Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.44
Revised: October 1, 2002
Accepted: October 12, 2002
Published online: January 15, 2003
AIM: To explore the inhibition of conjugated linoleic acid isomers in different purity (75% purity c9, t11-, 98% purity c9, t11- and 98% purity t10,c12-CLA) on the formation of forestomach neoplasm and cheopreventive mechanisms.
METHODS: Forestomach neoplasm model induced by B (a) P in KunMing mice was established. The numbers of tumor and diameter of each tumor in forestomach were counted; the mice plasma malondialdehyde (MDA) were measured by TBARS assay; TUNEL assay was used to analyze the apoptosis in forestomach neoplasia and the expression of MEK-1, ERK-1, MKP-1 protein in forestomach neoplasm were studied by Western Blotting assay.
RESULTS: The incidence of neoplasm in B (a) P group, 75% purity c9,t11-CLA group, 98% purity c9,t11-CLA group and 98% purity t10,c12-CLA group was 100%, 75.0% (P > 0.05), 69.2% (P < 0.05) and 53.8% (P < 0.05) respectively and the effect of two CLA isomers in 98% purity on forestomach neoplasia was significant; CLA showed no influence on the average tumor numbers in tumor-bearing mouse, but significantly decreased the tumor size, the tumor average diameter of mice in 75% purity c9,t11-CLA group, 98% purity c9,t11-CLA group and 98% purity t10,c12-CLA group was 0.157 ± 0.047 cm, 0.127 ± 0.038 cm and 0.128 ± 0.077 cm (P < 0.05) and 0.216 ± 0.088 cm in B (a) P group; CLA could also significantly increase the apoptosis cell numbers by 144.00 ± 20.31, 153.75 ± 23.25, 157.25 ± 15.95 (P < 0.05) in 75% purity c9,t11-CLA group, 98% purity c9,t11-CLA group and 98% purity t10,c12-CLA group (30.88 ± 3.72 in BP group); but there were no significant differences between the effects of 75% purity c9,t11-CLA and two isomers in 98% purity on tumor size and apoptotic cell numbers; the plasma levels of MDA in were increased by 75% purity c9,t11-CLA, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA. The 75% purity c9,t11-CLA showed stronger inhibition; CLA could also inhibit the expression of ERK-1 protein and promote the expression of MKP-1 protein, however no influence of CLA on MEK-1 protein was observed.
CONCLUSION: Two isomers in 98% purity show stronger inhibition on carcinogenesis. However, the inhibitory mechanisms of CLA on carcinogenesis is complicated, which may be due to the increased mice plasma MDA, the inducing apoptosis in tumor tissues. And the effect of CLA on the expression of ERK-1 and MKP-1 may be one of the mechanisms of the inhibition of CLA on the tumor.
- Citation: Chen BQ, Xue YB, Liu JR, Yang YM, Zheng YM, Wang XL, Liu RH. Inhibition of conjugated linoleic acid on mouse forestomach neoplasia induced by benzo (a) pyrene and chemopreventive mechanisms. World J Gastroenterol 2003; 9(1): 44-49
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/44.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.44
Conjugated linoleic acid (CLA) refers to a group of dienoic derivatives of linoleic acid that can be found in natural foods, such as the milk fat and meat of ruminant animals[1-4]. CLA can also be synthesized in the laboratory and is available commercially as a dietary supplement and has shown to be non-toxic[5]. In several animal experiments, supplementation of feeding with CLA showed an anticarcinogenic effect against chemical-induced cancers of the skin, forestomach, colon and breast[6-9]. Moreover, of the individual isomers of CLA, c9, t11 isomer has been implicated as most active biologically because it is the predominant isomer incorporated into the phospholipids of cell membrane, however recent evidence showed the t10,c12-CLA isomer might also exert biological activity[10]. To date, the sample used for animal experiment or cell experiment is the isomer mixture of conjugated linoliec acid which mainly containing c9, t11-, t10, c12-, t9, t11-, t10,t12-CLA. Potent anticarcinogenic effects have been attributed to a synthetic mixture of CLA containing c9,t11- and t9,c11-CLA (43%) and t10,c12-CLA (45.3%)[7,11-14]. For example, CLA used in Ha’s report contained c9,t11-,t10,c12-,t9,t11-,t10,t12-CLA which accounted for about 90% of the material[7]; Hubbard applied a mixture of CLA isomers with 32.5% c9,t11-CLA and 32.5% t10,c12-CLA isomers making up 66% to mammary tumor metastasis[14], etc. In summary, there were few reports assessing the effects of CLA monomer on the carcinogenesis in animal model.
