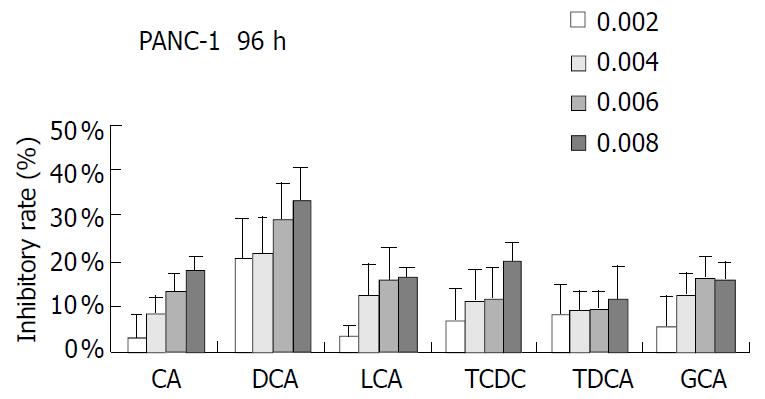
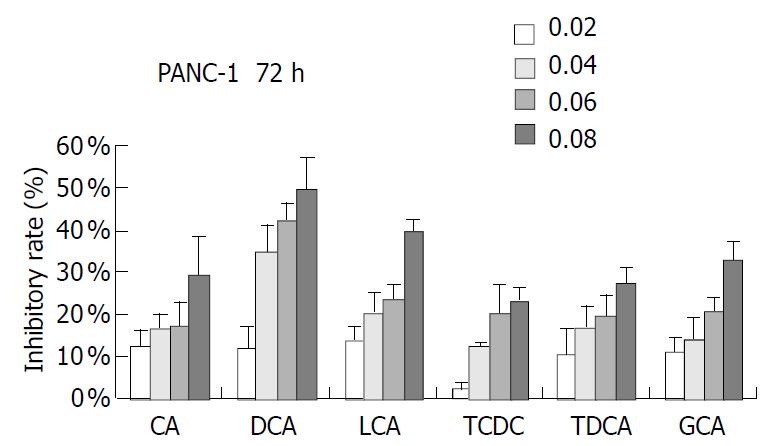
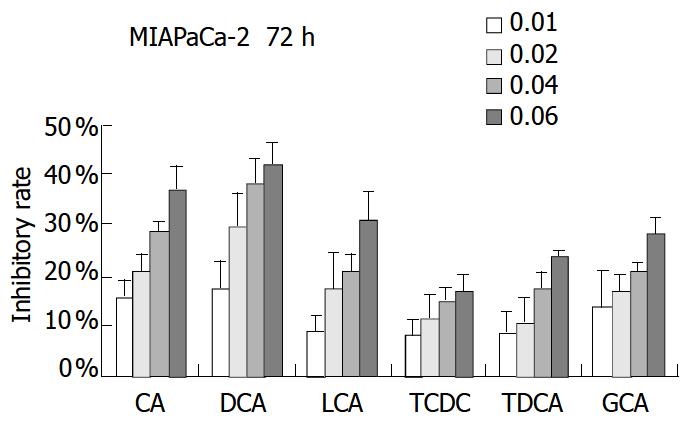
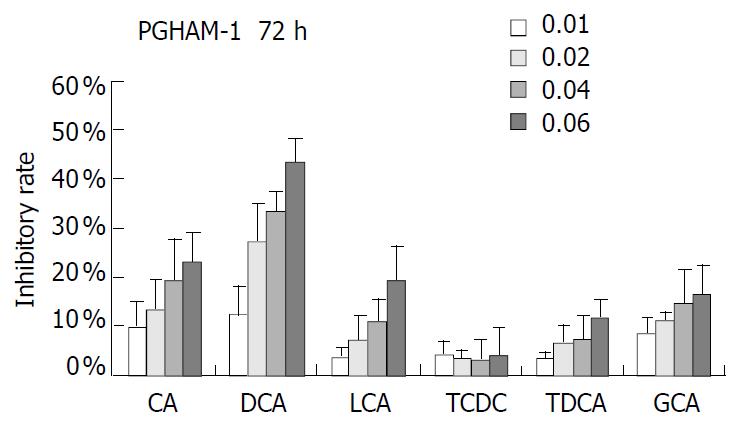
PANC-1 cell growth in 50 µmol/L-1000 µmol/L of bile acids added RPMI-1640 medium was significantly inhibited in only 24 h compared with control (data not shown). When PANC-1 cells were cultured in 0.05 mmol/L to 1.0 mmol/L bile acid modified medium for 48 h, including unconjugated bile acids CA, DCA over the concentration of 0.5 mmol/L, LCA over 1.0 mmol/L and conjugated bile acids GCA over 1.0 mmol/L greater than 50% growth inhibition was obtained (Figure 1). While PANC-1 treated with very low bile acid concentration of 2 µmol/L to 8 µmol/L for 96 h also produced certain inhibitory effect, but only exceeding 6 µmol/L did DCA show significant effect (Figure 2). All of three cell lines: PANC-1, MIA PaCa-2 and PGHAM-1 were incubated in 10 µmol/L-60 µmol/L bile acid supplemented media for 72 h, cell proliferation was inhibited to a certain degree. But the unconjugated bile acids CA, DCA and LCA showed powerful inhibitive capacity (Figure 3, Figure 4, Figure 5).
