Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2266
Revised: July 25, 2003
Accepted: August 2, 2003
Published online: October 15, 2003
AIM: The generation of oxygen free radicals has been implicated in the pathogenesis of experimental pancreatitis. The aim of this study was to determine the role of oxygen free radicals in patients with acute pancreatitis.
METHODS: The plasma levels of C-reactive protein (CRP), lipid peroxide (LPO), myeloperoxidase (MPO) and superoxide dismutase (SOD) were measured in 13 patients with acute pancreatitis and 14 healthy volunteers.
RESULTS: Among the patients with acute pancreatitis, there were higher plasma levels of LPO and MPO and lower SOD activity in patients with severe pancreatitis than in those with mild pancreatitis. However, there was no significant difference in the serum marker of oxidative stress no matter what the etiology was. The LPO level was especially correlated with the concentration of serum CRP and CT severity index.
CONCLUSION: The oxygen free radicals may be closely associated with inflammatory process and the severity of acute pancreatitis. Especially, the concentration of plasma LPO is a meaningful index for determining the severity of the disease.
- Citation: Park BK, Chung JB, Lee JH, Suh JH, Park SW, Song SY, Kim H, Kim KH, Kang JK. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol 2003; 9(10): 2266-2269
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2266.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2266
Oxygen free radicals are molecules produced continuously in cells by several mechanisms. The generation of oxygen free radicals is physiologic. In most circumstances, oxygen free radicals are neutralized immediately by enzymatic scavengers. However, when formation of oxygen free radicals overwhelms radical neutralization in cells, oxidative stress occurs. As they are very reactive, they react well with all biological substances such as proteins, polysaccharides and nucleic acids, resulting in tissue injury[1-3]. It has been suggested that oxygen free radicals are responsible for a wide variety of diseases or conditions[1,4-8].
Oxygen free radicals have been known to play an important role in the pathogenesis of pancreatitis of some experimental models[6-12]. Oxygen free radicals are involved in initiation of pancreatitis[11]. Also, it was reported that oxygen free radicals acted as important mediators of tissue damage in experimental acute pancreatitis[10]. Therapeutic effects of antioxidants and radical scavengers have been shown in experimental models of acute pancreatitis[11,13,14].
However, there is doubt that experimental models of acute pancreatitis will match clinically acute pancreatitis in humans. Although there were some data that reflected enhanced oxygen stress in patients with acute pancreatitis[15-17], the role of oxygen free radicals in patients with acute pancreatitis has not been well clarified.
Therefore, this study was conducted to evaluate the role of oxygen free radicals in human acute pancreatitis and to analyze the correlation between oxygen free radicals and the severity of acute pancreatitis.
Thirteen patients admitted to Medical Center of Yonsei University with a diagnosis of acute pancreatitis were included in this study. The diagnosis was established on the basis of acute abdominal pain, at least 3-fold elevated levels of serum amylase and computed tomography (CT). All patients had no previous history of acute pancreatitis. Fourteen healthy subjects without previous medical history were enrolled as controls. The mean age was 53.6 years in patients and 32.5 years in control group. The male to female ratio was 11:2 in patients and 10:4 in control groups.
Peripheral blood samples were taken on recruitment. After the blood was centrifuged, the plasma was stored at -70 °C until analysis. The blood samples of control group underwent the same process. C-reactive protein was measured by the routine method. Serum lipid peroxide (LPO) and myeloperoxide (MPO) as markers of oxygen free radicals were measured. Also the activity of superoxide dismutase (SOD) that diminished in oxidative stress due to its role as enzymatic scavenger was measured.
LPO was analyzed by measuring the amount of thiobarbituric acid reactive substances (TBARS). It was preceded that the standard solution was prepared by diluting 1000 nmol/mL tetraethoxy propane, which was made by mixing with tetraethoxy propane and tertiary distilled water. Tertiary distilled water was used as blank solution. Plasma, blank, and standard solution 200 μL, respectively, were mixed with 20% acetic acid. TBARS resulted from the reaction with thiobarbituric acid (TBA) 400 μL, was detected as reading fluorescence at 535 nm emission. The result was compared with standard curve.