Now the effect of CLA on carcinogenesis had been confirmed and there were evidences to support CLA action on the every stage of cancer, including initiation[15,16], post-initiation (promotion)[17,18], progression[19] and metastasis[14,20-22]. However the mechanisms through which CLA inhibits tumorigenesis are moot. Ha et al[7] suggested an antioxidant mechanism; Schonberg reported that the biochemical mechanisms by which CLA exerted its anticancer activity possibly including the formation of cytotoxic lipid peroxidation products, but this might not be sufficient to explain all the effects of these naturally occurring isomers of CLA[23]; Ip’s data showed an effect on growth and development of certain types of mammary cells[24,25]; Reduced formation of carcinogen-DNA adducts had been implicated[15,16]; Durgam’s work showed CLA inhibit cancer by influences on the oestrogen response system[26]; Others suggested inhibition mechanisms of CLA including its effects on eicosanoid metabolism[27-31], apoptosis[32,33], the gene expression such as stearoyl-CoA desaturase[34,35], PPRA[36-38], cyclin A, B(1), D(1) cyclin-dependent kinases inhibitors and (CDKI)(P16 and P21)[39] ect.
In our research group, we found that 75% purity c9,t11-CLA inhibit cancer incidence by 40%; at the same time that 98% purity c9,t11 and t10,c12-CLA showed stronger inhibition on human gastric cancer (SGC-7901) and breast cancer cells (MCF-7)[39-42]. Our study was designed to investigate the inhibition effects of synthetically-prepared individual isomer of CLA in different purity (75% purity c9,t11-CLA, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA) on the forestomach neolpasia induced by B (a) P and the mechanism whereby CLA acted as an anticarcinogen, especially in terms of lipid peroxidation, apoptosis and MAPKs pathway.
BP was purchased from Fluka Chemie AG of Switzerland. Salad oil was purchased from a local grocery. 75% purity c9,t11-CLA, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA were provided by Dr. Ruihai Liu at Cornell University.
KunMing mice, 6 to 7 wk of age, were purchased from Cancer Research Institute of HarBin Medical University in China. Two weeks later the animals were randomized by body weight and divided into 7 groups (15 mice/group). They were then subjected to a forestomach tumorigenesis as follows: each animal except animals in salad control group was given 0.2 ml salad oil solution per 20 mg body weight (1 mg BP in 0.2 ml of salad oil solution) by gavage and animals in salad control group were given 0.2 ml salad oil only twice every week for over after 4 wk. And the following diets were given after 2 weeks of giving BP and continue for 7 wk (Table 1). Beginning with the first intubation and continuing thereafter, the body weight and food intake were recorded twice weekly. All surviving mice were sacrificed 26 wk after the first dose of BP.
| Group | diets |
| Salad oil Control (A) | Standard diet + salad oil: fat (salad oil) concentration was 20% |
| BP Control (B) | Standard diet + salad oil: fat (salad oil) concentration was 20% |
| BP + 75% c9,t11-CLA (C) | Standard diet + salad oil + 75% c9,t11-CLA: Fat (salad oil) concentration was 20%, CLA 0.8% |
| BP + 98% c9,t11-CLA (D) | Standard diet + salad oil + 98% c9,t11-CLA: Fat (salad oil) concentration was 20%, CLA 0.5% |
| BP + 98% t10,c12-CLA (E) | Standard diet + salad oil + 98% t10,c12-CLA: Fat (salad oil) concentration was 20%, CLA 0.5% |
At termination of the study, the forestomach was removed. Tumor numbers and size were recorded, and then fixed in 10% formalin and paraffin-embedded; 4 µm sections were cut and stained with hematoxylin and eosin (HE).
