Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.69
Revised: February 2, 2002
Accepted: February 23, 2002
Published online: January 15, 2003
AIM: The imaging features of MRI and DSA, using the models of implanted and induced hepatoma, were investigated in rats.
METHODS: CBRH3 cancer cells were implanted for different liver site of rat liver and the diethylnitrosoamine was given orally to rats in order to induce liver cancer. Both experimental groups were detected by magnetic resonance imaging (MRI), digital subtraction angiography (DSA) and morphologic assay.
RESULTS: Hypointensity on T1WI and homogenous high signal intensity on T2WI in MRI, and ring-like abnormal stain on DSA were found in implanted cancer. Induced cancers appeared as homogeneous or heterogeneous hypointensity on T1WI (10 cases), and equal or slight high intensity on T2WI (8 cases), but some as hypointensity on T2WI (2 cases).
CONCLUSION: The imaging features of implanted cancers were similar to that of human liver metastases. Therefore, it could serve as an experimental model of human liver metastatic tumor. The imaging feature of induced cancers, whereas, were similar to that of human primary liver cancer. It could be use as an experimental model of human primary liver cancer.
- Citation: Yang JH, You TG, Li N, Qian QJ, Wang P, Yan ZL, Wu MC. Relationship between the imaging features and pathologic alteration in hepatoma of rats. World J Gastroenterol 2003; 9(1): 69-72
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/69.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.69
It is important to investigate the relationship between the biologic properties of liver tumor and the findings with modern imaging techniques. MRI affords the possibility in coronal and sagittal views, which are very helpful in liver surgery because surgical techniques are described in these planes[1-2]. In addition, targeted gene therapy, for instance, cytokines gene therapy, angiogenesis inhibitor and granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transduced tumor vaccine for patients, may be useful in developing anticancer drugs that prolong or stabilize theprogression of tumors with minimal systemic toxicities. These drugs may also be used as novel imaging and radiommunotherapeutic agents in cancer therapy[3-7].
However, little is known on experimental work of both scan imaging and liver cancer. The present study, using the model of implanted and induced hepatoma of rats, was undertaken to investigate the relationship between the morphologic alterations and imaging characteristics of magnetic resonance imaging (MRI) and digital subtraction angiography (DSA), and more to improve level of diagnostic and treatment for liver cancer. Finally, interleukin 2 was therapeutic effect on liver tumors[8], so the our further study of interleukin gene therapy for liver cancer was based on the present outcome of implanted and induced hepatoma.
MRI equipment was produced by Siemens AG (1.0 T). Its SE rank, 128*128 matrix, T1-weighted sequence (T1WI) were TR/TE 350-480/15 ms; T2-weighted sequence (T2WI) was TR/TE 2200/90 ms. Slice thickness was 2 mm and scan space was 0.5 mm. Both of coronal and sagittal scanning was fulfilled. Hepatic artery was scanned by DSA (Toshiba DFP-0.3A) with perfusing iodized oil (Lipiodol). Instruments and drugs were sterilized routinely.
Male Wister rats (200-250 g wt) were obtained from Animal Center of Chinese Academy of Sciences. Animals were maintained on a standard diet. Cellular strains of hepatoma CBRH3 were provided kindly by Prof. Xie Hong (Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science).
Hepatoma CBRH3 cells were injected into abdominal cavity of rat. Rats were sacrificed and tumors were removed from abdominal cavity 7-9 days late. The tumors were cut into pieces of 0.05-0.75 cm and then was inoculated into rat liver for one or more locations respectively. The tumor was grown up to diameter in 0.6-1 cm after 7-10 days of inoculation.
Male Wister rats (age for 8 weeks) took orally 1:10000 diethylnitrosamine for 80 days to induce liver cancer. The micro-nodules of hepatoma were developed 14 weeks late and 0.2-1 cm of tumor masses were observed 16-18 weeks late. There were poly-cysts in rat liver. This model was used to study for poly-nodules of hepatoma. The success rate of induced cancer was 100%[9,10].
Both of 10 implanted tumor rats (age of 9 days) and 10 induced tumor rats (age of 16-18 weeks) was divided to experimental group and control group, randomly. While rats were scanned by MRI, they were anesthetized by injecting ketamine into muscle. In addition, the hepatic artery and gastroduodenal artery were isolated carefully and micro-catheter was inserted into these artery for injection of iodine oil before DSA[11].
A stable model of rat hepatoma was established by either implanted tumor with cancer piece or inducing tumor with diethylnitrosamine.
