INTRODUCTION
Amyloidosis is an abnormal intercellular deposition of insoluble proteins that share a remarkably similar and stable core structure of β sheets[1]. It may be resulted from a heterogeneous group of disorders and result in impairment or even dysfunction of involved organs. Generally, amyloidosis is more commonly manifested as a systemic involvement of multiple tissues and organs including the heart, liver, spleen, kidneys, lymph nodes, adrenals, thyroid, as well as many others. In contrast, the clinical implication of a single organ or tissue is relatively rare in this pathological condition[2-6], in which the amyloidal deposit confined to the stomach is extremely scarce in the previous literatures[7-9]. Recently, we have experienced and cured a case of localized gastric amyloidosis and now report it as follows.
CASE REPORT
A 50-year-old female was admitted to our hospital on Aug 23, 2002, with chief complaints of recurrent epigastric discomfort for 10 years and a newly-appeared dull pain in the upper abdomen for 4 mo. The Inpatient No of this patient was 370655. Ever since being diagnosed as gastric ulcer and erosive gastritis with intestinal metaplasia 10 years ago by gastroscopy, she has not received any normal treatment except for long-term administration of metronidazole and omeprazole tablets herself. Prior to hospitalization, she was suggested to be of cancerization from gastric ulcer by gastroscopy at another medical institution. On admission, the patient displayed a good general condition and no positive signs including enlargement of superficial lymph node were revealed by physical check-up. Laboratory data showed negative results in the detection of serum anti-streptolysin O, rheumatoid factor and urine Bence-Jones protein. No abnormal signs were found on the chest radiograph. An upper gastrointestinal endoscopy revealed a gastric ulcer of 3 cm × 1 cm in size that was located at the posterior wall of small curvature at the inferior part of gastric corpus. The margin of the ulcer was heaped up and rugged, the ambient mucosa was erosive, friable and prone to bleeding. The base of the ulcer was shaggy and covered with fibrinous layers. The malignization of this ulcer was suggested by endoscopic ultrasonography with low echo findings that the sick part of gastric wall was markedly and unevenly thickened, and some parts of the submucosa were infiltrated. On the contrary, a diagnosis of gastric amyloidosis, along with chronic gastritis with intestinal metaplasia, proliferation of lymphatic tissue and negative finding of Helicobacter pylori, was made by the biopsy of gastric mucosa. Exploratory laparotomy was carried out on Sep 3, 2002, in which no abnormal signs including enlargement of lymph node were found except that part of tumor-like, stiff and diffusely-thickened gastric wall was recognized at the inferior part of gastric corpus. Subtotal gastrectomy and clearance of perigastric lymph nodes were performed. Final pathological diagnosis determined the lesion to be the deposition of amyloidal materials in the gastric mucosa, submucosa and blood vessel walls with intestinal metaplasia and atrophy of the gastric glands, and no malignancies or other tumors were found. When stained with hematoxylin-eosin (Figure 1) and Congo red (Figure 2) respectively, the amyloidal deposits displayed as amorphous, homogeneous, translucent and acidophilic material under light microscope. The amyloidal protein was further proved to be the AA type by the fact that it exhibited green birefrigence with Congo red staining under polarized light, which was disappeared when the specimens were pretreated with potassium permanganate. The patient got recovered and no complications occurred after operation. Multiple biopsies from esophagus, remnant stomach, duodenum, colon and bone marrow in the follow-up survey of 5 mo post operation showed no amyloidal deposition in these tissues and organs. Up to the present, no signs of recurrence have been found in this patient.
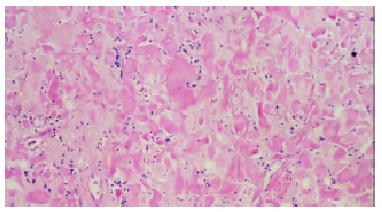
Figure 1 Stained with hematoxylin-eosin, amyloidal deposits in gastric mucosa and submucosa display amorphous, homogeneous, translucent and acidophilic materials under light microscope.
(Magnification × 100).
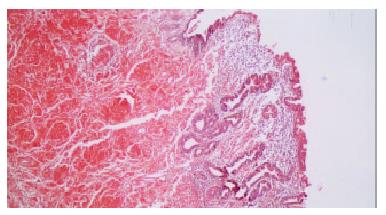
Figure 2 Stained with Congo red, deposition of amyloid could also be observed extending from gastric mucosa to submucosal layer.