The TBARS test was used to measure malonaldehyde (MDA). The mouse plasma MDA levels was determined by TBARs kits (Jiancheng Biotechnology Institute, NanJing, P.R. China).
In situ Cell Death Detection Kit, Fluorescein, were purchased from Boehringer Mannheim. Briefly, Fixed and permeabilized apoptotic tissue sections, incubated the tissue section with the TUNEL reaction mixture containing TdT and fluorescein-dUTP, detected the incorporated fluorescein with an anti-fluorescein antibody POD conjugate and at last visualized the immunocomplex with a substrate reaction were in light microscope. The apoptosis cell number was counted in 103 cells.
Three mice forestomaches in each group were homogenated and lysed in RIPA buffer (150 mM NaCl, 0.1% NP40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris, pH7.4) with protease inhibitor, leupeptin and aprotinin. Equal amounts of protein (80 µg/lane) were resolved by SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and immunobloted with an mouse anti-MEK-1, rabbit anti-ERK-1 and rabbit anti-MKP-1 antibody, then incubated with horseradish peroxidase secondary antibodies. Immunoreactive bands were detected using DAB (diaminobenzidine tetrahydrochloride) substrate and analyzed with a ChemiImagerTM 4000 Low Light Imaging System (Alpha Innotech Corporation, at the same time used GAPDH as house-keeping protein.
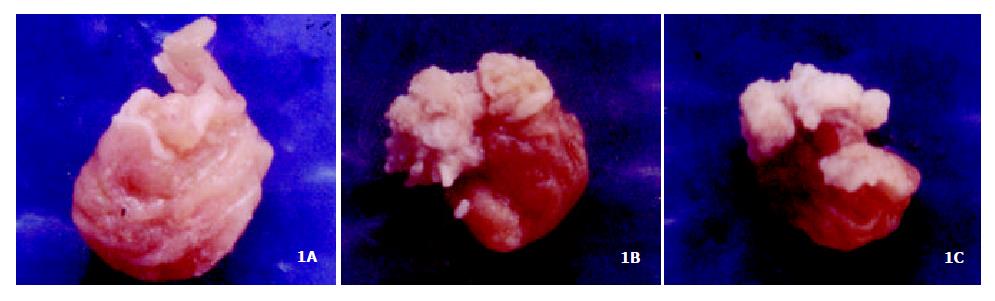
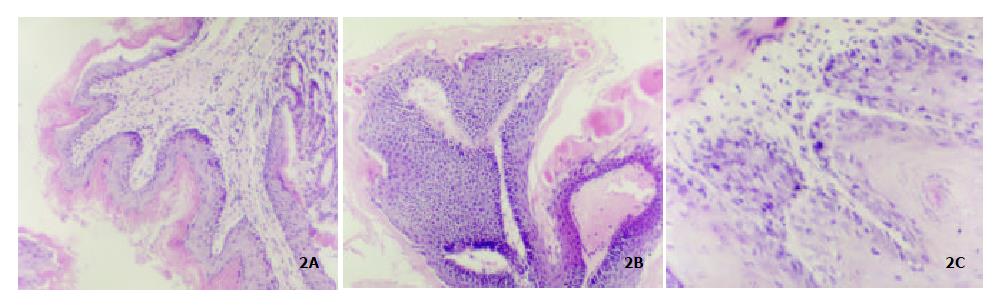
Figure 1A showed the normal forestomach with smooth surface and without tumor. However, the white-yellow cauliflower-like neoplasia in different size appeared in the forestomach of mice induced B (a) P (Figure 1B, Figure 1C). The structure of mouse forestomach in Figure 2A was normal and squamous epithelial cells and glandular epithelium cells were in order; The pathological analysis of B (a) P-induced forestomach neoplasia showed atypical hyperplasia in Figure 2B with stratified squamous epithelium excessively cornified, with focal proliferative basal cells and hypertrophic echinocyte; The basal cells, proliferating actively and out of order, grew through basement membrane and developed carcinoma in situ (Figure 2C).