MPO was analyzed as a marker of neutrophil activation by Bioxytech® MPO enzyme immunoassay kit (OXIS International Inc., Portland, USA). This assay detects the fluorescence of solution at 405 nm emission using sandwich enzyme-linked immunosorbent assay (ELISA) against biotin-labeled goat polyclonal anti-MPO.
SOD activity was measured by using Bioxytech® SOD-525TM assay kit (OXIS International Inc., Portland, USA). This assay detects the fluorescence of solution at 525 nm, based on the principle that autoxidation of tetracyclic cathechol accelerates in condition of SOD.
To determine the severity and prognosis of acute pancreatitis, the level of C-reactive protein (CRP) in serum was used for a single indicator, Ranson’s score for multiple indicator, and CT severity index[18] for morphological indicator. Atlanta classification[19] was used as a main determining index for the severity of acute pancreatitis.
We used chi-square test and independent sample t-test for comparison in each group. A P value less than 0.05 was considered significant.
The mean plasma level of LPO was 21.9 ± 26.5 nmol/mL in acute pancreatitis and 5.7 ± 1.7 nmol/mL in control group (P = 0.030). Also, the mean plasma level of MPO was 12.3 ± 11.7 nmol/mL in acute pancreatitis and 2.7 ± 1.2 nmol/mL in control group (P = 0.005). Oxygen free radicals significantly increased more in acute pancreatitis patients than in control group. The mean level of SOD activity was 48.2 ± 41.4 U/dL in acute pancreatitis patients and 100.4 ± 20.2 U/dL in control group (P = 0.002). The decrease of SOD activity was more prominent in acute pancreatitis than in control group (Table 1).
| Parameters | Control (n = 14) | Acute pancreatitis (n = 13) | Pvalue |
| Amylase (IU/dL) | 141.9 ± 179.3 | 393.3 ± 243.1 | 0.005 |
| LPO (nmol/mL) | 5.7 ± 1.7 | 21.9 ± 26.5 | 0.030 |
| MPO (nmol/mL) | 2.7 ± 1.2 | 12.3 ± 11.7 | 0.005 |
| SOD (U/dL) | 100.4 ± 20.2 | 48.2 ± 41.4 | 0.002 |
The etiologies of acute pancreatitis were gallstone (n = 5), alcohol (n = 4), hyperlipidemia (n = 1), and unexplained (n = 3). Comparing gallstone pancreatitis patients with alcohol induced pancreatitis, the plasma levels of CRP, LPO, MPO, and SOD showed no significant differences between the two groups (Table 2).
| Paramerters | Gallstone group (n = 5) | Alcohol group (n = 4) | Pvalue |
| Age (years) | 55.4 ± 19.7 | 44.5 ± 14.2 | 0.368 |
| Amylase (IU/dL) | 364.6 ± 271.9 | 366.5 ± 252.5 | 0.992 |
| Lipase (IU/dL) | 10004.0 ± 15873.2 | 6035.8 ± 8265.2 | 0.646 |
| CRP (mg/dL) | 6.0 ± 2.5 | 12.3 ± 9.8 | 0.296 |
| LPO (nmol/mL) | 15.6 ± 10.5 | 38.7 ± 45.1 | 0.385 |
| MPO (nmol/mL) | 11.3 ± 15.3 | 15.5 ± 14.3 | 0.689 |
| SOD (U/dL) | 59.2 ± 49.3 | 21.0 ± 17.1 | 0.171 |
According to the Atlanta classification, acute pancreatitis was classified as mild in 8 patients and as severe in 5 patients based on their clinical manifestations. There were no differences of age and sex between the two groups (Table 3). The mean level of serum CRP in patients with severe pancreatitis was higher than the one in patients with mild pancreatitis (12.3 ± 9.0 mg/dL vs 6.7 ± 4.5 mg/dL, P = 0.311). The mean level of SOD activity more markedly decreased in patients with severe pancreatitis than in patients with mild pancreatitis (27.3 ± 15.4 U/dL vs 65.7 ± 49.2 U/dL, P = 0.130).