Figure 1 Bile acids (mmol/L) on PANC-1 for 48 h.
Figure 2 Bile acids (mmol/L) on PANC-1 for 96 h.
Figure 3 Bile acids (mmol/L) on PANC-1 for 72 h.
Figure 4 Bile acids (mmol/L) on MIA PaCa-2-1 for 72 h.
Figure 5 Bile acids (mmol/L) on PGHAM-1 for 72 h.
The inhibitive effects of all six bile acids were ranked in the following order: DCA > CA > LCA > GCA > TDCA > TCDC. Bile acid inhibition on the growth of three pancreatic cancer cells all showed a dosage dependent pattern. Among these bile acids, DCA produced the most powerful and obvious dosage-dependent inhibitory effect. Meanwhile PANC-1 cells were incubated in DCA (10-60 µmol/L) added medium supplemented with dexamethasone (50 µg per well), DCA’s inhibitive effects could not be reversed by supplement of membrane stabilizer dexamethasone (data not shown).
Ultrastructural alteration of PANC-1 cells incubated in DCA added medium
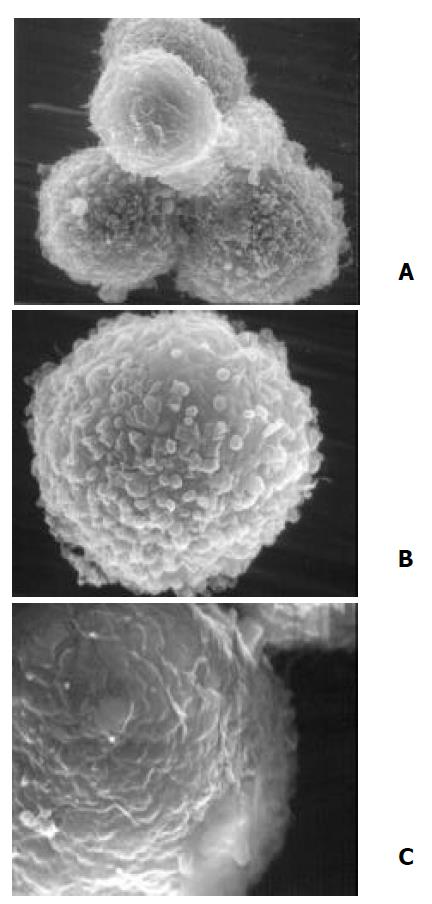
The untreated PANC-1 cells, as viewed by SEM, were characteristic of spherical in shape with plentiful microvilli which could be described as floss, spherical and leaf like microvilli (Figure 6A×7500). When PANC-1 cells were cultured in 20 µmol/L DCA modified medium for 48 h, the majority of microvilli turned into spherical villi and the density of microvilli decreased obviously (Figure 6B×13000). When PANC-1 cells were kept in 40 µmol/L DCA treated medium for 48 h, some cell surfaces were dotted with huge leaf-like microvilli or big microhills. Most cells lost their microvilli, becoming a bear ball with only some microvilli remnants, micro-trench and prominences (Figure 6C×20000).
Figure 6 Ultrastructural alteration of PANC-1 viewed by SEM.
