Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2596
Revised: July 14, 2003
Accepted: July 24, 2003
Published online: November 15, 2003
AIM: To detect the vascular endothelial growth factor (VEGF) and soluble splice variant 6 of CD44 (sCD44v6) levels in ascites and to explore their role in differentiating benign from malignant ascites.
METHODS: Cirrhotic ascites (n = 36), tuberculosis ascites (n = 8) and malignant ascites (n = 23) were collected and studied. Concentrations of soluble VEGF and sCD44v6 in various kinds of ascites (n = 67) were measured using a sandwich enzyme-linked immunoadsorbent assay.
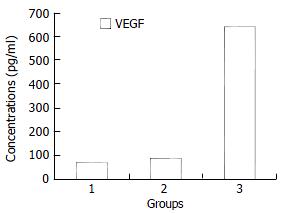
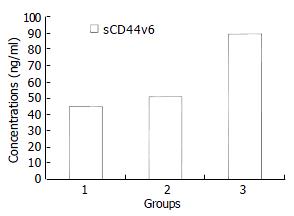
RESULTS: VEGF and sCD44v6 levels in malignant ascites were 640.74 ± 264.81 pg/mL and 89.22 ± 38.20 ng/mL, respectively, both of which were significantly higher than those in cirrhotic ascites and tuberculous ascites (q = 18.98, 11.89 and q = 8.92, 5.09; P < 0.01). However, the levels of VEGF and sCD44v6 in cirrhotic and tuberculous ascites had no significant difference (q = 0.48, 0.75; P > 0.05). Furthermore, VEGF levels in malignant ascites in patients with ovarian cancer were higher than those with gastric and colon cancer (q = 5.03, 6.79; P < 0.01, respectively). But differences of VEGF levels between gastric and colon cancer were not significant (q = 1.90, P > 0.05). Whereas, sCD44v6 levels in malignant ascites from patients with ovarian, gastric and colon cancer had no significant difference (q = 0.06, 0.91, 0.35; P > 0.05, respectirely). In comparison with cirrhotic and tuberculous ascites, when the upper limit of its VEGF mean levels 119.44 pg/mL (70.90 ± 48.54) and sCD44v6 mean levels 63.59 ng/mL (44.42 ± 19.17) was taken as the minimum cutoff limit, the sensitivity and specificity of VEGF and sCD44v6 of this assay to the diagnos is of malignant ascites were 91.3%, 90.9% and 73.9%, 88.7% respectively.
CONCLUSION: Elevated levels of VEGF and sCD44v6 may be useful in differential diagnosis of benign and malignant ascites.
- Citation: Dong WG, Sun XM, Yu BP, Luo HS, Yu JP. Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol 2003; 9(11): 2596-2600
- URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2596.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2596
Angiogenesis is an absolute requirement for neoplastic growth of solid tumors after tumors reach a critical size of 1-2 mm3[1], and is also essential for tumor invasion and metastasis, facilitates the shedding of tumor cells into surrounding blood vessels. Tumor cells have been shown to secrete a variety of angiogenic factors and thereby induce local formation of new blood capillaries. Among these factors, vascular endothelial growth factor (VEGF), also called vascular permeability factor (VPF), is a bifunctional cytokine and has the role in enhancing vascular permeability and stimulating endothelial growth[2-5], and is recognized as one of the most important molecules in the growth, invasion, metastasis and recurrence of human tumors[6-9].
However, tumor invasion and metastasis are considered to be a complex and multi-step process. Since the initial observation that a splice variant of CD44 (CD44v) could endow non-metastasizing cells with metastasis potential[10]. Many studies have demonstrated that CD44v, especially splice variant 6 of CD44 (CD44v6), probably promoting cancer cells to adhere to vascular endothelium and base membranes and enhancing moving ability of cancer cells, is most likely responsible for the invasion and metastasis of several tumor systems[11-15].