The effect of CLA on BP-induced neoplasia of the forestomach in female KunMing mice was shown in Table 2. The incidence of the tumor and average diameter of tumor in 98% purity c9,t11-CLA group and 98% purity t10,c12-CLA group were significantly lower than that in B (a) P group (P < 0.05). 75% Purity c9,t11-CLA only decreased the tumor incidence which was no significant (P > 0.05), but its influence on the average diameter being significant (P < 0.05). The average tumor number of each tumor-bearing mouse showed no statistical significance (P > 0.05).
| Group | No. of mice (No. of tumor-bearing mice)/treatment | Total number of tumors | Tumor incidence (%) | Tumors/tumor-bearing moused | Diameter/tumor (CM) |
| A | 14 (0) | 0 | 0 | 0 | 0 |
| B | 12 (12) | 31 | 100 | 2.58 ± 0.90 | 0.216 ± 0.088 |
| C | 12 (9) | 23 | 75.0a | 2.56 ± 0.73 | 0.157 ± 0.047e |
| D | 13 (9) | 22 | 69.2bc | 2.44 ± 0.53 | 0.127 ± 0.038e |
| E | 13 (7) | 21 | 53.8bc | 3.00 ± 0.58 | 0.128 ± 0.077e |
The plasma levels of MDA measured by the TBARS assay increased in mice treated by 75% purity c9,t11-CLA, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA (P < 0.05). Moreover, 75% purity c9,t11-CLA were more effective than purity 98% c9,t11-CLA and 98% purity t10,c12-CLA (Table 3).
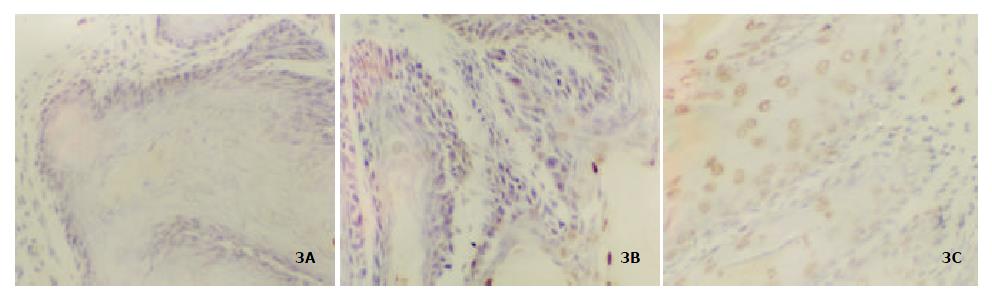
Figure 3 showed the apoptosis in forestomach neoplasia. Table 4 showed that 75% c9,t11-CLA, purity 98% c9,t11-CLA and 98% purity t10,c12-CLA can significantly induce apoptosis but with no statistical difference (P < 0.05).
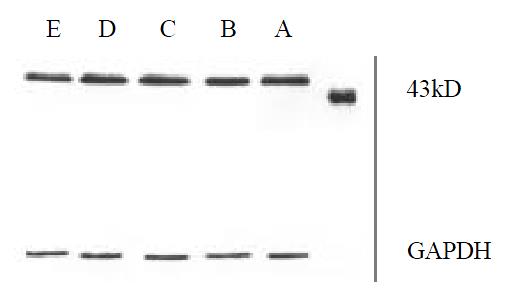
Figure 4, Figure 5, Figure 6 showed that 98% purity t10,c12-CLA decreased the expression of MEK-1 protein, but no influence was observed in mice treated by 75% purity c9,t11-CLA and 98% c9,t11-CLA. The expression of ERK-1 protein in 75% purity c9,t11-CLA group, 98% purity c9,t11-CLA group and 98% purity t10,c12-CLA group was inhibited significantly, and 98% purity c9,t11-CLA and 98% purity t10,c12-CLA showed more effective than 75% purity c9,t11-CLA. The expression of MKP-1 was increased in mice treated by 75% purity c9,t11-CLA, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA.