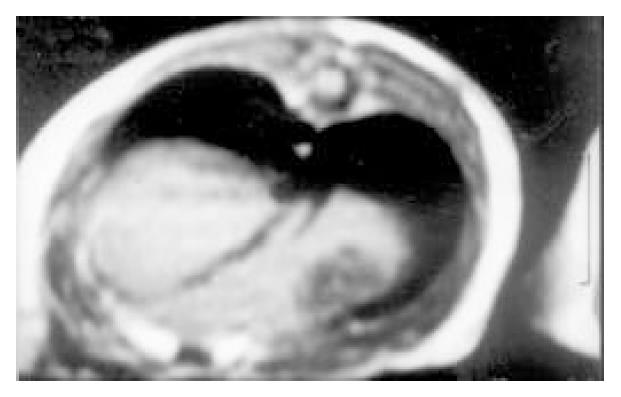
Imaging features of the implanted tumors in MRI and DSA and the pathology:The 10 implanted models showed 18 nodules of tumor in MRI and DSA. 11 nodules of them were round shape and 7 of them were ellipse. The margin of tumors was smooth. Implanted cancer showed homogeneous hypointensity on T1WI (Figure 1) and homogeneous hyperintensity on T2WI. The rat liver showed normal signal and normal organ shape.
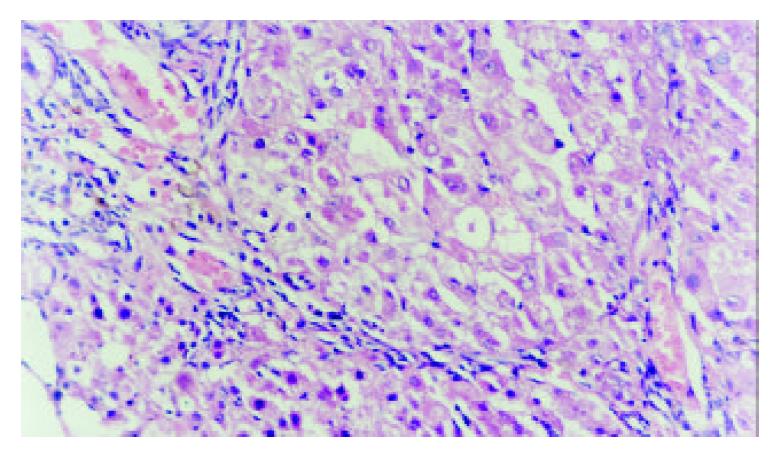
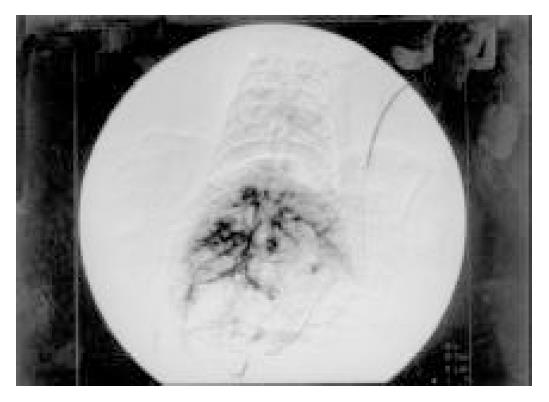
In DSA, the tumors showed no stain for blood vessels in the center of tumor (vacancy of vessel) and abnormal ring-like staining of vessel around tumor. The liver tissues were normal. Necrosis and colliquational cavity were shown on the core of tumors in all of 18 tumor mass and the yellow-gray tissue could be seen in cross section of tumors. The cancer cells were small or mediate in size with great nucleus. The parenchyma of liver was normal (Figure 2).