Malignant ascites is the direct and prominent manifestation of advanced carcinoma metastasized to the peritoneum[16]. Thus it is reasonable to hypothesize that VEGF and CD44v6 can be detected in malignant ascites. In the present study, we measured the concentration of VEGF and soluble CD44v6 (sCD44v6) using an enzyme-linked immunoadsorbent assay (ELISA) in various kinds of ascites in order to assess the value of VEGF and CD44v6 in identifying benign and malignant ascites.
A total of 67 inpatients with ascites were collected at Renmin Hospital of Wuhan University, Zhongnan Hospital of Wuhan University and Tumor Hospital in Hubei Province from July 2002 to March 2003 (Table 1). Informed consent of the patient and approval of the hospital were provided prior to collection of samples and medical records. All the cases were confirmed by cytologic examination of ascites, pathological examination, B-ultrasound and CT scan, etc.
| Diagnosis | No. of Patients | Mean age (range) | Female/male |
| Ascite | 67 | 47 (19-86) | 26/41 |
| Cirrhotic ascites | 36 | 48 (30-86) | 10/26 |
| Tuberculous ascites | 8 | 28 (19-33) | 4/4 |
| Carcinoma ascites | 23 | 66 (35-76) | 12/11 |
| Ovarian cancer | 8 | 60 (35-70) | 8/0 |
| Gastric cancer | 6 | 68 (38-74) | 1/5 |
| Colon cancer | 5 | 64 (48-71) | 2/3 |
| Hepatocellular cancer | 2 | - | 0/2 |
| Pancreatic cancer | 1 | - | 0/1 |
| Primary peritoneal carcinoma | 1 | - | 1/0 |
Ascites samples were collected during therapeutic or diagnostic paracentesis and centrifuged at 3000 rpm for 15 min at 4 °C. Cell free supernatants were collected and aliquots were stored at -70 °C before determination.
Cirrhotic, tuberculous and malignant ascites were defined as groups 1, 2 and 3, respectively. Malignant ascites from patients with ovarian, gastric and colon cancer were grouped as groups A, B and C, respectively.
Concentrations of VEGF in ascites were determined with an ELISA kit (R&D Systems) following the manufacturer’s guidelines. All samples were analyzed in the laboratory of the Department of Gastroenterology, Renmin Hospital, Wuhan University. For determination of VEGF, samples were analyzed in duplicate, human recombinant VEGF165 was diluted in series and used as a standard. VEGF concentrations were measured according to the standard curve. Samples with VEGF values beyond the standard curve were diluted and reanalyzed.
Levels of sCD44v6 in ascites were measured with a sCD44v6 ELISA kit (Bender MedSystems, Austria). Briefly, monoclonal antibody against CD44v6, VFF-7, was absorbed by microwells in 96-well microtiter plates. sCD44v6 in the sample or in the standard bound to antibodies was adsorbed by each microwell. Horseradish peroxidase-conjugated monoclonal antibody against CD44v6 was then added and bound to the sCD44v6 that had been captured by the first antibody. After incubation, unbound enzyme conjugated antibodies were removed by washing and a substrate solution was added to each well. A colorful reactive product was formed, the reaction was terminated by addition of acid, and absorbance was measured at 450 nanometers. A standard curve was prepared from six standard dilutions of sCD44v6, which allowed determination of the levels of sCD44v6 in our samples.
The data were presented as ¯x ± s. One-way analysis of variance was used for statistical analysis. Differences were considered significant when P value was less than 0.05.
Figure 1 shows VEGF levels in malignant ascites (640.74 ± 264.81 pg/mL), which were significantly higher than those in cirrhotic ascites (67.05 ± 51.91 pg/mL), tuberculous ascites (88.25 ± 24.12 pg/mL) (P < 0.01). However, there was no significant difference of VEGF levels between cirrhotic and tuberculous ascites (P > 0.05).
sCD44v6 levels in malignant ascites (89.22 ± 38.20 ng/mL) were higher than those in cirrhotic ascites (44.79 ± 18.02 ng/mL), tuberculous ascites (50.25 ± 12.57 ng/mL) (P < 0.01). But the difference of sCD44v6 levels in cirrhotic and tuberculous ascites was not statistically significant (P > 0.05) (Figure 2). We found both VEGF and sCD44v6 levels were increased in malignant ascites.