To date, all of the in vivo work with CLA has been done with a commercial free fatty acid preparation containing a mixture of c9, t11-, t10, c12-, c11, t13-isomers, although food CLA is predominatly the c9, t11-isomer present in triacylglycerols (80%-90%). Ha et al reported that dietary mixture of c9,t11-CLA, t10,c12-CLA, t9,t11-CLA and t10,t12-CLA significantly decreased the incidence of mouse neoplasm induced B (a) P (up to 20%)[7]. In DMBA-induced mammary adenocarcinoma model, the incidence in mice treated with dietary 0.05%, 0.1%, 0.25%, 0.5% CLA isomer mixture was 58%, 42%, 34%, 36%, respectively (that of the control was 56%)[9]. In this study, we found that the incidence in mice fed with diet containing 75% purity c9,t11-CLA (75%), 98% purity c9,t11-CLA (69.2%), 98% purity t10,c12-CLA (53.8%) had been decreased, moreover, 98% purity isomers showed significant influences in the inhibition of carcinogenesis; although 75% purity c9,t11-CLA decreased tumor incidence with no statistical significance, it still significantly decreased the tumor size; all of which suggested that the effect of CLA on carcinogenesis was possibly related to the CLA purity. In addition, the different isomers of CLA in different purities decreased the average diameter of the tumors, but no influence was observed in average tumor numbers of each tumor-bearing mouse, which might be one of characteristics of the inhibition of food components on carcinogenesis.
Lu reported that arachidonic acid and linoleic acid may promote HSC proliferation, but increased concentration can be cytotoxic to HSC[44]. As one of the positional and structural isomer of linoleic acid, more interests in conjugated linoleic acid promoting oxidation in cancer cells were paid upon. The study results in Stanton’s group showed that CLA could make breast cancer cell more susceptible to lipid peroxidation, moreover, the extent of lipid peroxidation of CLA treated cells was related to CLA-induced cytotoxicity against cancer cell lines. At the same time, they found that milk fat triglyceride-bound CLA, consisting primarily of the c9, t11 isomer, was also cytotoxic towards MCF-7 cells[45-47]. In our study, 75% purity c9,t11-CLA, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA increased the levels of MDA in mouse plasma and 75% purity c9,t11-CLA was most effective, but 75% purity c9,t11-CLA did not show strongest inhibition of carcinogenesis, which suggested that the cytotoxic effect of lipid peroxidation to tumor cells might be one of mechanisms by which CLA exerted its biochemical activity. And Schönberg also reported that the formation of MDA induced by 40 µmol·L-1 CLA in lung adenocarcinoma cell lines was completely abolished by 30 microM vitamin E, but the growth rates were only partially restored, which indicated that cytotoxic lipid peroxidation products were only in part responsible for the growth inhibitory effects of CLA[24]. Furthermore, the lipid peroxidation product induced played important role in apoptosis[48]. We found that purity 75% c9,t11-CLA, purity 98% c9,t11-CLA and 98% purity t10,c12-CLA induced significant apoptosis cells in forestomach neoplasia, but there were no differences between 75% purity c9,t11-CLA group, 98% purity c9,t11-CLA and 98% purity t10,c12-CLA groups. The MAPK family consists of at least three different subgroups which include: ERKs, JNKs (SAPKs), and p38 MAPK kinase[49-53]. The Ras-Raf-MEK1/2-ERK1/2 pathway has been explained more clearly. Lavoie found that the transcription of cyclin D1 requires the long-term activation of ERK, which suggests that ERK can regulate cell cycle. In one report, there is a homeostasis between JNK/SAPKs and ERK systems: when ERK cascade is predominant in lymphocyte, cells will proliferate; by contraries, JNK/SAPK cascade will activate cell apoptosis[54]. It is found that the abnormalities of Ras/Raf/MAPK cascade reaction may contribute to malignant transformation of hepatocytes and activation of MAPK proteins may be an early event in hepatocellular carcinogenesis[55]. In summary, The activation of Ras-Raf-MEK-ERK can promote cell proliferation and inhibit cell apoptosis. In addition, The product of the immediate early gene MAP kinase phosphatase (MKP-1), is able to dephosphorylate phosphoserine/threonine as well as phosphotyrosine residues, and shows selectivity for ERKs 1 and 2 in vitro, with lower activity toward other MAP kinases such as JNK and P38 MAP kinase[56]. MKP-1 inactivates ERK following growth factor stimulation in intact cells and also suppresses signaling downstream of ERK at the level of gene transcription and proliferation[57], most likely through its inhibitory effects on MAP kinase. In our study, we found that CLA could inhibit the expression of ERK-1 protein, and at the same time inactivate the ERK-1 by increasing the expression of MKP-1, which might be one of mechanisms of CLA anticarcinogen.