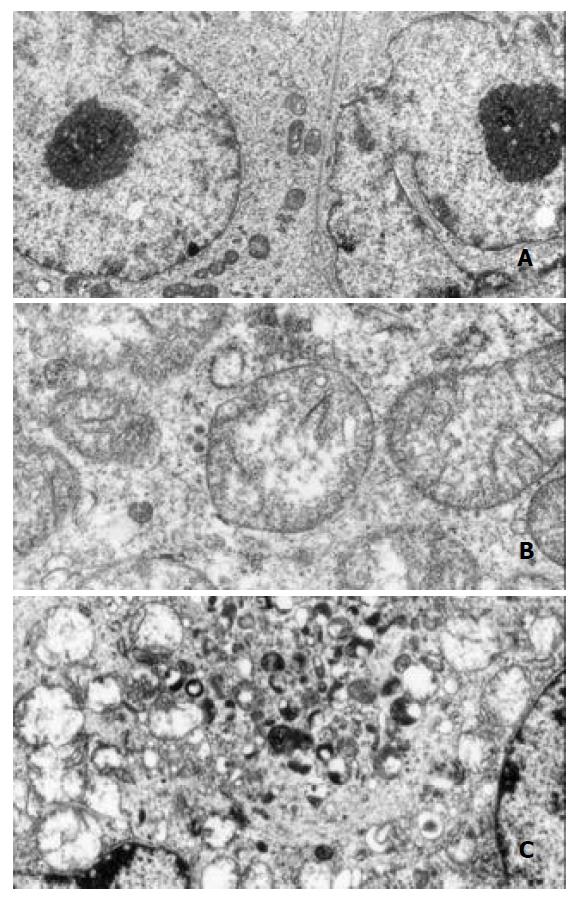
Observation of intracellular ultrastructure with TEM showed the cells in control group had normal distribution of organelles, with the integrity of mitochondria and other organelles preserved. The nuclei were surrounded by double membrane envelope (Figure 7A×9600). The cells growing in 20 µmol/L DCA modified medium for 48 h exhibited greatly extended mitochondria and dilated rough endoplasmic reticulum. All the organelles located in cells disorderly and the obviously dilated mitochondria contained twisted and broken cristae. Injured organelles were frequently seen in plasm (Figure 7B×30000). When DCA concentration was increased to 40 µmol/L and incubated with cells for 48 h, exceedingly dilated mitochondria and vesicles formed by endoplasmic reticulum could be observed, some mitochondria lysed in cytoplasm were also seen. The lysosomes were also damaged and broken, which caused other surrounding organelles lysed in cytoplasm. The major portion of cytoplasm was occupied by vacuoles. The nuclear membrane double layer structure turned into indistinct and disconnected envelope. Rough granular masses against the inner surface of nuclear membrane could easily be observed (Figure 7C×12000).
Figure 7 Ultrastructural alteration of PANC-1 viewed by TEM.
Cell cycles of PANC-1
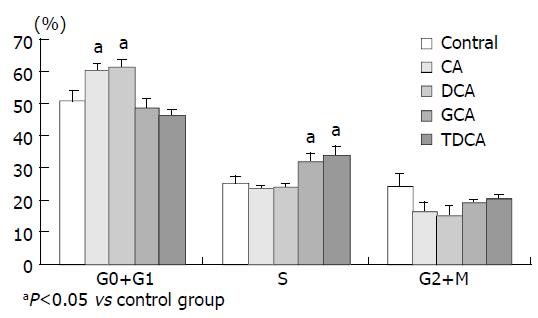
The cell cycles of PANC-1 cells incubated in 40 μM of bile acids added media for 24 h were shown as follows. DCA and CA increased the percentage of G0 + G1 phases cells, while GCA and TDCA elevated the S phase cell number compared with the control (P < 0.05) (Figure 8).
Figure 8 Cell cycles of PANC-1 cells incubated in 40 μM of bile acids added media for 24 h.
DISCUSSION
Pancreatic cancer frequently causes obstructive jaundice and elevates bile acid level in serum. For example, in non-pruritic jaundiced serum GCA concentration was 33.44 ± 16.90 µmol/L[14]. Bile acids are steroid metabolites of cholesterol functioning as trophic factors for gut epithelium and as detergents for absorption of cholesterol and fat-soluble vitamins. The cytotoxicity of bile acids on various normal human cells[15,16] and a few malignant cells[17,18] has been demonstrated. It has been previously confirmed that bile acids could stimulate the growth of colonic epithelial cells but not that of colonic cancer cell lines[19]. While Debruyne et al[20] found recently that bile acids such as CA, chenodeoxycholic acid (CDCA), DCA, LCA could stimulate the invasion of HCT-8/E11 and PCmsrc human colorectal cancer cells into collagen type I gels. Is there any interaction of elevated bile acids on human pancreatic cancer progression However, how bile acids influence pancreatic cancer remains a subject of controversy. In order to investigate the direct effects of bile acids on pancreatic cancer, three typical pancreatic cancer cell lines(PANC-1, MIA PaCa-2 and PGHAM-1) and six common bile acids were selected and used in this study. Cultured pancreatic cancer cells have provided a model of pancreatic cancer free of other probable influencing factors such as immunoreaction and serum albumin in combination with bile acids in the body. MTT colorimetric assay is a very useful method for evaluating cell survival and proliferation. The reliability and sensitivity of MTT assay have been demonstrated to be comparable to that of tritiated thymidine incorporation in cell proliferation and cytotoxicity assays[21]. To a certain extent, the results of MTT reflect the final effects of cell proliferation or cytotoxicity instead of only earlier events detected by tritiated thymidine incorporation.