| Mild group | Severe group | |
| No. of cases | 8 | 5 |
| Age (years) | 53.5 ± 17.1 | 53.8 ± 15.7 |
| Sex (M:F) | 7:1 | 5:1 |
| Etiology | ||
| Gallstone | 2 | 3 |
| Alcohol | 2 | 2 |
| Others* | 4 | 0 |
| No. of mortality | 0 | 0 |
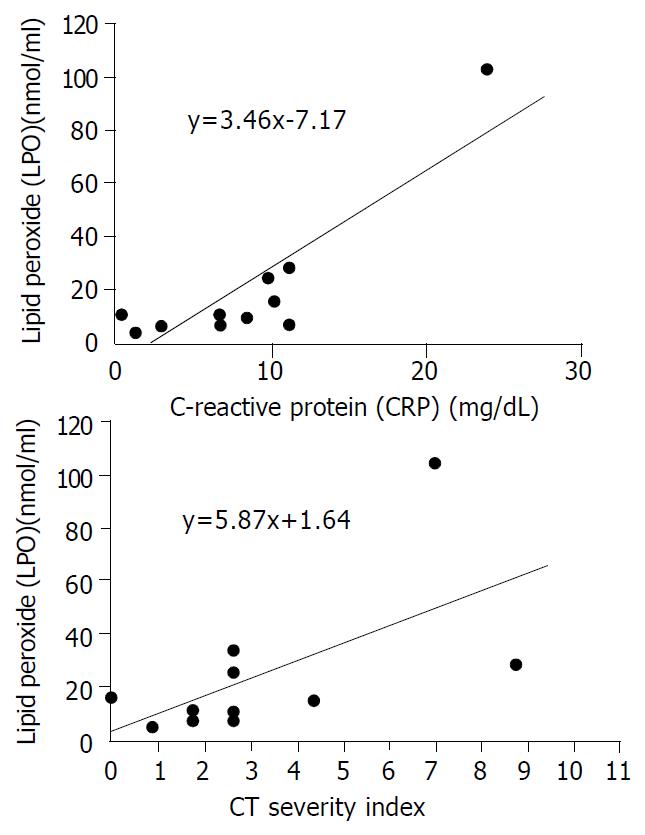
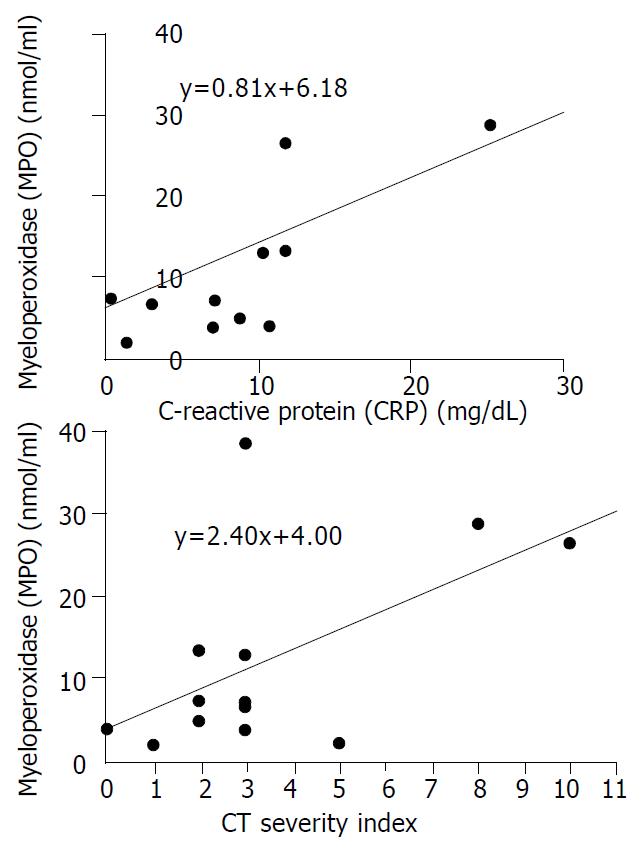
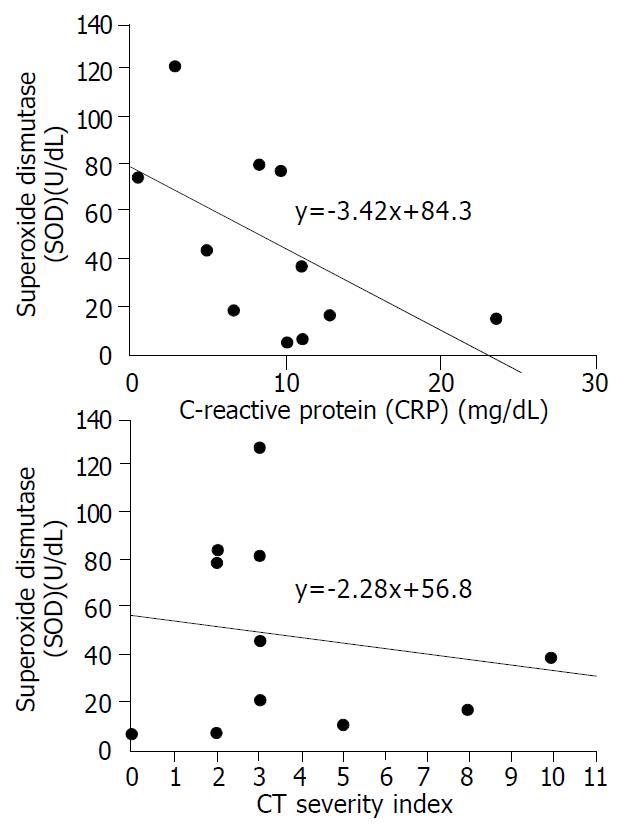
The mean plasma levels of LPO and MPO were higher in patients with severe pancreatits than those in patients with mild pancreatitis (LPO: 38.7 ± 38.1 nmol/mL vs 11.5 ± 6.8 nmol/mL, P = 0.068, MPO: 20.1 ± 16.1 nmol/dL vs 7.5 ± 4.1 nmol/dL, P = 0.053). The significant difference was for CT severity index between the two groups (5.8 ± 3.1 in severe pancreatitis, 2.0 ± 1.1 in mild pancreatitis, P = 0.008) (Table 4). To analyze the relationship between oxygen free radicals and CT severity index, serum CRP that is widely used as an index for severity of pancreatitis, plasma LPO showed a close correlation with CT severity index and serum CRP (r2 = 0.373, and 0.675, P = 0.027, and 0.001, respectively) (Figure 1). Plasma MPO showed a close correlation with CT severity index but not with serum CRP (r2 = 0.319 and 0.202, P = 0.044 and 0.143, respectively) (Figure 2). But SOD activity showed no significant correlation with either CT severity index or serum CRP (r2 = 0.026 and 0.307, P = 0.638 and 0.096, respectively ) (Figure 3). CT severity index was correlated with serum CRP and Ranson’s score (r2 = 0.334 and 0.382, P = 0.049 and 0.024, respectively).