In summary, it is confirmed that CLA shows inhibition on forestomach neoplasia induced by B (a) P, which is possibly related with CLA purity. Although the inhibition of different isomers (c9,t11- and t10,c12-CLA) on carcinogenesis is different, they show no significant difference. Moreover, the inhibition mechanism of CLA is complicated and difficult to be explained by an mechanism.
We thank Dr. Rui-Hai Liu at Cornell University for helps and 75% purity c9,t11-CLA, 98% purity c9,t11-CLA, 98% purity t10,c12-CLA.
| 1. | Ha YL, Grimm NK, Pariza MW. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8:1881-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 555] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Kritchevsky D. Antimutagenic and some other effects of conjugated linoleic acid. Br J Nutr. 2000;83:459-465. [PubMed] |
| 3. | Pariza MW, Park Y, Cook ME. Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med. 2000;223:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Pariza MW, Park Y, Cook ME. Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999;52:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Scimeca JA. Toxicological evaluation of dietary conjugated linoleic acid in male Fischer 344 rats. Food Chem Toxicol. 1998;36:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Belury MA, Nickel KP, Bird CE, Wu Y. Dietary conjugated linoleic acid modulation of phorbol ester skin tumor promotion. Nutr Cancer. 1996;26:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Ha YL, Storkson J, Pariza MW. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990;50:1097-1101. [PubMed] |
| 8. | Xu M, Dashwood RH. Chemoprevention studies of heterocyclic amine-induced colon carcinogenesis. Cancer Lett. 1999;143:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212-1215. [PubMed] |
| 10. | Sébédio JL, Gnaedig S, Chardigny JM. Recent advances in conjugated linoleic acid research. Curr Opin Clin Nutr Metab Care. 1999;2:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ip C, Chin SF, Scimeca JA, Pariza MW. Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res. 1991;51:6118-6124. [PubMed] |
| 12. | Schut HA, Cummings DA, Smale MH, Josyula S, Friesen MD. DNA adducts of heterocyclic amines: formation, removal and inhibition by dietary components. Mutat Res. 1997;376:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Pariza MW, Hargraves WA. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1985;6:591-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 150] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Hubbard NE, Lim D, Summers L, Erickson KL. Reduction of murine mammary tumor metastasis by conjugated linoleic acid. Cancer Lett. 2000;150:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Josyula S, Schut HA. Effects of dietary conjugated linoleic acid on DNA adduct formation of PhIP and IQ after bolus administration to female F344 rats. Nutr Cancer. 1998;32:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Josyula S, He YH, Ruch RJ, Schut HA. Inhibition of DNA adduct formation of PhIP in female F344 rats by dietary conjugated linoleic acid. Nutr Cancer. 1998;32:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Ip C, Jiang C, Thompson HJ, Scimeca JA. Retention of conjugated linoleic acid in the mammary gland is associated with tumor inhibition during the post-initiation phase of carcinogenesis. Carcinogenesis. 1997;18:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Kimoto N, Hirose M, Futakuchi M, Iwata T, Kasai M, Shirai T. Site-dependent modulating effects of conjugated fatty acids from safflower oil in a rat two-stage carcinogenesis model in female Sprague-Dawley rats. Cancer Lett. 2001;168:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 19. | Ip C, Scimeca JA, Thompson H. Effect of timing and duration of dietary conjugated linoleic acid on mammary cancer prevention. Nutr Cancer. 1995;24:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Xue Y, Chen B, Zheng Y, Yuan L. [Effects of conjugated linoleic acid on the metastasis of mouse melanoma B16-MB]. Weisheng Yanjiu. 2001;30:37-39. [PubMed] |