(Magnification × 50).
DISCUSSION
Amyloidosis, a disorder marked by the deposition of amyloid in various organs and tissues of the body, is usually associated with a variety of chronic diseases such as rheumatoid arthritis, tuberculosis, multiple myeloma and many others. Its classifications have been notoriously unsatisfactory for donkey's years because the definition of this disorder was initially based on the morphological features, in which the amyloidosis was categorized according to the tissue distribution of amyloid (e.g. systemic versus localized amyloidosis) and the presence or absence of the identifiable predisposing factors (e.g. secondary versus primary amyloidosis). As the unique feature of amyloidal substance was, the component of the precursor protein that forms the fibrillar deposit has been now accepted as the basis for the classification of amyloidosis[10]. Up to the present, several types of the precursor proteins such as serum amyloid A (SAA), amyloid immunoglobulin light chains (AL), abnormal transthyretin (ATTR), β2 microglobulin (β2-M), amyloid precursor protein etc have been identified in amyloidosis.
Gastrointestinal tract is one of the regions to be commonly involved in the systemic amyloidosis. However, amyloidosis confined to the stomach is a rare occurrence. Although the detailed mechanism for the deposition of amyloidal materials in a specific tissue or organ remains unclear, the excessive accumulation of proteinaceous metabolites in local tissue might be a possible explanation[11]. The patient in our report suffered from gastric ulcer and gastritis for more than 10 years, which might cause a local disorder in protein metabolism and lead to localized deposition of amyloidal materials.
The clinical manifestations of amyloidosis were often uncharacteristic and varied with the involved organs. As for localized gastric amyloidosis, a variety of common gastrointestinal symptoms such as epigastric discomfort, poor appetite, hematemesis, hematochezia and gastric perforation might occur in the process of this disease because of involvement of local autonomic nervous system[7] and gastric wall structure damage[8]. Although localized gastric amyloidosis might associate with gastric malignancies in some cases[6,12,13], its non-tumorous form usually tended to be misdiagnosed as gastric tumors due to the likeness of gross appearance in endoscopic and imaging examinations. In this respect, biopsy has been suggested to be the only means to confirm the diagnosis[15]. The fact that pretreatment with potassium permanganate made biopsy specimens unstained by Congo red is helpful to determine the amyloidal component as AA type rather than AL protein. Scintigraphy with radiolabeled serum amyloid P (SAP) component could provide support for the diagnosis of amyloidosis in patients with negative histological studies[11] and distinguish localized lesion from systemic amyloidosis[14]. Besides, immunohistochemical staining or immunofixation electrophoresis of biopsy specimens with the specific antibodies might guarantee the accurate classification of this disease[15-17].
The prognosis of amyloidosis depends on both the specific types of lesions and the involved organs. Systemic amyloidosis is usually with an unfavorable prognosis while the localized types of this disease such as the localized gastric amyloidosis have a relatively better outcome. Untreated AL amyloidosis often had the worst prognosis with a median survival time of one to two years[18], especially when cardiac involvement occurred. Patients with ATTR amyloidosis might survive up to 15 years from diagnosis but this time also varies with the specific mutation and the time of diagnosis - the younger the age of presentation the worse the outcome. However, the prognosis of patients with AA type was affected mainly by the underlying conditions[1,15]. Currently, there is no specific therapy for systemic amyloidosis. The treatment strategy has been directed both to support the affected organs and to deal with the underlying specific disease[19] in an attempt to reduce the deposition of amyloidal substances and improve prognosis, in which several supportive protocols and chemotherapeutic drugs including melphalan, iodinated anthracycline 4-iodo-4-deoxydoxorubicin, dimethylsulfoxide and colchicines have been widely used, although their effectiveness in ameliorating this disease has remained to be determined[15]. With the advances in molecular biology, some promising attempts have been made to reduce inflammatory response and amyloidal deposits by blocking the signal conduction of RAGE-NF-κB in monocytes/macrophages[20]. In patients with localized amyloidosis, thorough resection of the foci and their circumambient lymph nodes as performed in our case is probably the preferable therapeutic modality and the key measures to prevent postoperative recurrence. Up to the present, no signs of recurrence have been found in the follow-up survey of our patient.