| Parameters | Mild group (n = 8) | Severe group (n = 5) | Pvalue |
| Amylase (IU/dL) | 374.7 ± 252.4 | 423.0 ± 253.1 | 0.745 |
| Lipase (IU/dL) | 10082.8 ± 12565.6 | 30008.0 ± 3314.9 | 0.167 |
| CRP (mg/dL) | 6.7 ± 4.5 | 12.3 ± 9.0 | 0.311 |
| LPO (nmol/mL) | 11.5 ± 6.8 | 38.7 ± 38.1 | 0.068 |
| MPO (nmol/mL) | 7.5 ± 4.1 | 20.1 ± 15.4 | 0.053 |
| SOD (U/dL) | 65.7 ± 49.2 | 27.3 ± 15.4 | 0.130 |
| Ranson’s score | 1.4 ± 0.7 | 2.8 ± 1.9 | 0.177 |
| CT severity index | 2.0 ± 1.1 | 5.8 ± 3.1 | 0.008 |
Acute pancreatitis led to various degrees of interstitial edema, acinar cell damage, hemorrhage and necrosis[8]. Although the inflammation initiates in pancreas, the disease may lead to systemic multi-organ failure. Several factors (complement activation, cytokines, oxygen free radicals, ischemia, and autodigestion of pancreatic enzyme) have been known to be involved in the pathogenesis of acute pancreatitis. But the role of these factors remains still unclear. Among them, oxygen free radicals could damage extracellular tissue by degrading hyaluronic acid and collagen in the intercellular matrix and directly attack biological membrane through the peroxidation of structurally and functionally important lipids[20]. Furthermore, they could denature enzymes and other important proteins, and damage nucleic acid. In addition, they could indirectly trigger the accumulation of polymorphonulclear (PMN) leukocyte in the tissue. Activated PMN leukocytes could secrete various enzymes such as myeloperoxidase, protease, and elastase[21]. As a result, the inflammatory reaction accelerated. Also, oxygen free radicals could indirectly stimulate arachidonic acid metabolism with increased production of prostaglandins, thromboxane, and leukotrienes, and eventually could lead to microcircular derangement and cellular damage[2,15].
There are some reports concerning mechanisms of enhanced production of oxygen free radicals. Xanthine oxidase, PMN leukocyte, and cytochromes P-450 have been introduced as the source of oxygen free radicals[3,12]. However, the true sources of oxygen free radicals that are responsible for the pathogenesis of acute pancreatitis have not yet been identified. Since the first study by Sanfey et al[9], many studies have demonstrated the role of oxygen free radicals in the pathogenesis of acute pancreatitis in experimental models and patients. Oxygen free radicals have been known to mediate an important step in the initiation of acute pancreatitis[11]. But most results have been derived from experimental animal models in which acute pancreatitis was induced by stress, cerulein injection, choline-deficient diet, or taurocholate injection into pancreatic duct. Only a few data have been derived from patients. Furthermore, there are many limitations on studying patients with acute pancreatitis in clinical settings.
Because of the nature of their high reactivity, oxygen free radicals are difficult to measure directly. Direct measurement of oxygen free radicals with electron spin resonance (ESR) technique was limited to in vitro studies due to short half time of free radicals and toxicity of compounds[22]. For these reasons, the alternative of measuring stable metabolites was met with broad acceptance. In most studies, measurements of the effects of radical reaction with biological substances (i.e., lipid peroxides and changes of glutathione metabolism) have replaced the assessment of oxygen free radicals.
In acute pancreatitis patients, increased production of lipid peroxidation, decreased level of vitamin C, and direct correlation between oxidative stress and severity of acute pancreatitis have been reported[15-17]. Equally, our results showed that plasma levels of LPO and MPO increased more in acute pancreatitis patients than in normal control group, and that increase of LPO and MPO was more prominent in severe pancreatitis than in mild pancreatitis, and that SOD activity, as a scavenger of oxygen free radical, was lower in acute pancreatitis patients than in normal control group. From these results, we can suggest that oxygen-derived free radicals play a pivotal role in the pathogenesis of acute pancreatitis in patients. In our study, it was probable that lipid peroxidation was correlated with severity of acute pancreatitis. There was a close correlation of lipid peroxidation with serum CRP and CT severity index. Therefore, there is some possibility that LPO could be a predictor of severity of acute pancreatitis. Similarly it was shown that lipid peroxidation was significantly increased in patients with septic shock[23]. In contrast, Abu-Zidan et al[16] have reported that lipid peroxidation was highly correlated with the severity of pancreatitis but not a good predictor of it. This discordance may be due to different method of measuring lipid peroxidation and study design.
In our study, the level of SOD activity, enzymatic scavenger, was significantly decreased in the plasma of patients with acute pancreatitis, and although not significant, it was related to the severity of acute pancreatitis. The effects of antioxidant, such as vitamins C and E, in experimental models have been reported[11,13,14]. However, the results have varied according to different models. Because in these most studies, antioxidant therapy was performed before acute pancreatitis was induced, the results were unlikely to be applied to clinical conditions. As well, the therapeutic effect of antioxidants and radical scavenger in patients has not been demonstrated yet.