| 21. | Chen BQ, Xue YB, Feng WJ, Zheng YM, Liu RH. The effects of conjugated linoleic acid on the adhension and migration of B16-MB mouse melanoma cells.. J Health Toxicology. 2001;1:20-23. |
| 22. | Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 1998;18:1429-1434. [PubMed] |
| 23. | Visonneau S, Cesano A, Tepper SA, Scimeca JA, Santoli D, Kritchevsky D. Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res. 1997;17:969-973. [PubMed] |
| 24. | Schønberg S, Krokan HE. The inhibitory effect of conjugated dienoic derivatives (CLA) of linoleic acid on the growth of human tumor cell lines is in part due to increased lipid peroxidation. Anticancer Res. 1995;15:1241-1246. [PubMed] |
| 25. | Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, Barbano D, Bauman D. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr. 1999;129:2135-2142. [PubMed] |
| 26. | Durgam VR, Fernandes G. The growth inhibitory effect of conjugated linoleic acid on MCF-7 cells is related to estrogen response system. Cancer Lett. 1997;116:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Cunningham DC, Harrison LY, Shultz TD. Proliferative responses of normal human mammary and MCF-7 breast cancer cells to linoleic acid, conjugated linoleic acid and eicosanoid synthesis inhibitors in culture. Anticancer Res. 1997;17:197-203. [PubMed] |
| 28. | Liu KL, Belury MA. Conjugated linoleic acid modulation of phorbol ester-induced events in murine keratinocytes. Lipids. 1997;32:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Igarashi M, Miyazawa T. The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochim Biophys Acta. 2001;1530:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Thompson H, Zhu Z, Banni S, Darcy K, Loftus T, Ip C. Morphological and biochemical status of the mammary gland as influenced by conjugated linoleic acid: implication for a reduction in mammary cancer risk. Cancer Res. 1997;57:5067-5072. [PubMed] |
| 31. | Miller A, Stanton C, Devery R. Modulation of arachidonic acid distribution by conjugated linoleic acid isomers and linoleic acid in MCF-7 and SW480 cancer cells. Lipids. 2001;36:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Ip MM, Masso-Welch PA, Shoemaker SF, Shea-Eaton WK, Ip C. Conjugated linoleic acid inhibits proliferation and induces apoptosis of normal rat mammary epithelial cells in primary culture. Exp Cell Res. 1999;250:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Ip C, Ip MM, Loftus T, Shoemaker S, Shea-Eaton W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9:689-696. [PubMed] |
| 34. | Choi Y, Park Y, Storkson JM, Pariza MW, Ntambi JM. Inhibition of stearoyl-CoA desaturase activity by the cis-9,trans-11 isomer and the trans-10,cis-12 isomer of conjugated linoleic acid in MDA-MB-231 and MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2002;294:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Choi Y, Park Y, Pariza MW, Ntambi JM. Regulation of stearoyl-CoA desaturase activity by the trans-10,cis-12 isomer of conjugated linoleic acid in HepG2 cells. Biochem Biophys Res Commun. 2001;284:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Thuillier P, Anchiraico GJ, Nickel KP, Maldve RE, Gimenez-Conti I, Muga SJ, Liu KL, Fischer SM, Belury MA. Activators of peroxisome proliferator-activated receptor-alpha partially inhibit mouse skin tumor promotion. Mol Carcinog. 2000;29:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Moya-Camarena SY, Vanden Heuvel JP, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res. 1999;40:1426-1433. [PubMed] |