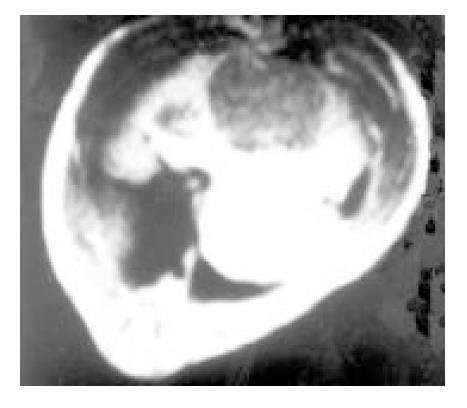
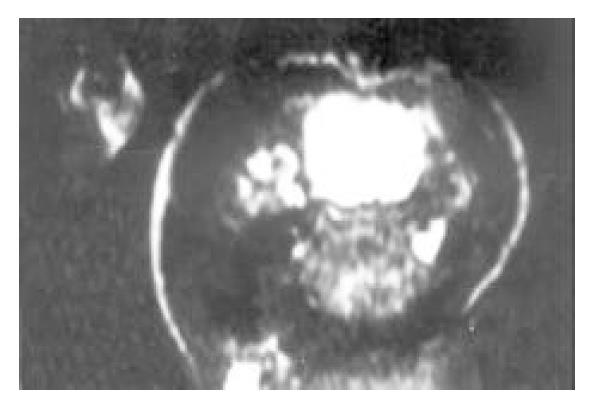
The scanning pictures of MRI and DSA and the pathological alteration in induced cancer: Liver cirrhosis with different degree and multiple nodules of cancer were observed in 10 tumor cases. Eight among 10 tumors showed homogeneous slight hypo-intensity on T1WI (Figure 3) and inhomogeneous slight hyper-intensity on T2WI with different shapes, but some of them were with normal signal or more contrast enhancement (Figure 4). Two among 10 tumor livers were shown inhomogeneous slight low signal on T1WI, but equal signal was found in interval. Hypointensity was observed on T2WI.
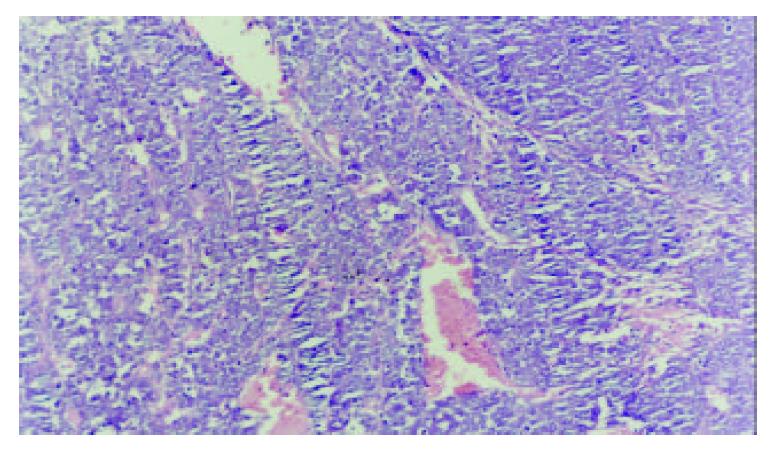
In DSA the distribution of internal hepato-vascularity was disorder and the angio-clumps were different in sizes in tumor (Figure 5). Pathology: Tumor liver showed poly-nodules, tumor diffusion and inequality of size. The tumor was light yellow. In microscopy examination, distribution of tumor and liver tissue was in interval. The cancer tissue showed plenty and dilation of vessel. The cell plasma of cancer was not abundant. The arrangement of tumor cell was nest and bunch. Liver tissue was easy for proliferation of nodule with acid-like change and water-alteration. The dilation of lymphatic tube and the proliferation of bile duct were shown between hepato-lobule. Hepato-lobule was divided by proliferation of fibre. The artery and vein were not distinguished because of dilation of vessel (Figure 6).
The available MR image photos were taken because technique is as follow. (1) Technique of eye surface loop: To avoid disturbing factors for image definition that focus on the location of rat liver, this technique was used. (2) Folium technique keeps away the cabin effect on conceal of small tumor nodules. (3) False shadow caused by move of body act and breath was prevented by suitable anesthesia for rats. On DSA image, deep anesthesia and careful anatomy of hepatic artery with catheter were necessary. But it is difficulty to puncture femoral artery. In order to find available condition of exposure time, we try to test more times[12].
The component of tumor is of long longitudinal relaxation time (T1) and long transverse relaxation time (T2) since the implanted cancer show low signal on T1WI and equal signal on T2WI in MRI. Equal signal intensity implies that there are no tissue component in the core of tumor apart from necrosis, liquefaction and cystic change. Therefore, the situation of blood supply was not described due to no-contrast enhancement of Gadolium-DTPA[13,14].
On DSA, the imaging of blood vessel around tumor was found such as folium annularity, whereas central liquefaction and necrosis were existed in tumor. Thus, cancer cell on the border of tumor grows actively because of blood supply in plenty and cancer cell in center of tumor does not grow because of insufficient blood supply. There is no liver cirrhosis because signal intensity of liver tissue in MRI and distribution of blood vessel in DSA are normal compared to normal liver parenchyma[19].