In this study, PANC-1 cells were incubated in 50 µmol/L to 1.0 mmol/L bile acid supplemented medium for 48 h, cell growth was inhibited to some extent at low concentration of bile acids and was also significantly inhibited by the higher concentration of bile acids. When bile acids concentration was reduced to only 2-8 µmol/L and the incubation time was prolonged to 96 h, cell proliferation was also observed. In 6-8 µmol/L of DCA supplemented medium significant inhibition could be obtained. Cell proliferation of PANC-1, MIA PaCa-2 and PGHAM-1 was significantly inhibited by bile acids supplemented media at certain concentrations for 72 h. The inhibitive effects of all six bile acids were ranked in the following order: DCA > CA > LCA > GCA > TDCA > TCDC. The inhibition exhibited a dosage-dependent manner, especially for DCA. Among these three cell lines, MIA PaCa-2 seemed to show the most sensitive response to all of the bile acids. The concentrations of bile acids resembled the elevation of some bile acids in jaundiced serum. Our investigation indicated that free bile acids in jaundiced serum might inhibit pancreatic cell growth and proliferation. Furthermore, we found that when PANC-1 cells were incubated in 40 μM of DCA or CA added media for 24 h, the percentage of G0+G1 phase cells was significantly increased compared with the control group (P < 0.05). This implied that DCA and CA could induce cell cycle arrest, resulting in reduced proliferation and apoptosis of PANC-1 cells. Concerning its mechanism, Martinez et al[22] believed that cytotoxicity of DCA might be produced through induction of apoptosis via a protein kinase C-dependent signaling pathway.
According to our ultrastructural findings, the membrane and organelles were definitely damaged by supplement of bile acid DCA in medium, the inhibitory effects of bile acids on these pancreatic cancer cell lines might mainly depend on their cytotoxicity. Usually, cytotoxicity of bile acid is resulted from its hydrophobic features and hydrophobic-hydrophilic balance in serum. Lipophilic unconjugated bile acids have been shown to flip-flap rapidly across artificial lipid bilayers, so that unconjugated bile acids might enter the cytosol of every kind of cells even at a low concentration[23]. It has also been demonstrated that DCA or CDCA administration in rat colon could enhance cell membrane phospholipid turnover[24]. Hydrophobic bile acids, such as DCA, could damage cells by lysing membranes[25] and impairing mitochondrial function as well as increasing the generation of reactive oxygen radicals which can cause membrane lipid peroxidation and attack nucleic acids[26]. Velardi et al have demonstrated that the cytotoxicity of bile acids is partially dependent on the cell membrane composition. Because cell membrane components such as glycolipids, receptors, and transport proteins vary in different cell lines depending on cell origin and differentiation, cell lines may also differ in regard to their response to bile acids. The over susceptibility of MIA PaCa-2 to bile acids might be due to their unique membrane lipid components. Under SEM, we also found that DCA could reduce microvilli density of PANC-1 cells. The microvilli on the surface of malignant cells could contribute to movement, attachment and invasion of malignant cells[27]. Reduction of microvilli might potentially inhibit PANC-1 cell invasion.
Biliary drainage has been shown to be an appropriate, definitive therapy for biliary obstruction due to unresectable pancreatic and peripancreatic malignancies. Some surgeons preferred to choose billiary drainage as a preoperative treatment to reduce the level of jaundice. They expected patients to get a better operation tolerance before the radical cure. However, patients with jaundice had a higher risk of bleeding complications while those with preoperative biliary drainage (PBD) had more infective complications[28]. Some researchers demonstrated that preoperative biliary drainage increased the risk of developing intraoperative infectious morbidity and postoperative infectious morbidity and mortality following pancreaticoduodenectomy. Preoperative biliary drainage should be avoided whenever possible in patients with potentially resectable lesions. Consequently, the effect of bile acids on pancreatic cancer should be evaluated. It is concluded that bile acids could inhibit proliferation of PANC-1, MIA PaCa-2 and PGHAM-1 cell lines in vitro[29]. This may imply that bile acid can inhibit the growth of pancreatic cancer. Although the observation that bile acids inhibited proliferation of cultured cell lines could not completely reflect the complex pathophysiology of pancreatic cancer patients accompanied with obstructive jaundice, this study, at least, provides a clue that bile acids in jaundiced serum may have potentially inhibitory effects on pancreatic cancer progression. However, our investigation is an in vitro one, further studies are needed.
ACKNOWLEDGEMENTS
We would like to express our thanks to Mr. Masahiko Onda and Mr. Eiji Uchida, from the First Department of Surgery, Nippon Medical School, Tokyo, Japan, for their valuable help in the work.