Statistical comparison of VEGF and sCD44v6 levels in these kinds of malignant ascites was not performed due to the limited number of hepatocellular cancer (n = 2), pancreatic cancer (n = 1) and primary peritoneal carcinoma (n = 1).
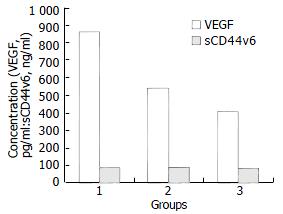
Figure 3 shows VEGF levels in ascites from patients with ovarian cancer (866.25 ± 208.46 pg/mL), which were higher than those with gastric cancer (541.30 ± 123.17 pg/mL) and colon cancer (402.80 ± 140.10 pg/mL), respectively (P < 0.01). There was no significant difference of VEGF levels between gastric and colon cancer (P > 0.05). Whereas, no statistical difference of sCD44v6 levels in ascites of patients with ovarian cancer (89.42 ± 25.70 ng/mL), gastric cancer (83.91 ± 32.62 ng/mL) and colon cancer (80.10 ± 9.97 ng/mL) was found (P > 0.05).
Additionally, in our study, 2 out of 4 unidentified ascites cases were identified by peritoneum biopsy as metastatic ovarian cancer and primary peritoneal carcinoma. In these two cases, the levels of VEGF exceeded 1200 pg/mL, and sCD44v6 levels exceeded 100 ng/mL. Another 2 cases of gastric and colon cancer, were identified by gastroscopy and colonoscopy, respectively. The concentration of VEGF and sCD44v6 in these 2 cases exceeded 650 pg/mL and 80 ng/mL respectively.
Using benign ascites including cirrhotic and tuberculous ascites as control, and the upper limit of its VEGF mean levels, 119.44 pg/mL (70.90 ± 48.54), as positive boundary value, 21 out of 23 malignant ascites cases and 4 out of 44 benign ascites cases exceeded the boundary line. So the sensitivity and specificity of this assay to the diagnosis of malignant ascites were 91.3% (21/23) and 90.9% (40/44), respectively, and calculated positive value, negative value and accurate rate were 84.0% (21/25), 95.2% (40/42) and 91.0% (61/67), respectively. With same method, the sensitivity, specificity, positive value, negative value and accurate rate of sCD44v6 to the diagnosis of malignant ascites were 73.9% (17/23), 88.7% (39/44), 77.3% (17/22), 86.4% (39/45) and 83.4% (56/67), respectively.
Human VEGF could be expressed in at least 5 isoforms, which have 206, 189, 165, 145 and 121 amine acids, respectively[17]. It is a multifunctional cytokine that has potent angiogenic activity and enhances microvascular permeability by direct action on vascular endothelium, promoting tumor growth and metastasis[18,19]. Strong VEGF expression has been demonstrated in various solid tumor types, including gastric[20-24], colorectal[25,26] and ovarian carcinomas[27,28]. Recently, substantial evidences indicated that serum concentration of VEGF was increased in cancerous patients[29-32]. Although some studies showed that VEGF levels were high in malignant ascites[30,33], its value to the diagnosis of malignant ascites has not been elucidated.