Necrosis at core of tumor and the proliferated nodule of tumor cell were found around blood sinus and dilation of blood vessel after 9 days of implanted cancer[16,17]. The change in MRI and DSA was accord to pathology.
Resent study has suggested that a random clonal origin of hepatocyte carcinoma from mature hepatocytes is seen in the diethylnitrosamine model of hepatocarcinogenesis[18].
Induced cancer is of two different signal intensities including 1. slight low signal intensity on T1WI and slight high signal intensity on T2WI in 8 cases and 2. inhomogeneous low signal on T1WI and low signal on T2WI with regular appearance in 2 cases. We hypothesize that both signals is caused by the difference in hepatoma component and hepatoma cellular arrange. Amount of fribrocyte, apart from tumor cells, is found in these 2 cases which result in extended longitudinal relaxation time (T1) and contracted transverse relaxation time (T2) with low signal on T1WI and low signal on T2WI. Irregular liver shape and liver nodule proliferation in deferent degree with liver cyst could be manifested on MRI because of liver cirrhosis and degeneration resulting from taking carcinogen for long time. To differentiate with cirrhosis nodules and tumor nodules, some investigators reported that the early-enhancing single nodule with cirrhosis is high predictive to hepatocyte cancer in patient. This phenomenon is also seen in our data of photo and microscopy examintion[20,21]. On DAS, the tumor stain is displayed by reason of disorder of blood vessel and angio-clamp[22].
Primary liver cancer Based on the liver cirrhosis, primary liver cancer display the slight low signal intensity on T1WI and equal or slight high signal intensity following extended echo time without raise of signal in MRI[23]. In DSA, the plenty amateur vessel is observed[24].
Liver metastases MRI shows the low signal intensity on T1WI, high signal on T2WI and annular enhancement by Gadolium. In DSA, annular staining is displayed without background of cirrhosis[15].
Compared with manifestation of MRI and DSA, studies confirm that implanted hepatoma lead that the signal intensity is high following the extended echo time and annular staining around tumor is occurred without liver cirrhosis[25].
In induced cancer, liver cirrhosis with slight high signal or low signal due to extended echo time without raise of signal is observed in MRI. The abundant malignant vessel stain is indicated that it is similar to human primary liver cancer[26].
In conclusion, the imaging features of implanted cancers were similar to human liver metastases in manifestation of MRI and DSA. Therefor, it could serve as an experimental model of human liver metastatic tumor. The imaging feature of induced cancers, whereas, were similar to that of human primary liver cancer. It could be use as an experimental model of human primary liver cancer.
| 1. | Foley LM, Towner RA, Painter DM. In vivo image-guided (1)H-magnetic resonance spectroscopy of the serial development of hepatocarcinogenesis in an experimental animal model. Biochim Biophys Acta. 2001;1526:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Eliat PA, Lechaux D, Gervais A, Rioux-Leclerc N, Franconi F, Lemaire L, Dazord L, Catros-Quemener V, de Certaines JD. Is magnetic resonance imaging texture analysis a useful tool for cell therapy in vivo monitoring. Anticancer Res. 2001;21:3857-3860. [PubMed] |
| 3. | Scappaticci FA. Mechanisms and future directions for angiogenesis-based cancer therapies. J Clin Oncol. 2002;20:3906-3927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Kawai K, Tani K, Yamashita N, Tomikawa S, Eriguchi M, Fujime M, Okumura K, Kakizoe T, Clift S, Ando D. Advanced renal cell carcinoma treated with granulocyte-macrophage colony-stimulating factor gene therapy: a clinical course of the first Japanese experience. Int J Urol. 2002;9:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Parkes AT, Speirs V. British Cancer Research Meeting, 30 June-3 July 2002, Glasgow. Breast Cancer Res. 2002;4:202-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Gu J, Lin T, Huang X, Roth JA, Fang B. Mechanisms involved in development of resistance to adenovirus-mediated proapoptotic gene therapy in DLD1 human colon cancer cell line. Gene Ther. 2002;9:1262-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, Lo KM. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Zhao W, Kobayashi M, Ding W, Yuan L, Seth P, Cornain S, Wang J, Okada F, Hosokawa M. Suppression of in vivo tumorigenicity of rat hepatoma cell line KDH-8 cells by soluble TGF-beta receptor type II. Cancer Immunol Immunother. 2002;51:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Balansky RM, Ganchev G, D'Agostini F, De Flora S. Effects of N-acetylcysteine in an esophageal carcinogenesis model in rats treated with diethylnitrosamine and diethyldithiocarbamate. Int J Cancer. 2002;98:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Rao MS, Kashireddy P. Effect of castration on dehydroepiandrosterone-induced hepatocarcinogenesis in male rats. Anticancer Res. 2002;22:1409-1411. [PubMed] |