There was no difference of oxygen free radicals between alcohol-induced pancreatitis and gallstone pancreatitis in our study. In many various experimental models and patients with acute pancreatitis, oxygen free radicals have been related to the disease[2,15,24,25]. Thus in our opinion, the role of oxygen free radicals may not be different no matter what the etiology of the disease is.
In conclusion, oxygen-derived free radicals may be closely associated with inflammatory process and the severity of acute pancreatitis, and the plasma level of LPO is a meaningful index for determining the severity of the disease.
| 1. | Kerr ME, Bender CM, Monti EJ. An introduction to oxygen free radicals. Heart Lung. 1996;25:200-209; quiz 200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Schoenberg MH, Birk D, Beger HG. Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995;62:1306S-1314S. [PubMed] |
| 3. | Schulz HU, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736-2750. [PubMed] |
| 4. | Janero DR. Ischemic heart disease and antioxidants: mechanistic aspects of oxidative injury and its prevention. Crit Rev Food Sci Nutr. 1995;35:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Thomas MJ. The role of free radicals and antioxidants: how do we know that they are working? Crit Rev Food Sci Nutr. 1995;35:21-39. [PubMed] |
| 6. | Correa P. The role of antioxidants in gastric carcinogenesis. Crit Rev Food Sci Nutr. 1995;35:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Machlin LJ. Critical assessment of the epidemiological data concerning the impact of antioxidant nutrients on cancer and cardiovascular disease. Crit Rev Food Sci Nutr. 1995;35:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Bulkley GB. The role of oxygen free radicals in human disease processes. Surgery. 1983;94:407-411. [PubMed] |
| 9. | Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Am Surg. 1984;200:405-413. |
| 10. | Rau B, Poch B, Gansauge F, Bauer A, Nüssler AK, Nevalainen T, Schoenberg MH, Beger HG. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann Surg. 2000;231:352-360. [PubMed] |
| 11. | Schoenberg MH, Büchler M, Gaspar M, Stinner A, Younes M, Melzner I, Bültmann B, Beger HG. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Sanfey H, Bulkley GB, Cameron JL. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985;201:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Sarr MG, Bulkley GB, Cameron JL. Temporal efficacy of allopurinol during the induction of pancreatitis in the ex vivo perfused canine pancreas. Surgery. 1987;101:342-346. [PubMed] |
| 14. | Shikata A, Suganuma M, Marugami Y, Sakurai Y, Ochiai M, Kamei K, Funabiki T, Shinohara R. The role of oxyhen-derived free radicals and their scavengers in experimental acute pancreatitis. Digestion. 1996;57:264. |
| 15. | Tsai K, Wang SS, Chen TS, Kong CW, Chang FY, Lee SD, Lu FJ. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850-855. [RCA] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Abu-Zidan FM, Bonham MJ, Windsor JA. Severity of acute pancreatitis: a multivariate analysis of oxidative stress markers and modified Glasgow criteria. Br J Surg. 2000;87:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Bonham MJ, Abu-Zidan FM, Simovic MO, Sluis KB, Wilkinson A, Winterbourn CC, Windsor JA. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg. 1999;86:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 980] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 19. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1746] [Article Influence: 52.9] [Reference Citation Analysis (1)] |
| 20. | Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1201] [Cited by in RCA: 1120] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 21. | Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978;298:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1699] [Cited by in RCA: 1591] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 22. | Schoenberg MH, Büchler M, Beger HG. Oxygen radicals in experimental acute pancreatitis. Hepatogastroenterology. 1994;41:313-319. [PubMed] |
| 23. | Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 321] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Uden S, Schofield D, Miller PF, Day JP, Bottiglieri T, Braganza JM. Antioxidant therapy for recurrent pancreatitis: biochemical profiles in a placebo-controlled trial. Aliment Pharmacol Ther. 1992;6:229-240. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Gut A, Shiel N, Kay PM, Segal I, Braganza JM. Heightened free radical activity in blacks with chronic pancreatitis at Johannesburg, South Africa. Clin Chim Acta. 1994;230:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Edited by Wang XL