In the present study, we found that VEGF protein levels in malignant ascites were markedly higher than those in benign ascites, which were consistent with the previous results[30,33], and possessed a high sensitivity and specificity to the diagnosis of malignant ascites. Meanwhile, we also found that VEGF levels in malignant ascites in patients with ovarian cancer were higher than those in patients with gastric or colon cancer. No significant difference of VEGF levels was observed among those with gastric and colon cancer. Additionally, although the number of cases studied in our experiment was small, extremely increased VEGF levels in ascites of patients with ovarian cancer (n = 8) and primary peritoneal carcinoma (n = 1) could be measured. These data suggested that VEGF levels in ascites could reflect tumor biological behavior to a great extent, and cells of ovarian cancer and primary peritoneal carcinoma might secrete VEGF into peritoneal cavity directly[27]. Most importantly, detection of VEGF levels could provide a new approach for differential diagnosis of benign and malignant ascites, which remains a knotty problem all the time[34,35]. Moreover, detecting VEGF levels may contribute to the diagnosis of primary cancer that causes malignant ascites to a certain extent.
CD44 is an integral cell membrane glycoprotein, and is known to function in homing of lymphocytes, cell adhesion, activation of leukocytes and migration of cells. At least 20 variants (v) of CD44 have been reported due to the alternative splicing of 10 exons (v1-v10) that encode the membrane’s proximal portion of the extracellular domain[36-38]. NH2-terminal function area of CD44 on the surface of cells could join the hyaluronate in the basement membrane to extracellular matrix, thus regulate the movement and function of cells. By this mechanism, neoplastic cells could adhere to the extracellular matrix and basement membrane of the host cell, resulting in invasion and metastasis of malignancy. On the other hand, the degraded products of hyaluronic acid could motivate the growth of local vessels, providing the basis for invasion and metastasis[39,40]. Many studies have reported that expression of CD44v, especially CD44v6, was correlated with invasion and metastasis of certain type of human cancer[11,40-47], including gastric cancer[48-50], colorectal cancer[51-53], ovarian cancer[54] and prostate cancer[55]. Furthermore, serum concentrations of sCD44v6 were found to be significantly increased in patients with advanced carcinoma[13,15,56]. To our knowledge, however, concentration of sCD44v6 has not been examined in malignant ascites, this might be the first study to document sCD44v6 in malignant ascites.
We found sCD44v6 levels were high in malignant ascites, and relatively low in nonmalignant ascites. It implies that elevated CD44v6 appears to be correlated to the invasion and metastasis of cancer cells into peritoneal cavity. But it is unclear why CD44v6 is closely associated with malignant ascites. The ability of CD44v6 to bind peritoneal mesothelial surfaces of abdominal cavity, and a subsequent cancer cell implantation may contribute to it. At the same time, our results showed a higher sensitivity and specificity of sCD44v6 to the diagnosis of malignant ascites. However, no evidence is available to show that detection of sCD44v6 could contribute to the determination of a potential primary cancer causing malignant ascites. It is reasonable to consider sCD44v6 may be a diagnostic index of malignant ascites.
In summary, VEGF and sCD44v6 are detectable in ascites and are significantly elevated in malignant ascites. Prospective monitoring of VEGF and sCD44v6 levels in ascites would be helpful in differential diagnosis of benign and malignant ascites.