| 11. | Thorlacius H, Larmark M, Randell M, Hultberg B, Jeppsson B. Isolated liver perfusion permits administration of high doses of chemotherapeutic agents. Comparison with hepatic artery infusion. Eur Surg Res. 2001;33:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Liu Y, Cao W, Liu Y, Fan A. [Establishment of modified model of Vx-2 carcinoma in rabbit liver and DSA imagining features of the tumor]. Zhonghua Ganzangbing Zazhi. 2002;10:149. [PubMed] |
| 13. | Krupski G, Ameis D, Cataldegirmen G, Herbst H, Henschel MG, Nicolas V, Rogiers X, Bücheler E. [Native magnetic resonance tomography imaging of the Morris hepatoma (MH-7777A) in the rat]. Rofo. 2001;173:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Lauenstein TC, Goehde SC, Herborn CU, Treder W, Ruehm SG, Debatin JF, Barkhausen J. Three-dimensional volumetric interpolated breath-hold MR imaging for whole-body tumor staging in less than 15 minutes: a feasibility study. AJR Am J Roentgenol. 2002;179:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Turler A, Schaefer H, Schaefer N, Wagner M, Maintz D, Qiao JC, Hoelscher AH. Experimental low-level direct current therapy in liver metastases: influence of polarity and current dose. Bioelectromagnetics. 2000;21:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Kuppen PJ, Gorter A, Hagenaars M, Jonges LE, Giezeman-Smits KM, Nagelkerke JF, Fleuren G, van de Velde CJ. Role of NK cells in adoptive immunotherapy of metastatic colorectal cancer in a syngeneic rat model. Immunol Rev. 2001;184:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Nakamoto T, Inagawa H, Nishizawa T, Honda T, Kanou J, Nagasue N, Soma G. Antitumor effects of isolated hypoxic hepatic perfusion (IHHP) with high-dose TNF against colonic liver metastases in a rat model. Anticancer Res. 2002;22:2455-2459. [PubMed] |
| 18. | Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Guo WJ, Li J, Ling WL, Bai YR, Zhang WZ, Cheng YF, Gu WH, Zhuang JY. Influence of hepatic arterial blockage on blood perfusion and VEGF, MMP-1 expression of implanted liver cancer in rats. World J Gastroenterol. 2002;8:476-479. [PubMed] |
| 20. | Dean CE, Benjamin SA, Chubb LS, Tessari JD, Keefe TJ. Nonadditive hepatic tumor promoting effects by a mixture of two structurally different polychlorinated biphenyls in female rat livers. Toxicol Sci. 2002;66:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Stoker J, Romijn MG, de Man RA, Brouwer JT, Weverling GJ, van Muiswinkel JM, Zondervan PE, Laméris JS, Ijzermans JN. Prospective comparative study of spiral computer tomography and magnetic resonance imaging for detection of hepatocellular carcinoma. Gut. 2002;51:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Carroll NM, Alexander HR. Isolation perfusion of the liver. Cancer J. 2002;8:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Jeong YY, Mitchell DG, Kamishima T. Small (< 20 mm) enhancing hepatic nodules seen on arterial phase MR imaging of the cirrhotic liver: clinical implications. AJR Am J Roentgenol. 2002;178:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Vogl TJ, Balzer JO, Mack MG, Bett G, Oppelt A. Hybrid MR interventional imaging system: combined MR and angiography suites with single interactive table. Feasibility study in vascular liver tumor procedures. Eur Radiol. 2002;12:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Teng GJ, He SC, Guo JH, Cai XL, Gao GR. Preoperative transcatheter hepatic arterial embolization for hepatic malignancy. Invest Radiol. 1993;28:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | An Y, Bie P, Dong J. [Hepatic artery and portal vein dual perfusion chemotherapy in combination with injection of lipiodol-ethanol in treatment of advanced primary hepatocellular carcinoma]. Zhonghua Waike Zazhi. 2001;39:593-595. [PubMed] |
Edited by Pang LH