| 38. | Moya-Camarena SY, Van den Heuvel JP, Belury MA. Conjugated linoleic acid activates peroxisome proliferator-activated receptor alpha and beta subtypes but does not induce hepatic peroxisome proliferation in Sprague-Dawley rats. Biochim Biophys Acta. 1999;1436:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901). World J Gastroenterol. 2002;8:224-229. [PubMed] |
| 40. | Liu JR, Chen BQ, Deng H, Han XH, Liu RH. Cellular Apoptosis Induced by Conjugated Linoleic Acid in Human Gastric Cancer (SGC-7901) Cells.. Gongye Weisheng Yu Zhiyebing. 2001;27:129-133. |
| 41. | Liu JR, Chen BQ, Xue YB, Han XH, Yang YM, Liu RH. Conju-gated linoleic acid inhibits the growth of mammarg carcinoma cells.. Zhonghua Yufang Yixue. 2001;35:244-247. |
| 42. | Zhu Y, Qiou J, Chen BQ, Liu RH. The inhibitory effect of conjugated linoleic acid on mice forestomach neoplasia induced by benzo (a) pyrene.. Zhonghua Yufang Yixue. 2001;35:19-22. |
| 43. | Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo (a) pyrene (B (a) P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60-65. [PubMed] |
| 44. | Lu LG, Zeng MD, Li JQ, Hua J, Fan JG, Qiu DK. Study on the role of free fatty acids in proliferation of rat hepatic stellate cells (II). World J Gastroenterol. 1998;4:500-502. [PubMed] |
| 45. | Devery R, Miller A, Stanton C. Conjugated linoleic acid and oxidative behaviour in cancer cells. Biochem Soc Trans. 2001;29:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | O'Shea M, Stanton C, Devery R. Antioxidant enzyme defence responses of human MCF-7 and SW480 cancer cells to conjugated linoleic acid. Anticancer Res. 1999;19:1953-1959. [PubMed] |
| 47. | O'Shea M, Devery R, Lawless F, Murphy J, Stanton C. Milk fat conjugated linoleic acid (CLA) inhibits growth of human mammary MCF-7 cancer cells. Anticancer Res. 2000;20:3591-3601. [PubMed] |
| 48. | Hawkins RA, Sangster K, Arends MJ. Apoptotic death of pancreatic cancer cells induced by polyunsaturated fatty acids varies with double bond number and involves an oxidative mechanism. J Pathol. 1998;185:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 49. | Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 635] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 50. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4033] [Article Influence: 161.3] [Reference Citation Analysis (33)] |
| 51. | Meng AH, Ling YL, Zhang XP, Zhao XY, Zhang JL. CCK-8 inhibits expression of TNF-alpha in the spleen of endotoxic shock rats and signal transduction mechanism of p38 MAPK. World J Gastroenterol. 2002;8:139-143. [PubMed] |
| 52. | Fleischer F, Dabew R, Göke B, Wagner AC. Stress kinase inhibition modulates acute experimental pancreatitis. World J Gastroenterol. 2001;7:259-265. [PubMed] |
| 53. | Wu K, Liu BH, Zhao DY, Zhao Y. Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2001;7:83-87. [PubMed] |
| 54. | Jarvis WD, Fornari FA, Auer KL, Freemerman AJ, Szabo E, Birrer MJ, Johnson CR, Barbour SE, Dent P, Grant S. Coordinate regulation of stress- and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol Pharmacol. 1997;52:935-947. [PubMed] |
| 55. | Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33-36. [PubMed] |
| 56. | Dai T, Rubie E, Franklin CC, Kraft A, Gillespie DA, Avruch J, Kyriakis JM, Woodgett JR. Stress-activated protein kinases bind directly to the delta domain of c-Jun in resting cells: implications for repression of c-Jun function. Oncogene. 1995;10:849-855. [PubMed] |
| 57. | Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 942] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
Edited by Wu XN