| 1. | Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1364] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 2. | Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3536] [Cited by in RCA: 3581] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 4. | Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2734] [Cited by in RCA: 2693] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 5. | Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 918] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 6. | Che X, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, Aikou T. Tumor angiogenesis related to growth pattern and lymph node metastasis in early gastric cancer. Chin Med J (. Engl). 1998;111:1090-1093. [PubMed] |
| 7. | Erenoglu C, Akin ML, Uluutku H, Tezcan L, Yildirim S, Batkin A. Angiogenesis predicts poor prognosis in gastric carcinoma. Dig Surg. 2000;17:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 8. | Yoshikawa T, Yanoma S, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Angiogenesis inhibitor, TNP-470, suppresses growth of peritoneal disseminating foci. Hepatogastroenterology. 2000;47:298-302. [PubMed] |
| 9. | Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, Kuroshima K, Aikou T. Angiogenesis as an unfavorable factor related to lymph node metastasis in early gastric cancer. Ann Surg Oncol. 1998;5:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1240] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 11. | Sleeman J, Moll J, Sherman L, Dall P, Pals ST, Ponta H, Herrlich P. The role of CD44 splice variants in human metastatic cancer. Ciba Found Symp. 1995;189:142-151; discussion 151-156, 174-176. [PubMed] |
| 12. | Harn HJ, Ho LI, Chang JY, Wu CW, Jiang SY, Lee HS, Lee WH. Differential expression of the human metastasis adhesion molecule CD44V in normal and carcinomatous stomach mucosa of Chinese subjects. Cancer. 1995;75:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Harn HJ, Ho LI, Shyu RY, Yuan JS, Lin FG, Young TH, Liu CA, Tang HS, Lee WH. Soluble CD44 isoforms in serum as potential markers of metastatic gastric carcinoma. J Clin Gastroenterol. 1996;22:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Fichtner I, Dehmel A, Naundorf H, Finke LH. Expression of CD44 standard and isoforms in human breast cancer xenografts and shedding of soluble forms into serum of nude mice. Anticancer Res. 1997;17:3633-3645. [PubMed] |
| 15. | Zeimet AG, Widschwendter M, Uhl-Steidl M, Müller-Holzner E, Daxenbichler G, Marth C, Dapunt O. High serum levels of soluble CD44 variant isoform v5 are associated with favourable clinical outcome in ovarian cancer. Br J Cancer. 1997;76:1646-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Enck RE. Malignant ascites. Am J Hosp Palliat Care. 2002;19:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] |
| 18. | Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 671] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 19. | Brock TA, Dvorak HF, Senger DR. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol. 1991;138:213-221. [PubMed] |
| 20. | Tao HQ, Lin YZ, Wang RN. Significance of vascular endothelial growth factor messenger RNA expression in gastric cancer. World J Gastroenterol. 1998;4:10-13. [PubMed] |
| 21. | Konno H, Baba M, Tanaka T, Kamiya K, Ota M, Oba K, Shoji A, Kaneko T, Nakamura S. Overexpression of vascular endothelial growth factor is responsible for the hematogenous recurrence of early-stage gastric carcinoma. Eur Surg Res. 2000;32:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 22. | Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol. 2001;78:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (3)] |
| 23. | Kabashima A, Maehara Y, Kakeji Y, Sugimachi K. Overexpression of vascular endothelial growth factor C is related to lymphogenous metastasis in early gastric carcinoma. Oncology. 2001;60:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Xue JT, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF (121) in gastric carcinoma MGC803 cell line. World J Gastroenterol. 2000;6:281-283. [PubMed] |
| 25. | Zhao MF, Mao H, Zhen JX, Yuan YW. Effect of vascular endothelial growth factor on adhesion of large intestine cancer cell HT-29. Shijie Huaren Xiaohua Zazhi. 2000;8:646-649. |
| 26. | Ishigami SI, Arii S, Furutani M, Niwano M, Harada T, Mizumoto M, Mori A, Onodera H, Imamura M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br J Cancer. 1998;78:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 254] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Santin AD, Hermonat PL, Ravaggi A, Cannon MJ, Pecorelli S, Parham GP. Secretion of vascular endothelial growth factor in ovarian cancer. Eur J Gynaecol Oncol. 1999;20:177-181. [PubMed] |
| 28. | Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, Sakahara H, Mori T. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 262] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Dirix LY, Vermeulen PB, Pawinski A, Prové A, Benoy I, De Pooter C, Martin M, Van Oosterom AT. Elevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patients. Br J Cancer. 1997;76:238-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, Unger C, Marmé D, Gastl G. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Zhang HT, Hu S. Relationship between VEGF in the sera and invasion and metastasis of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2003;11:344-345. |
| 32. | Mao ZB, Xiao MB, Huang JF, Ni HB, Ni RZ, Wei Q, Zhang H. Expression of VEGF in the sera of patients with gastric cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:1220-1221. |
| 33. | Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 188] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Aslam N, Marino CR. Malignant ascites: new concepts in pathophysiology, diagnosis, and management. Arch Intern Med. 2001;161:2733-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Tamsma JT, Keizer HJ, Meinders AE. Pathogenesis of malignant ascites: Starling's law of capillary hemodynamics revisited. Ann Oncol. 2001;12:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160-12164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 775] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 37. | Harn HJ, Isola N, Cooper DL. The multispecific cell adhesion molecule CD44 is represented in reticulocyte cDNA. Biochem Biophys Res Commun. 1991;178:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Herrlich P, Zöller M, Pals ST, Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 1993;14:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: A novel role for CD44 in the process of peritoneal implantation. Cancer Res. 1997;57:1228-1232. [PubMed] |
| 40. | Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62:2281-2286. [PubMed] |
| 41. | Chen GY, Wang DR. The expression and clinical significance of CD44v in human gastric cancers. World J Gastroenterol. 2000;6:125-127. [PubMed] |
| 42. | Xin Y, Zhao FK, Zhang SM, Wu DY, Wang YP, Xu L. Relationship beteeen CD44v6 expression and prognosis in gastriccarcinoma patients. Shijie Huaren Xiaohua Zazhi. 1999;7:210-214. |
| 43. | Gu HP, Ni CR, Zhang RZ. Relationship of expressions of CD15, CD44v6 and nm23 H1 mRNA with metastasis and prognosis of colon carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:887-891. |
| 44. | Mi JQ, Zhang ZH, Sheng MC. Significance of CD44v6 protein expression in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:156-158. |
| 45. | Liu YH, Liu JZ, Xiao B, Wang SX. The clinical significance of CD44v6 abnormal expression in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:89-90. |
| 46. | Wu LY, Hao YD, Shi ML. Relationship between CD44v6 expression and biological behavior of gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:1034. |
| 47. | Xiao CZ, Dai YM, Yu HY, Wang JJ, Ni CR. Relationship between expression of CD44v6 and nm23-H1 and tumor invasion and metastasis in hepatocellular carcinoma. World J Gastroenterol. 1998;4:412-414. [PubMed] |
| 48. | Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Sun XW, Shen BZ, Shi MS, Dai XD. Relationship between CD44v6 expression and risk factors in gastric carcinoma patients. Shijie Huaren Xiaohua Zazhi. 2002;10:1129-1132. |
| 50. | Chen ZF, Deng CS, Xia B, Zhu YQ, Zeng J, Gong LL. Expression of heat shock protein 60, CD44v6 splice variant in human gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:988-991. |
| 51. | Masaki T, Goto A, Sugiyama M, Matsuoka H, Abe N, Sakamoto A, Atomi Y. Possible contribution of CD44 variant 6 and nuclear beta-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinoma. Cancer. 2001;92:2539-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 52. | Xu SH, Feng JG, Li DC, Mou HZ, Lou RC. Relationship between CD44 in the peripheral blood of patients with colorectalcancer and clinicopathological features. Shijie Huaren Xiaohua Zazhi. 2000;8:432-435. |
| 53. | Cai Q, Lu HF, Sun MJ, Du X, Fan YZ, Shi DR. Expression of CD44 v3 and v6 proteins in human colorectal carcinoma and its relevance with prognosis. Shijie Huaren Xiaohua Zazhi. 2000;8:1255-1258. |
| 54. | Schiffenbauer YS, Meir G, Maoz M, Even-Ram SC, Bar-Shavit R, Neeman M. Gonadotropin stimulation of MLS human epithelial ovarian carcinoma cells augments cell adhesion mediated by CD44 and by alpha (v)-integrin. Gynecol Oncol. 2002;84:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Ekici S, Ayhan A, Kendi S, Ozen H. Determination of prognosis in patients with prostate cancer treated with radical prostatectomy: prognostic value of CD44v6 score. J Urol. 2002;167:2037-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Saito H, Tsujitani S, Katano K, Ikeguchi M, Maeta M, Kaibara N. Serum concentration of CD44 variant 6 and its relation to prognosis in patients with gastric carcinoma. Cancer. 1998;83:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Edited by Ren SY and Wang XL